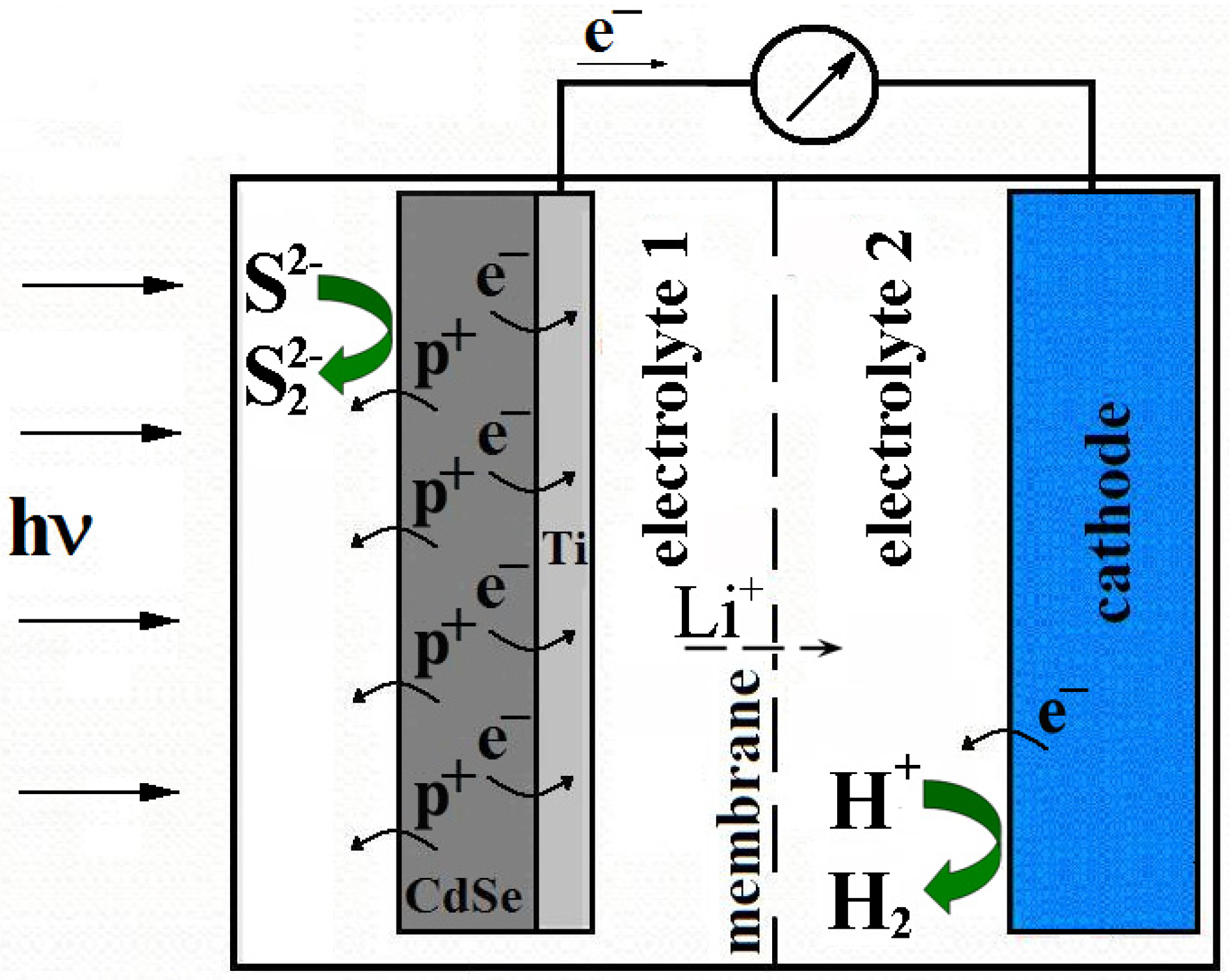
Photoelectrochemical Hydrogen Production System Using Li-Conductive Ceramic Membrane
Abstract
:1. Introduction
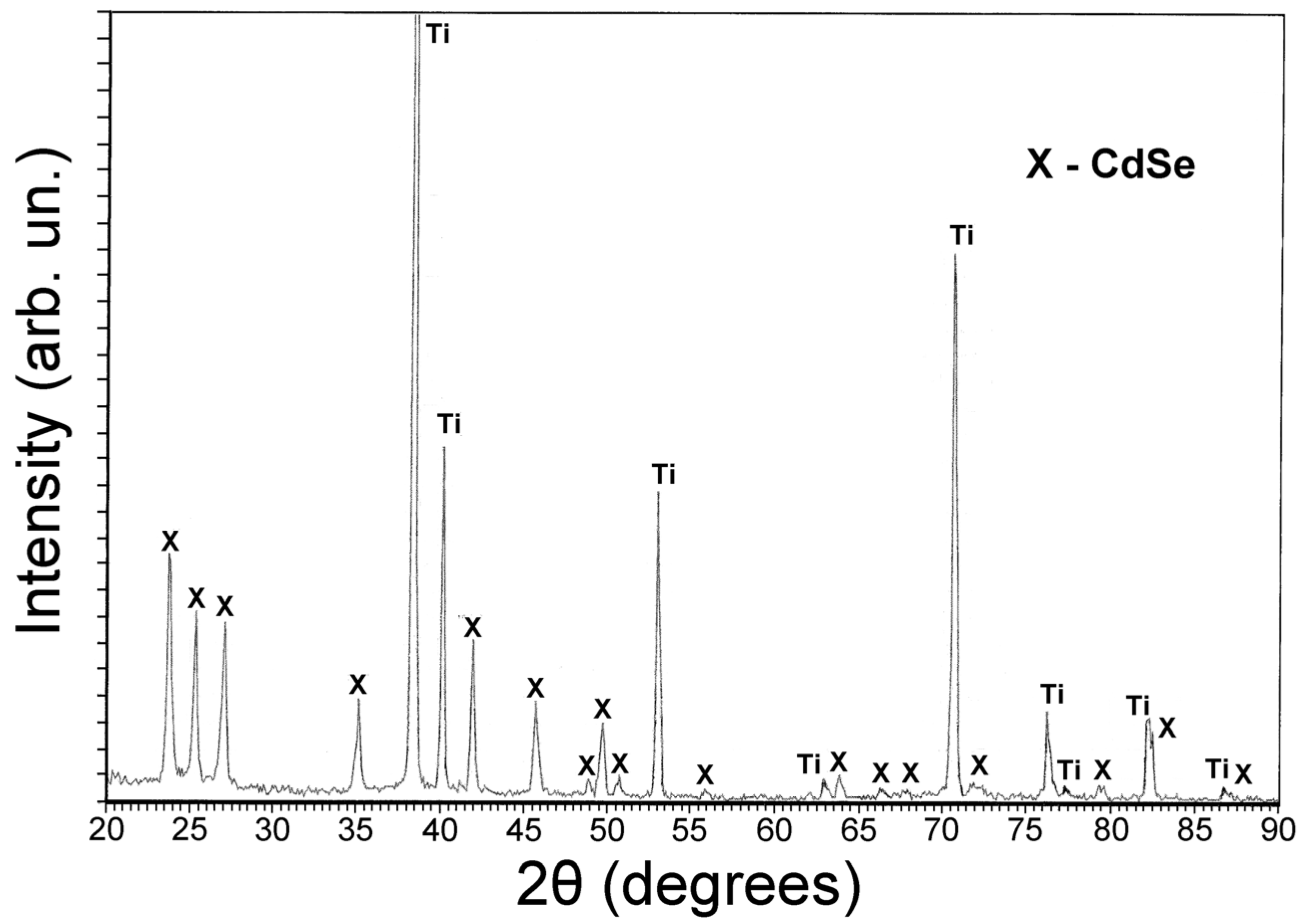
2. Experimental Section
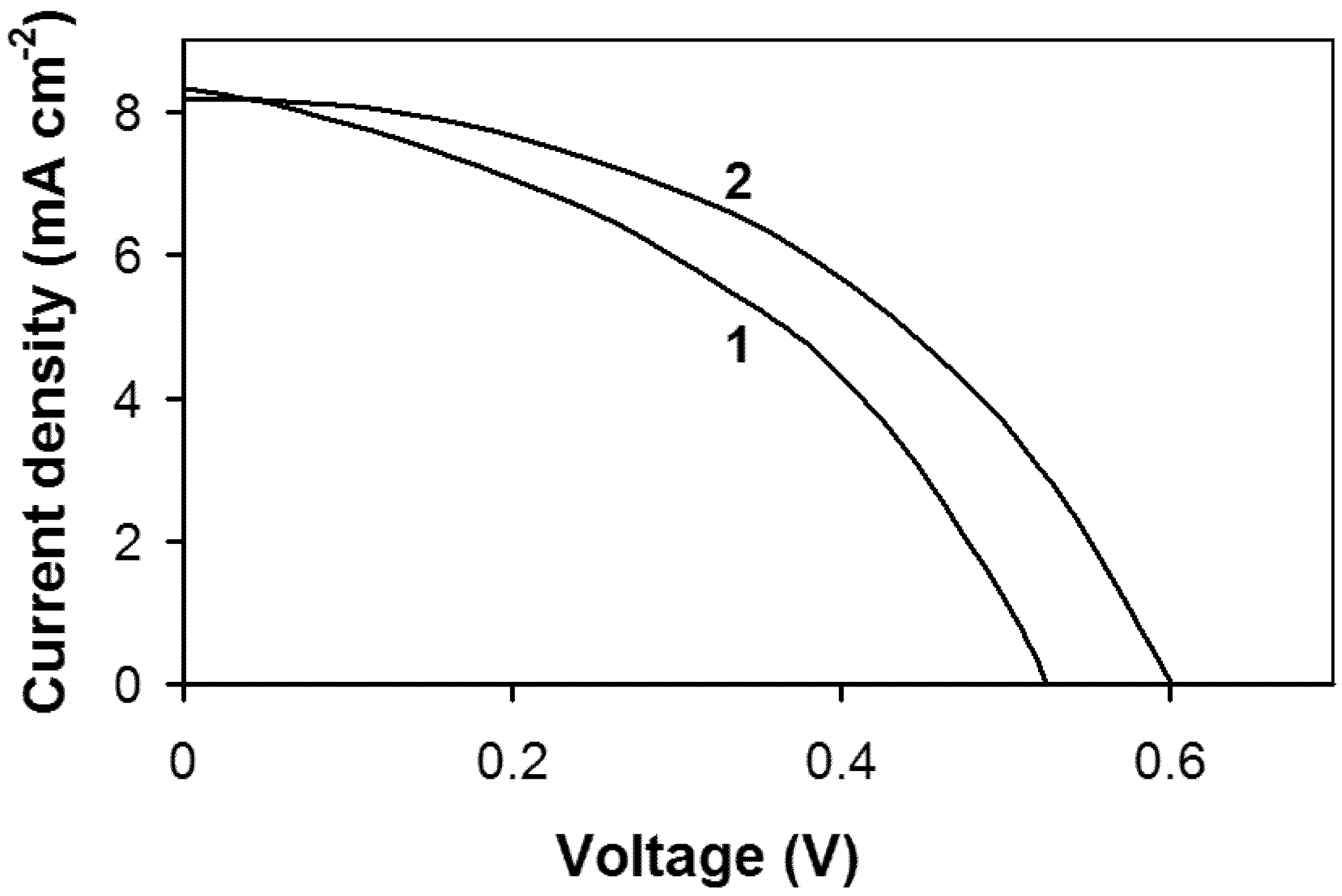
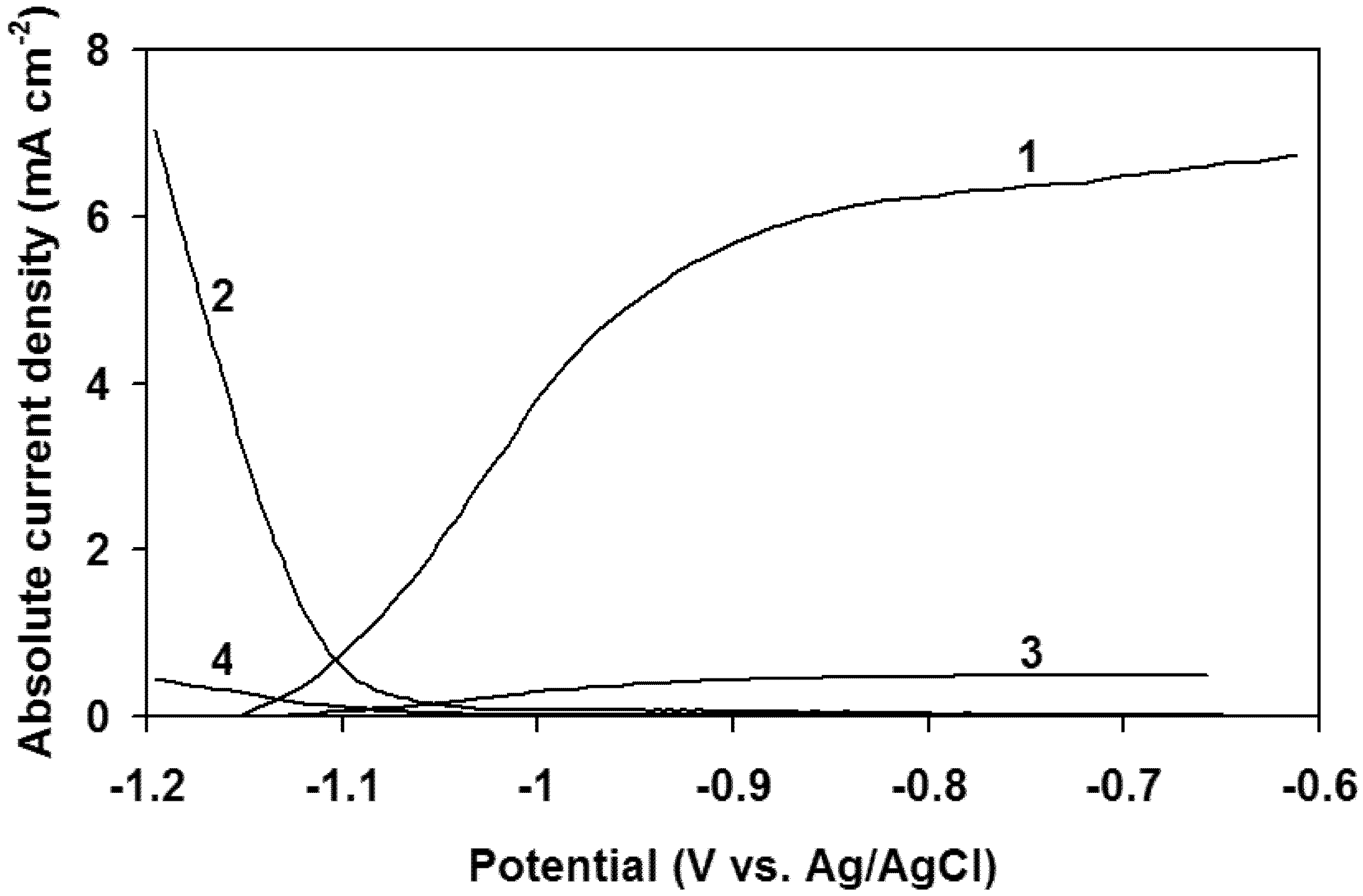
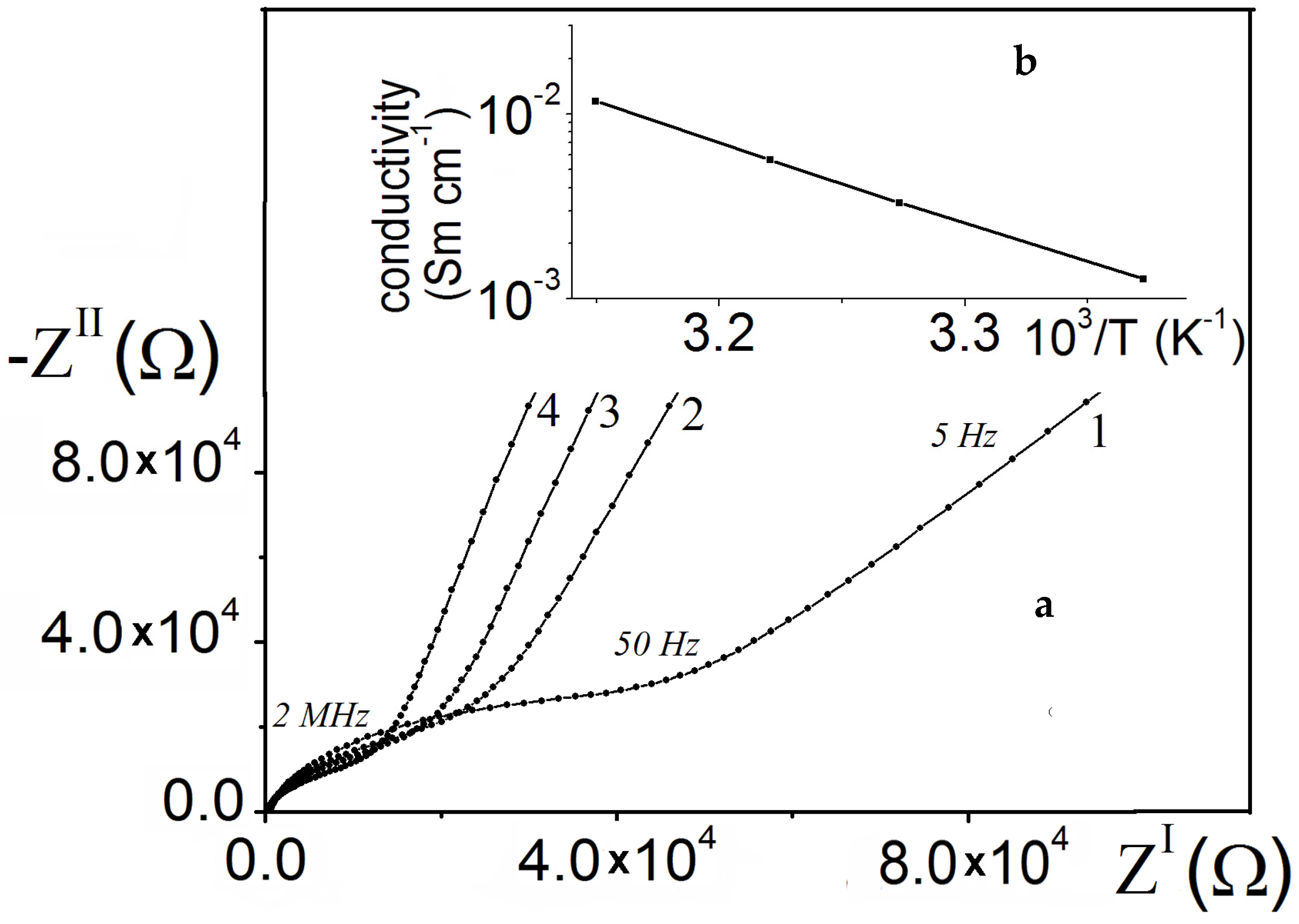
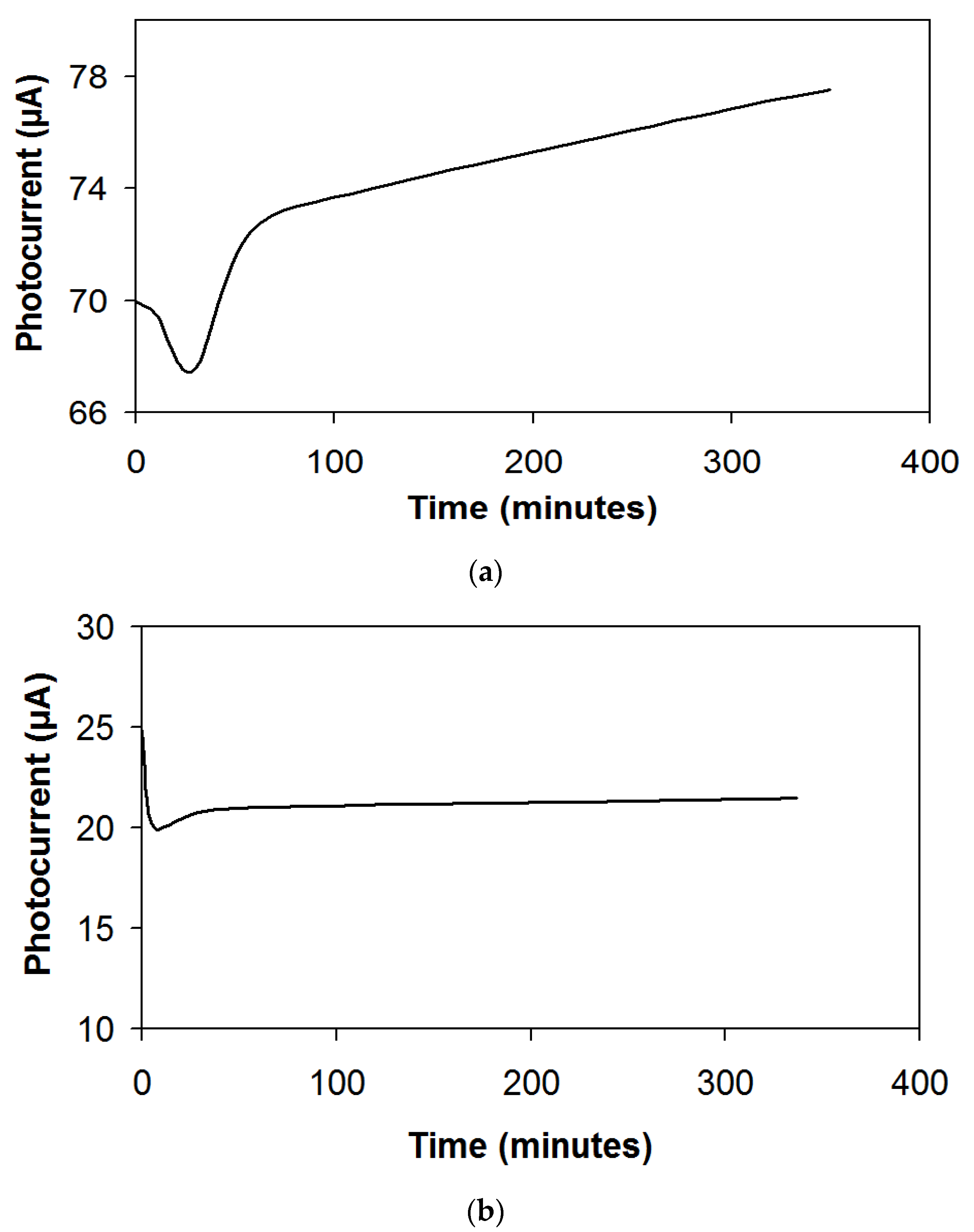
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rusetskyi, I.A.; Danilov, M.O.; Fomanyuk, S.S.; Slobodyanyuk, I.A.; Vorobets, V.S.; Kolbasov, G.Y. Photoelectrochemical properties of the composites based on TiO2 nanotubes, CdSe and graphene oxide. Res. Chem. Intermed. 2019, 45, 4121–4132. [Google Scholar] [CrossRef]
- Kasap, S.; Capper, P. (Eds.) Springer Handbook of Electronic and Photonic Materials; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; p. 1088. [Google Scholar] [CrossRef] [Green Version]
- Heller, A.; Miller, В. Some recent progress in semiconductor-liquid junction solar cells. Electrochem. Acta 1980, 25, 29–41. [Google Scholar] [CrossRef]
- Russak, M.A.; Reichman, J.; Witzke, H.; Deb, S.K.; Chen, S.N. Thin Film CdSe Photoanodes for Electrochemical Photovoltaic Cells. J. Electrochem. Soc. 1980, 127, 725–733. [Google Scholar] [CrossRef]
- Tenne, R. The Effect of Added Salts on the Stability of Cd-Chalcogenide/Polysulfide Photoelectrochemical Cells. J. Electrochem. Soc. 1982, 129, 143–145. [Google Scholar] [CrossRef]
- Miller, B.; Heller, A.; Robbins, M.; Menezes, S.; Chang, K.C.; Thomson, J., Jr. Solar Conversion Efficiency of Pressure Sintered Cadmium Selenide Liquid Junction Cells. J. Electrochem. Soc. 1977, 124, 1019–1022. [Google Scholar] [CrossRef]
- Rusetskii, I.A.; Shcherbakova, L.G.; Danilov, M.O.; Slobodyanyuk, I.A.; Kolbasov, G.Y.; Fomanyuk, S.S.; Solonin, Y.M. Accumulation of Solar Hydrogen in the Photoelectrochemical System Based on CdSe Photoanode and MH Cathode. ECS Trans. 2018, 87, 335–342. [Google Scholar] [CrossRef]
- Pleskov, Y.V.; Gurevich, Y.Y. Semiconductor Photoelectrochemistry; Consultants Bureau: New York, NY, USA; London, UK, 1986; p. 422. ISBN 0-306-10983-2. [Google Scholar]
- Grätzel, M. (Ed.) Energy Resources through Photochemistry and Catalysis; Academic Press: New York, NY, USA; London, UK, 1983; p. 588. ISBN 9780323145145. [Google Scholar]
- Shcherbakova, L.G.; Dan‘ko, D.B.; Muratov, V.B.; Kossko, I.A.; Solonin, Y.M.; Kolbasov, G.Y.; Rusetskii, I.A. Metal hydride use for solar energy accumulation. In NATO Security through Science Series—A: Chemistry and Biology. Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Veziroglu, T.N., Zaginaichenko, S.Y., Schur, D.V., Baranowski, B., Shpak, A.V., Skorokhod, V.V., Kale, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 699–706. [Google Scholar] [CrossRef]
- Solonin, Y.; Dan’ko, D.; Galiy, O.; Kossko, I.; Kolbasov, G.; Rusetskii, I. Hydrogen Storage in Metal Hydride Under Action of Sunlight. In Fuel Cell Technologies: State and Perspectives. NATO Science Series II. Mathematics, Physics and Chemistry; Sammes, N., Smirnova, A., Vasylyev, O., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 202, pp. 193–198. [Google Scholar] [CrossRef]
- Antropov, L.I.; Beknazarov, A. Theoretical Electrochemistry; University Press of the Pacific: Honolulu, HI, USA, 2001; p. 568. ISBN 0898753236 9780898753233. [Google Scholar]
- Dobos, D. Electrochemical Data: A Handbook for Electrochemists in Industry and Universities; Elsevier Elsevier Science & Technology: Amsterdam, The Netherlands, 1975; p. 339, ISBN-10: 0444998632; ISBN-13: 978-0444998637. [Google Scholar]
- Lidiard, A.B. Ionic Conductivity. In Electrical Conductivity II. Elektrische Leitungsphänomene II. Handbuch der Physik. Encyclopedia of Physics; Springer: Berlin/Heidelberg, Germany, 1957; Volume 4/20, pp. 246–349. [Google Scholar] [CrossRef]
- Belous, A.G.; Novitskaya, G.N.; Polyanetskaya, S.V.; Gornikov, Y.I. Investigation into complex oxides of La2/3-xLi3xTiO3 composition. Izv. Akad. Nauk SSSR, Neorg. Mater. 1987, 23, 470–472. (In Russian) [Google Scholar] [CrossRef]
- Inaguma, Y.; Chen, L.Q.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Rivera, A.; Leon, C.; Santamaria, J.; Várez, A.; Paris, M.A.; Sanz, J. Li3xLa2/3-xTiO3 fast ionic conductors. Correlation between lithium mobility and structure. J. Non-Cryst. Solids 2002, 307–310, 992–998. [Google Scholar] [CrossRef]
- Sanz, J.; Alonso, J.; Várez, A. Structural analysis of Li-ion conducting perovskites Li0,5-xNaxLa0,5TiO3. Inst. Laue-Langevin Annu. Rep. 2002, 34–35. [Google Scholar]
- Pham, Q.N.; Bohnke, O.; Bohnke, C. Potentiometric measurements and impedance characteristics of Li0,30La0,57TiO3 membrane in lithium anhydrous solutions. Electrochim. Acta 2006, 51, 6186–6193. [Google Scholar] [CrossRef]
- Slobodyanyuk, I.; Rusetskyi, I.; Shcherbakova, L.; Danilov, M.; Kolbasov, G.; Solonin, Y. The photoelectrochemical cell with hydrogen accumulation at the conditions of natural insolation. Fr.-Ukr. J. Chem. 2018, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Belous, A.; Yanchevskiy, O.; V’yunov, O.; Bohnke, O.; Bohnke, C.; Le Berre, F.; Fourquet, J.-L. Peculiarities of Li0.5La0.5TiO3 Formation during the Synthesis by Solid-State Reaction or Precipitation from Solutions. Chem. Mater. 2004, 16, 407–417. [Google Scholar] [CrossRef]
- Harris, D.; Winnick, J.; Kohl, A.P. Cation Effect on the CdSe - Liquid Junction. J. Electrochem. Soc. 1993, 140, 2581–2588. [Google Scholar] [CrossRef]
- Licht, S.; Hodes, G.; Manassen, J. Numerical analysis of aqueous polysulfide solutions and its application to cadmium chalcogenide/polysulfide photoelectrochemical solar cells. Inorg. Chem. 1986, 25, 2486–2489. [Google Scholar] [CrossRef]
- Rivera, A.; Leόn, C.; Santamaría, J.; Várez, A.; V’yunov, O.I.; Belous, A.G.; Alonso, J.A.; Sanz, J. Percolation-Limited Ionic Diffusion in Li0.5-xNaxLa0.5TiO3 Perovskites (0 ≤ x ≤ 0.5). Chem. Mater. 2002, 14, 5148–5152. [Google Scholar] [CrossRef]
- Herrero, C.; Várez, A.; Rivera, A.; Santamaría, J.; Leόn, C.; V’yunov, O.; Belous, A.; Sanz, J. Influence of Vacancy Ordering on the Percolative Behavior of (Li1-xNax)3yLa2/3-yTiO3 Perovskites. J. Phys. Chem. B. 2005, 109, 3262–3268. [Google Scholar] [CrossRef]
- Sanjuan, M.L.; Laguna, M.A.; Belous, A.G.; V’yunov, O.I. On the Local Structure and Lithium Dynamics of La0.5(Li,Na)0.5TiO3 Ionic Conductors. A Raman Study. Chem. Mater. 2005, 17, 5862–5866. [Google Scholar] [CrossRef]
- Boyano, I.; Mainar, A.R.; Blázquez, J.A.; Kvasha, A.; Bengoechea, M.; de Meatza, I.; García-Martín, S.; Varez, A.; Sanz, J.; García-Alvarado, F. Reduction of Grain Boundary Resistance of La0. 5Li0.5TiO3 by the Addition of Organic Polymers. Nanomaterials 2021, 11, 61. [Google Scholar] [CrossRef]
- Anderson, E.L.; Buhlmann, P. Electrochemical Impedance Spectroscopy of Ion-Selective Membranes: Artifacts in Two-, Three-, and Four-Electrode Measurements. Anal. Chem. 2016, 88, 9738–9745. [Google Scholar] [CrossRef]
- Kashkovskiy, R.; Strelnikova, K.; Fedotova, A. Application of electrochemical impedance spectroscopy to study hydrogen sulphide corrosion of steel and its inhibition: A review. Corros. Eng. Sci. Technol. 2019, 54, 493–515. [Google Scholar] [CrossRef]
- Belous, A.G. Lithium ion conductors based on the perovskite La2/3-xLi3xTiO3. J. Eur. Ceram. Soc. 2001, 21, 1797–1800. [Google Scholar] [CrossRef]
- Belous, A.G.; Kolbasov, G.Y.; Boldyrev, E.I.; Kovalenko, L.L. Lithium–Air Cell with Lanthanum–Lithium Titanate Ceramic Electrolyte. Russ. J. Electrochem. 2015, 51, 1162–1167. [Google Scholar] [CrossRef]
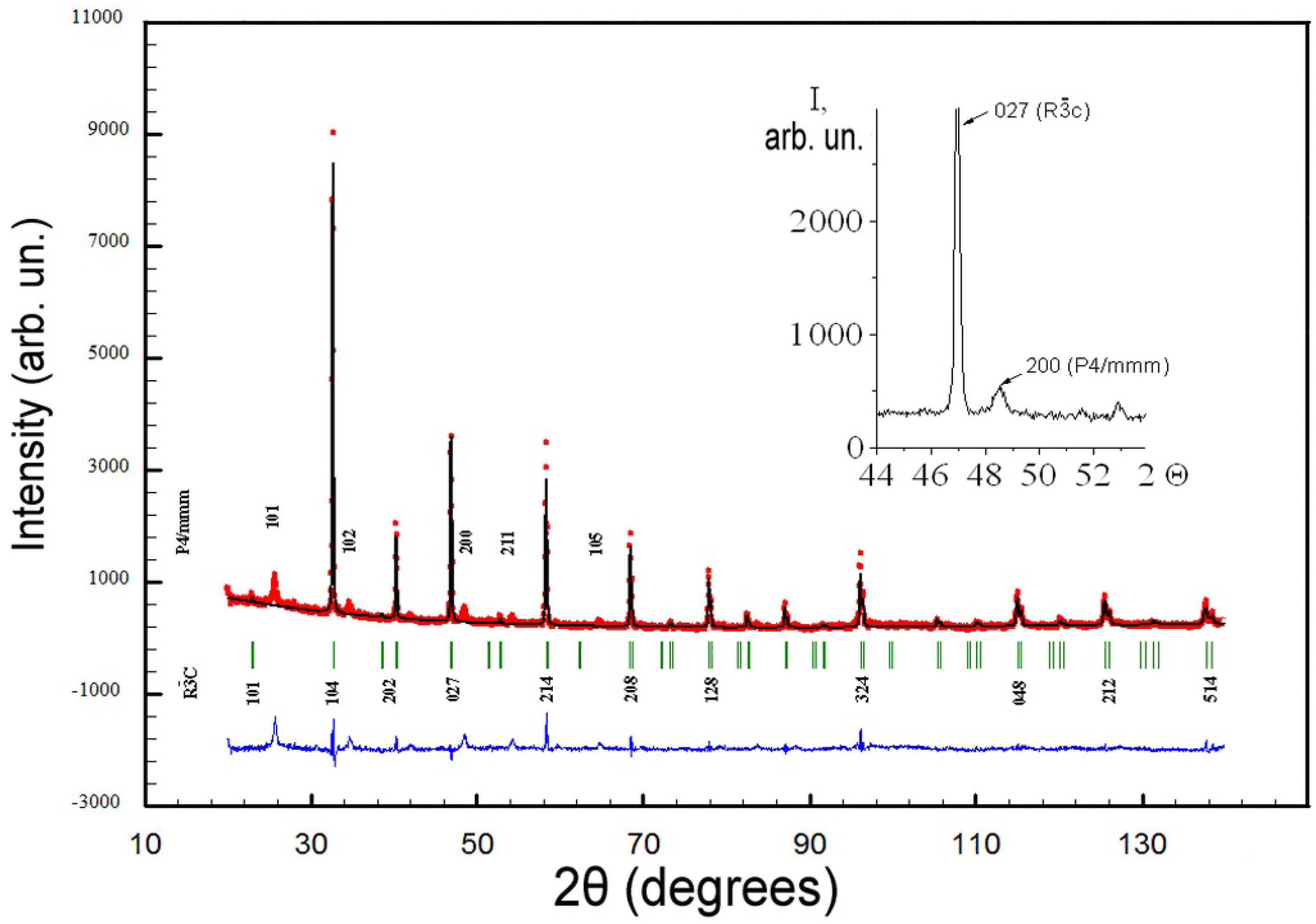



| R3c Phase | P4/mmm | |
|---|---|---|
| Unit cell parameters | ||
| a, A | 5.4836(4) | 5.4720(3) |
| c, A | 13.44(1) | 7.7735(6) |
| V, A3 | 349.9(3) | 232.76(3) |
| Coordinates of ions * | ||
| Ti z/c | 0 | 0.251(4) |
| O1 x/a | 0.53(3) | ½ |
| O3/O4 z/c | - | 0.21(2) |
| Reliability factor | ||
| X2 | 9.53 | 9.53 |
| RB, % | 6.74 | 13.5 |
| Rf, % | 8.86 | 10.5 |
| Rexp,% | 9.17 | 9.17 |
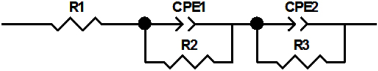 | |||
|---|---|---|---|
| Membrane Thickness 0.70 mm | Membrane Thickness 1.35 mm | ||
| electrolyte 30% KOH + 2 M LiOH | |||
| R1 (Ohm) | 82.32 | R1 (Ohm) | 164.2 |
| CPE1 | 3.895 × 10−7 | CPE1 | 2.579 × 10−7 |
| R2 (Ohm) | 5069 | R2 (Ohm) | 9911 |
| CPE2 | 1.038 × 10−4 | CPE2 | 0.8163 × 10−4 |
| R3 (Ohm) | 1.3573 × 105 | R3 (Ohm) | 2.0037 × 105 |
| electrolyte 30% H2SO4 + 1 M Li2SO4 | |||
| R1 (Ohm) | 82.09 | R1 (Ohm) | 157.3 |
| CPE1 | 4.3954 × 10−7 | CPE1 | 2.3086 × 10−7 |
| R2 (Ohm) | 4985 | R2 (Ohm) | 9588 |
| CPE2 | 4.2605 × 10−5 | CPE2 | 3.3769 × 10−5 |
| R3 (Ohm) | 3.7751 × 105 | R3 (Ohm) | 2.0456 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusetskyi, I.A.; Kovalenko, L.L.; Danilov, M.O.; Slobodyanyuk, I.A.; Fomanyuk, S.S.; Smilyk, V.O.; Belous, A.G.; Kolbasov, G.Y. Photoelectrochemical Hydrogen Production System Using Li-Conductive Ceramic Membrane. Membranes 2022, 12, 1189. https://doi.org/10.3390/membranes12121189
Rusetskyi IA, Kovalenko LL, Danilov MO, Slobodyanyuk IA, Fomanyuk SS, Smilyk VO, Belous AG, Kolbasov GY. Photoelectrochemical Hydrogen Production System Using Li-Conductive Ceramic Membrane. Membranes. 2022; 12(12):1189. https://doi.org/10.3390/membranes12121189
Chicago/Turabian StyleRusetskyi, Ihor A., Leonid L. Kovalenko, Michail O. Danilov, Ivan A. Slobodyanyuk, Sergii S. Fomanyuk, Vitaliy O. Smilyk, Anatolii G. Belous, and Gennadii Ya. Kolbasov. 2022. "Photoelectrochemical Hydrogen Production System Using Li-Conductive Ceramic Membrane" Membranes 12, no. 12: 1189. https://doi.org/10.3390/membranes12121189






