Microbial Responses to Various Types of Chemical Regents during On-Line Cleaning of UF Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Microorganisms
2.2. Chemical Treatment Assay
2.3. Cross-Flow Ultrafiltration Test
2.4. Determination of EPS and Intracellular ROS of Microorganisms
2.5. Determination of Microbial Viability and Visualization of Biofilm
2.6. Determination of Microbial Surface Properties
3. Results and Discussion
3.1. Properties of Microorganisms after Exposure to Different Chemical Cleaning Agents
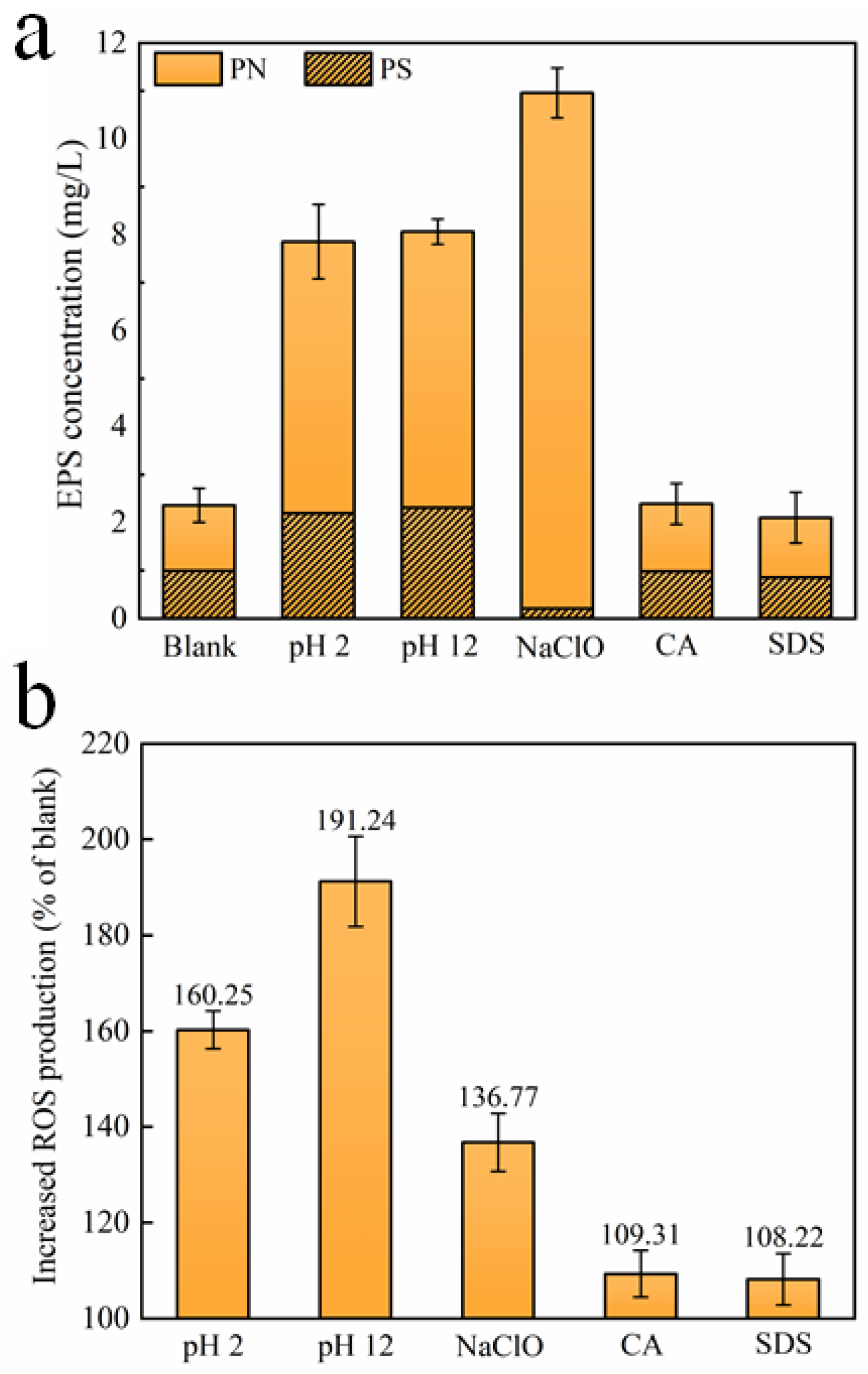
3.2. EPS and ROS Productions after Exposure to Different Chemical Cleaning Agents
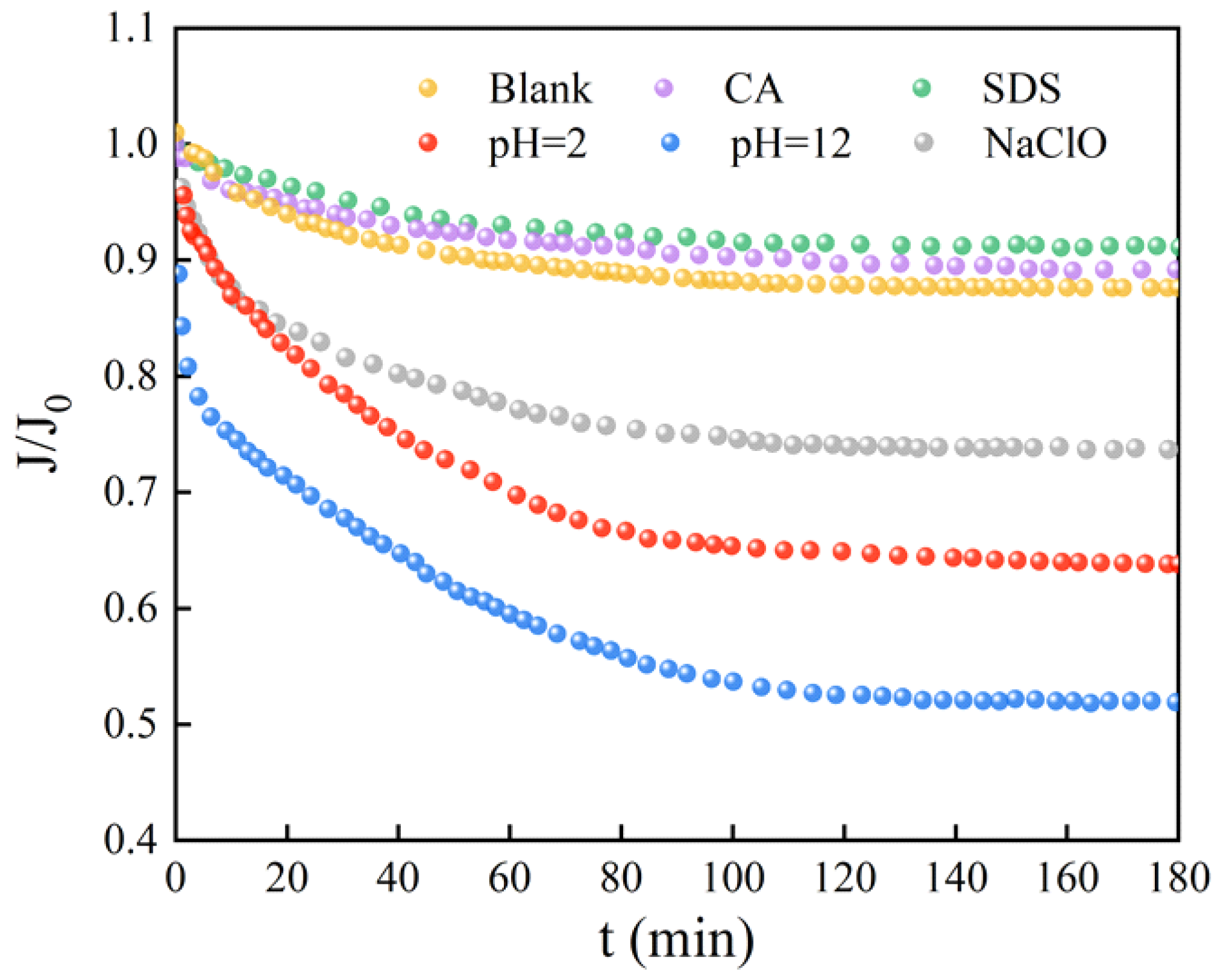
3.3. Membrane-Fouling Potentials of Microorganisms after Exposure to Different Chemical Cleaning Agents
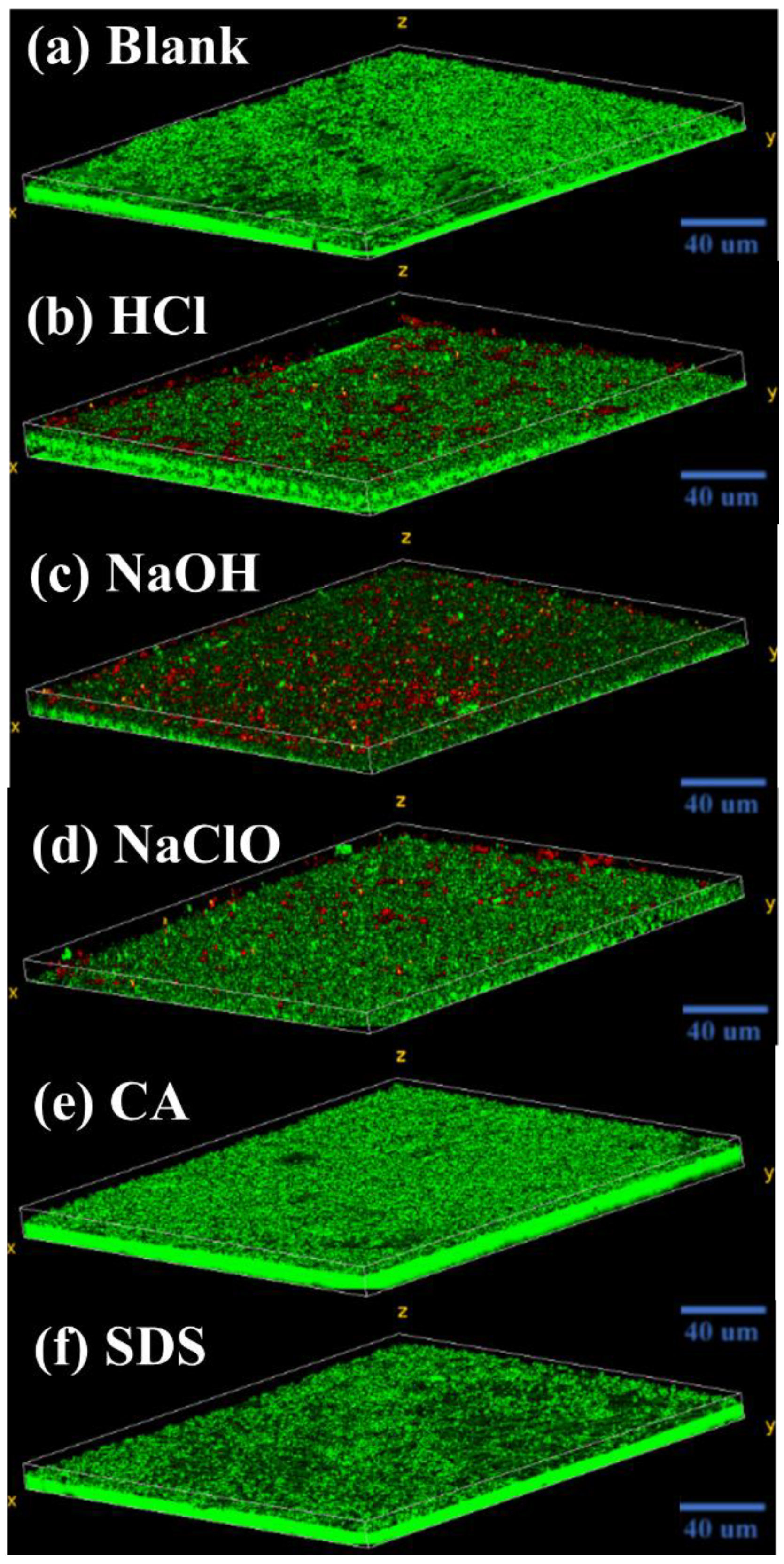
3.4. Morphology of Biofilm Layer
4. Conclusions
- (i)
- All of five cleaning agents do not significantly affect the zeta potential of microorganisms. HCl, NaOH, and NaClO markedly increase cell surface hydrophilicity, while CA and SDS have minimal impact. HCl, NaOH, NaClO, and SDS greatly reduce the mean size of microorganisms;
- (ii)
- Compared to suspended microorganisms without treatment, CA and SDS show a limited effect on EPS and ROS production. HCl, NaOH, and NaClO increase EPS release that could accelerate biofilm formation, and trigger intracellular ROS production that could lead to the death of a large quantity of microorganisms. The concentrations of PS and PN upon exposure to HCl and NaOH increase significantly. However, the concentration of PS upon exposure to NaClO decreases;
- (iii)
- During filtration, driven by a self-protecting strategy, surviving microorganisms after exposure to HCl, NaOH, and NaClO tend to rapidly colonize onto the membrane surface, assisted by abundantly released EPS, eventually forming a living cell layer covered by dead cells, which causes a significant flux decline;
- (iv)
- The severity of membrane biofouling seems closely corelated to the intracellular ROS production and PS content in the released EPS. Under the threat of NaOH, microorganisms with the highest intracellular ROS production and PS release cause the most serious membrane fouling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, Y.; Xie, P.; Wang, Z.; Wang, J.; Ding, J.; Dewil, R.; Van der Bruggen, B. Application of UV/chlorine pretreatment for controlling ultrafiltration (UF) membrane fouling caused by different natural organic fractions. Chemosphere 2021, 263, 127993. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Cai, W.; Gao, Z.; Yu, S.; Lv, M.; Shi, Y.; Wang, J. New insights into membrane fouling formation during ultrafiltration of organic wastewater with high salinity. J. Membr. Sci. 2021, 635, 119446. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process. Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Chamberland, J.; Lessard, M.; Doyen, A.; Labrie, S.; Pouliot, Y. Biofouling of ultrafiltration membrane by dairy fluids: Characterization of pioneer colonizer bacteria using a DNA metabarcoding approach. J. Dairy Sci. 2017, 100, 981–990. [Google Scholar] [CrossRef]
- Yu, S.; Wang, J.; Zhao, Z.; Cai, W. Simultaneous coupling of fluidized granular activated carbon (GAC) and powdered activated carbon (PAC) with ultrafiltration process: A promising synergistic alternative for water treatment. Sep. Purif. Technol. 2022, 282, 120085. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Chang, Y.; Jiang, S. Surface grafted sulfobetaine polymers via atom transfer radical polymerization as superlow fouling coatings. J. Phys. Chem. B 2006, 110, 10799–10804. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed]
- Trägårdh, G. Membrane cleaning. Desalination 1989, 71, 325–335. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Lee, H.; Amy, G.; Cho, J.; Yoon, Y.; Moon, S.; Kim, I.S. Cleaning strategies for flux recovery of an ultrafiltration membrane fouled by natural organic matter. Water Res. 2001, 35, 3301–3308. [Google Scholar] [CrossRef]
- Vanysacker, L.; Bernshtein, R.; Vankelecom, I.F.J. Effect of chemical cleaning and membrane aging on membrane biofouling using model organisms with increasing complexity. J. Membr. Sci. 2014, 457, 19–28. [Google Scholar] [CrossRef]
- Cui, L.; Chen, P.; Zhang, B.; Zhang, D.; Li, J.; Martin, F.L.; Zhang, K. Interrogating chemical variation via layer-by-layer SERS during biofouling and cleaning of nanofiltration membranes with further investigations into cleaning efficiency. Water Res. 2015, 87, 282–291. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, Z.; Cai, W. Chemically induced alteration in PAC characteristics and its influences on PAC/UF water treatment: Implications for on-line membrane cleaning with NaClO. Sep. Purif. Technol. 2022, 294, 121130. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Y. Oxidative stress induced membrane biofouling and its implications to on-line chemical cleaning in MBR. Chem. Eng. J. 2018, 334, 1917–1926. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, M. Polyethersulfone (PES)–silver composite UF membrane: Effect of silver loading and PVP molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Y. Comparative study of dissolved organic matter generated from activated sludge during exposure to hypochlorite, hydrogen peroxide, acid and alkaline: Implications for on-line chemical cleaning of MBR. Chemosphere 2018, 193, 295–303. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Y. Enhanced membrane biofouling potential by on-line chemical cleaning in membrane bioreactor. J. Membr. Sci. 2016, 511, 84–91. [Google Scholar] [CrossRef]
- D’Souza, N.; Mawson, J. Membrane cleaning in the dairy industry: A review. Crit. Rev. Food Sci. Nutr. 2005, 45, 125–134. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Wang, X.; Zheng, X.; Ma, J.; Wu, Z. Microbial responses to membrane cleaning using sodium hypochlorite in membrane bioreactors: Cell integrity, key enzymes and intracellular reactive oxygen species. Water Res. 2016, 88, 293–300. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, J.; Wang, Z.; Østerhus, S.W. Backpulsing technology applied in MF and UF processes for membrane fouling mitigation: A review. J. Membr. Sci. 2019, 587, 117136. [Google Scholar] [CrossRef]
- Some, S.; Mondal, R.; Mitra, D.; Jain, D.; Verma, D.; Das, S. Microbial pollution of water with special reference to coliform bacteria and their nexus with environment. Energy Nexus 2021, 1, 100008. [Google Scholar] [CrossRef]
- Duan, X.; Liao, X.; Chen, J.; Xie, S.; Qi, H.; Li, F.; Yuan, B. THMs, HAAs and NAs production from culturable microorganisms in pipeline network by ozonation, chlorination, chloramination and joint disinfection strategies. Sci. Total Environ. 2020, 744, 140833. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, H.; Zhang, S.; Xiong, Y.; Zheng, M.; Zhu, T.; Park, M.; Magnuson, J.T.; Zheng, C.; El-Din, M.G. Remediation of surface water contaminated by pathogenic microorganisms using calcium peroxide: Matrix effect, micro-mechanisms and morphological-physiological changes. Water Res. 2022, 211, 118074. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: The cost benefit of optimisation. Water Res. 2010, 44, 1389–1398. [Google Scholar] [CrossRef]
- Chen, V.; Li, H.; Li, D.; Tan, S.; Petrus, H.B. Cleaning strategies for membrane fouled with protein mixtures. Desalination 2006, 200, 198–200. [Google Scholar] [CrossRef]
- Cai, W.; Liu, J.; Zhang, X.; Ng, W.J.; Liu, Y. Generation of dissolved organic matter and byproducts from activated sludge during contact with sodium hypochlorite and its implications to on-line chemical cleaning in MBR. Water Res. 2016, 104, 44–52. [Google Scholar] [CrossRef]
- Cai, W.; Han, J.; Zhang, X.; Liu, Y. Formation mechanisms of emerging organic contaminants during on-line membrane cleaning with NaOCl in MBR. J. Hazard. Mater. 2020, 386, 121966. [Google Scholar] [CrossRef]
- Frosth, S.; Lewerin, S. Survival of streptococcus equi subsp. equi in normal saline versus phosphate-buffered saline and at two different temperatures. Equine Vet. J. 2019, 83, 102814. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, J.; Li, Y.; Chen, Q.; Xie, W.; Wang, J. Characterizing membrane fouling formation during ultrafiltration of high-salinity organic wastewater. Chemosphere 2022, 287, 132057. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hamadi, F.; Latrache, H. Comparison of contact angle measurement and microbial adhesion to solvents for assaying electron donor–electron acceptor (acid–base) properties of bacterial surface. Colloids Surf. B 2008, 65, 134–139. [Google Scholar] [CrossRef] [PubMed]
- McEldowney, S.; Fletcher, M. Variability of the influence of physicochemical factors affecting bacterial adhesion to polystyrene substrata. Appl. Environ. 1986, 52, 460–465. [Google Scholar] [CrossRef]
- Neu, T.R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol. Rev. 1996, 60, 151–166. [Google Scholar] [CrossRef]
- Zhou, Z.; He, X.; Zhou, M.; Meng, F. Chemically induced alterations in the characteristics of fouling-causing bio-macromolecules—Implications for the chemical cleaning of fouled membranes. Water Res. 2017, 108, 115–123. [Google Scholar] [CrossRef]
- Desmond, P.; Huisman, K.; Sanawar, H.; Farhat, N.; Traber, J.; Fridjonsson, E.; Johns, M.; Flemming, H.; Picioreanu, C.; Vrouwenvelder, J. Controlling the hydraulic resistance of membrane biofilms by engineering biofilm physical structure. Water Res. 2022, 210, 118031. [Google Scholar] [CrossRef]
- Arts, I.; Gennaris, A.; Collet, J. Reducing systems protecting the bacterial cell envelope from oxidative damage. FEBS Lett. 2015, 589, 1559–1568. [Google Scholar] [CrossRef]
- Kweon, J.H.; Jung, J.H.; Lee, S.R.; Hur, H.W.; Shin, Y.; Choi, Y.H. Effects of consecutive chemical cleaning on membrane performance and surface properties of microfiltration. Desalination 2012, 286, 324–331. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Chen, Q.; Song, X.; Wang, J.; Cai, W. Microbial Responses to Various Types of Chemical Regents during On-Line Cleaning of UF Membranes. Membranes 2022, 12, 920. https://doi.org/10.3390/membranes12100920
Gao Z, Chen Q, Song X, Wang J, Cai W. Microbial Responses to Various Types of Chemical Regents during On-Line Cleaning of UF Membranes. Membranes. 2022; 12(10):920. https://doi.org/10.3390/membranes12100920
Chicago/Turabian StyleGao, Zeyuan, Qiuying Chen, Xiaolan Song, Jingwei Wang, and Weiwei Cai. 2022. "Microbial Responses to Various Types of Chemical Regents during On-Line Cleaning of UF Membranes" Membranes 12, no. 10: 920. https://doi.org/10.3390/membranes12100920
APA StyleGao, Z., Chen, Q., Song, X., Wang, J., & Cai, W. (2022). Microbial Responses to Various Types of Chemical Regents during On-Line Cleaning of UF Membranes. Membranes, 12(10), 920. https://doi.org/10.3390/membranes12100920







