High Flux Nanofiltration Membranes with Double-Walled Carbon Nanotube (DWCNT) as the Interlayer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of the Substrate with DWCNT
2.3. Preparation of NF Membranes
2.4. Membranes Characterization
2.5. Membrane Filtration Performance
3. Results and Discussion
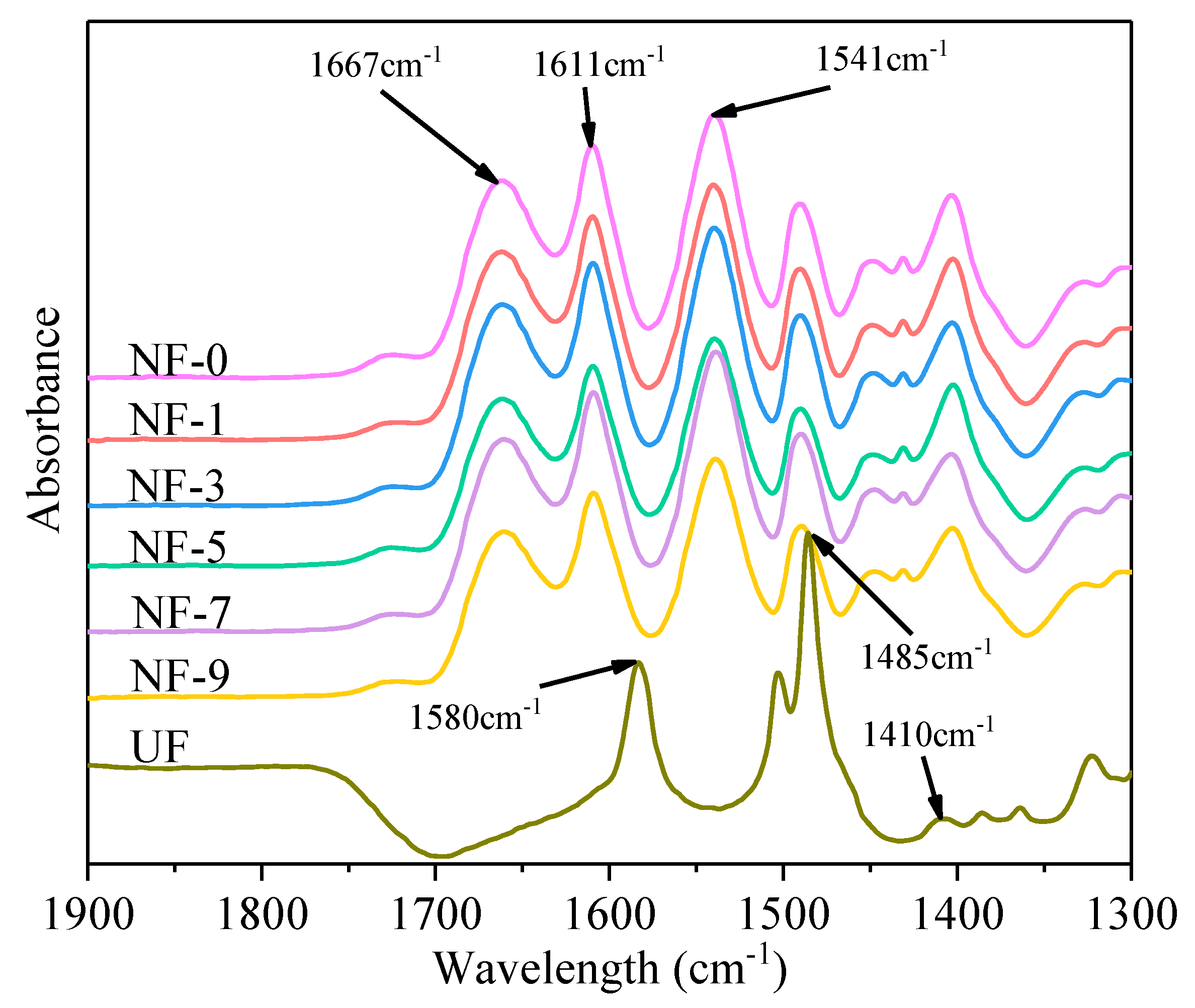
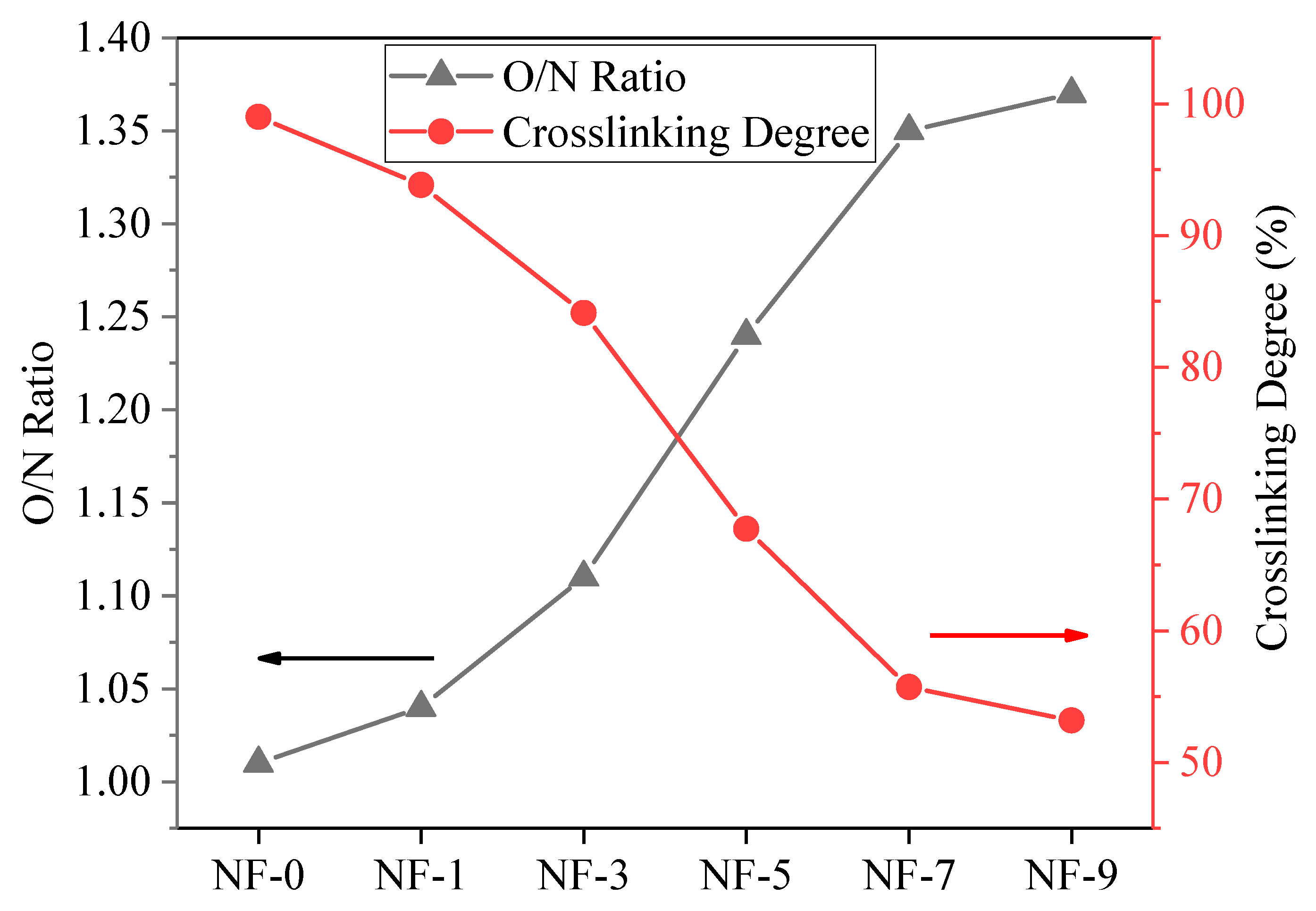
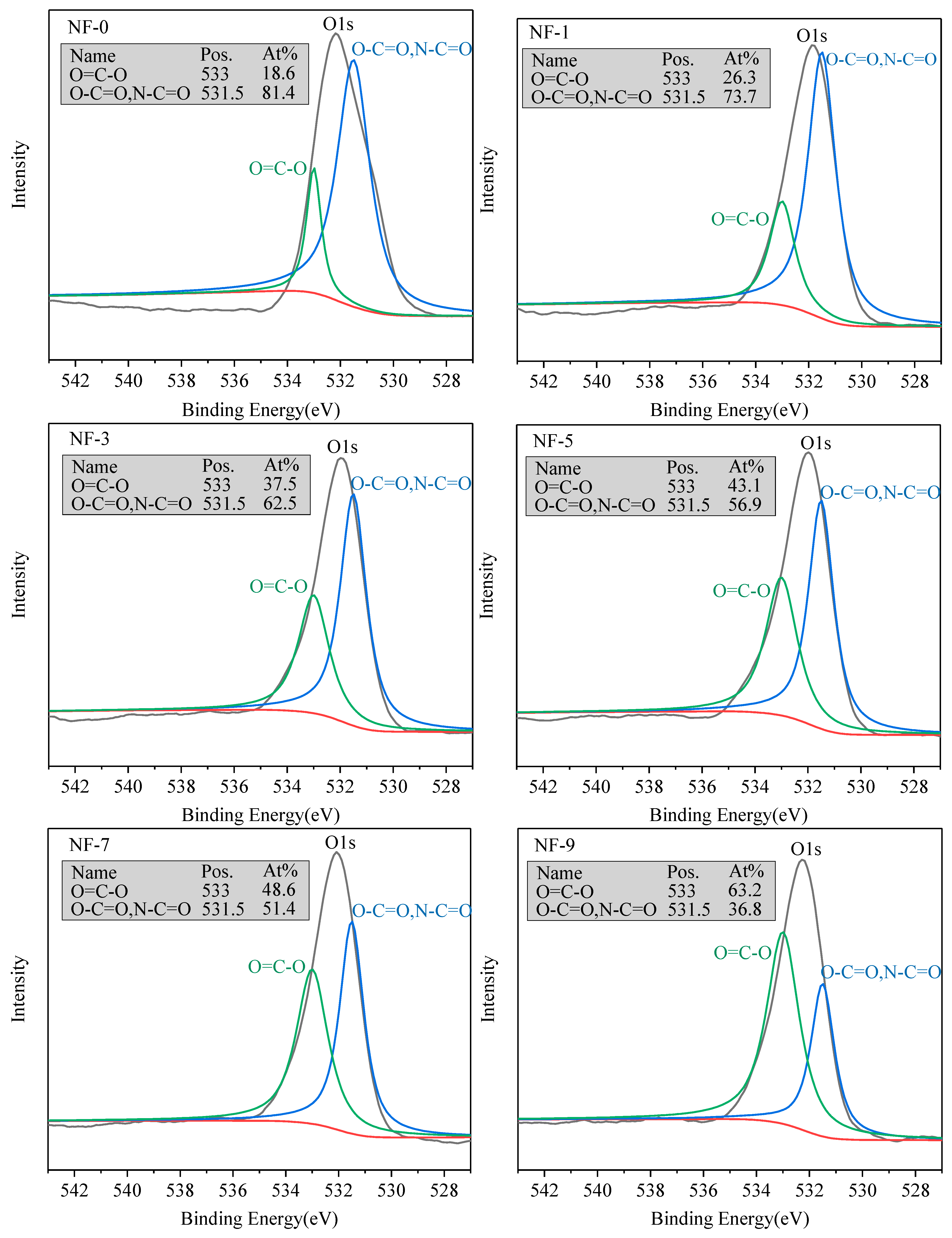
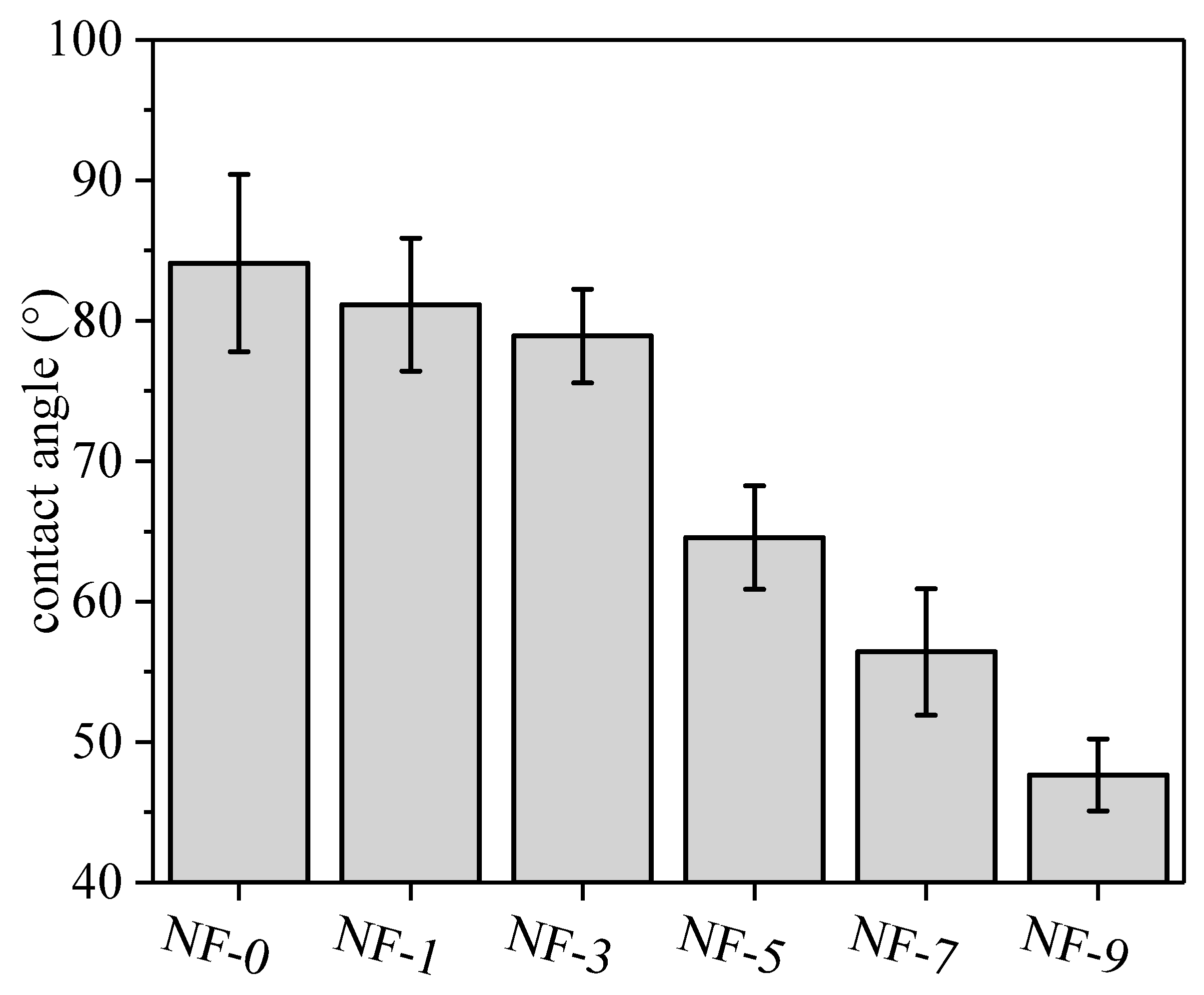
3.1. Surface Properties of Membranes
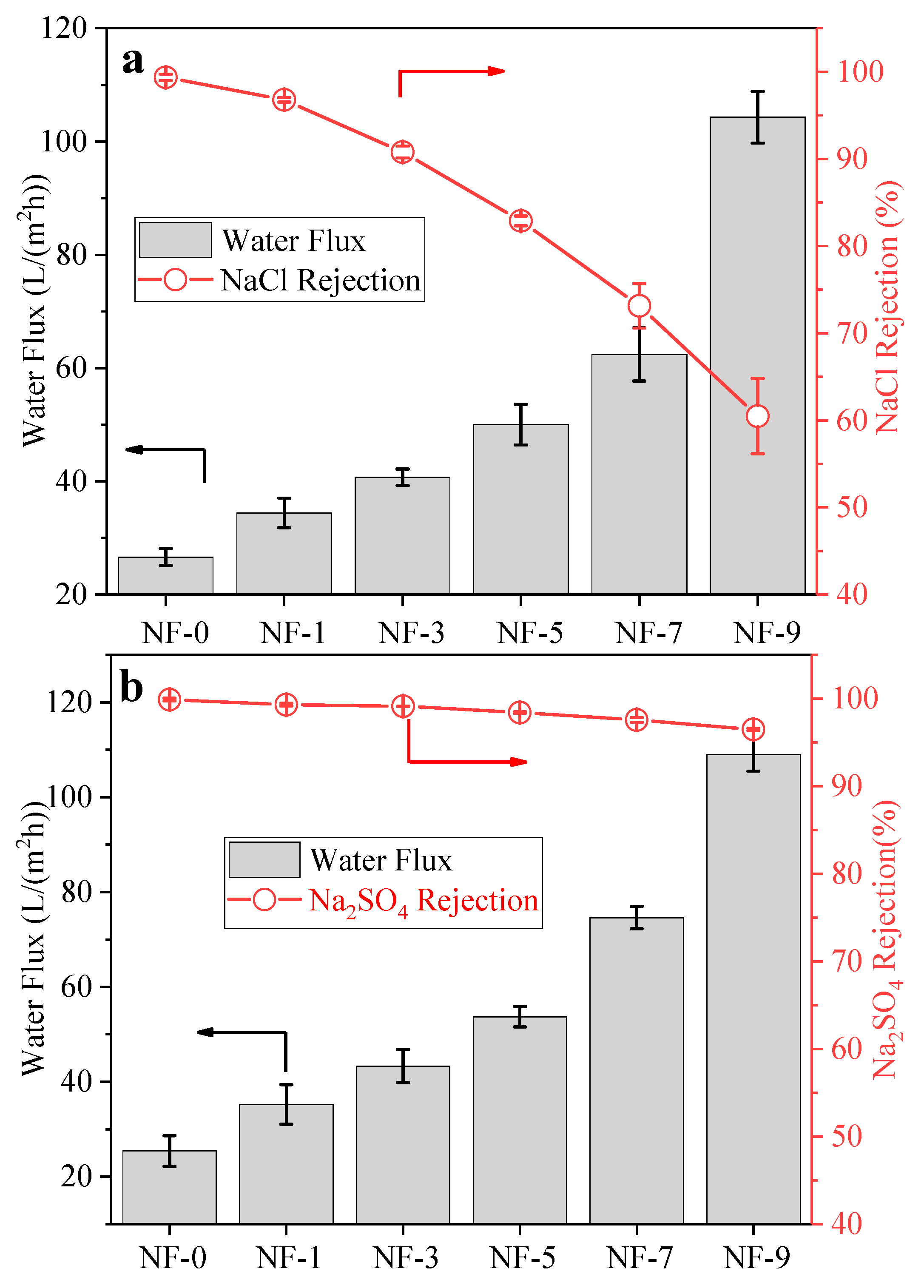
3.2. Membrane Performance Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, X.; Wei, S.; Liu, S.; Li, R.; Zhang, Z.; Liu, Y.; Zhang, X.; Lou, M.; Chen, G.; Li, F. Metal-Coordinated Nanofiltration Membranes Constructed on Metal Ions Blended Support toward Enhanced Dye/Salt Separation and Antifouling Performances. Membranes 2022, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Amadou-Yacouba, Z.; Mendret, J.; Lesage, G.; Zaviska, F.; Brosillon, S. Impact of Pre-Ozonation during Nanofiltration of MBR Effluent. Membranes 2022, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.; Jang, J.; Kang, Y.; Eisa, T.; Chae, K.-J.; Kim, I.S.; Yang, E. Enhancing the Dye-Rejection Efficiencies and Stability of Graphene Oxide-Based Nanofiltration Membranes via Divalent Cation Intercalation and Mild Reduction. Membranes 2022, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Masibi, E.G.; Makhetha, T.A.; Moutloali, R.M. Effect of the Incorporation of ZIF-8@ GO into the Thin-Film Membrane on Salt Rejection and BSA Fouling. Membranes 2022, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Xue, Y.-R.; Xu, Z.-K. Alginate hydrogel assisted controllable interfacial polymerization for high-performance nanofiltration membranes. Membranes 2021, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, C.; Fan, M.; Liu, M.; Shen, S. Optimization of Nanofiltration Hollow Fiber Membrane Fabrication Process Based on Response Surface Method. Membranes 2022, 12, 374. [Google Scholar] [CrossRef]
- Al-Rashdi, B.; Somerfield, C.; Hilal, N. Heavy metals removal using adsorption and nanofiltration techniques. Sep. Purif. Rev. 2011, 40, 209–259. [Google Scholar] [CrossRef]
- Chang, F.; Liu, W.; Wang, X. Comparison of polyamide nanofiltration and low-pressure reverse osmosis membranes on as (III) rejection under various operational conditions. Desalination 2014, 334, 10–16. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Saurina, J.; Granados, M.; Cortina, J.L. Integration of Nanofiltration and Reverse Osmosis Technologies in Polyphenols Recovery Schemes from Winery and Olive Mill Wastes by Aqueous-Based Processing. Membranes 2022, 12, 339. [Google Scholar] [CrossRef]
- Javier, L.; Pulido-Beltran, L.; Vrouwenvelder, J.S.; Farhat, N.M. Permeation Increases Biofilm Development in Nanofiltration Membranes Operated with Varying Feed Water Phosphorous Concentrations. Membranes 2022, 12, 335. [Google Scholar] [CrossRef]
- Vives, M.B.; Thuvander, J.; Arkell, A.; Lipnizki, F. Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process. Membranes 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Szekely, G.; Bandarra, J.; Heggie, W.; Sellergren, B.; Ferreira, F.C. Organic solvent nanofiltration: A platform for removal of genotoxins from active pharmaceutical ingredients. J. Membr. Sci. 2011, 381, 21–33. [Google Scholar] [CrossRef]
- Siew, W.E.; Livingston, A.G.; Ates, C.; Merschaert, A. Molecular separation with an organic solvent nanofiltration cascade–augmenting membrane selectivity with process engineering. Chem. Eng. Sci. 2013, 90, 299–310. [Google Scholar] [CrossRef]
- Tres, M.V.; Ferraz, H.C.; Dallago, R.M.; Di Luccio, M.; Oliveira, J.V. Characterization of polymeric membranes used in vegetable oil/organic solvents separation. J. Membr. Sci. 2010, 362, 495–500. [Google Scholar] [CrossRef]
- García-Martín, N.; Pérez-Magariño, S.; Ortega-Heras, M.; González-Huerta, C.; Mihnea, M.; González-SanJosé, M.L.; Palacio, L.; Prádanos, P.; Hernandez, A. Sugar reduction in musts with nanofiltration membranes to obtain low alcohol-content wines. Sep. Purif. Technol. 2010, 76, 158–170. [Google Scholar] [CrossRef]
- Salgado, C.; Palacio, L.; Carmona, F.; Hernández, A.; Prádanos, P. Influence of low and high molecular weight compounds on the permeate flux decline in nanofiltration of red grape must. Desalination 2013, 315, 124–134. [Google Scholar] [CrossRef]
- Catarino, M.; Mendes, A. Dealcoholizing wine by membrane separation processes. Innov. Food Sci. Emerg. Technol. 2011, 12, 330–337. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Ambats, G.; Presser, T.S.; Davis, R.A. Removal of selenium from contaminated agricultural drainage water by nanofiltration membranes. Appl. Geochem. 1996, 11, 797–802. [Google Scholar] [CrossRef]
- Akbari, A.; Desclaux, S.; Rouch, J.-C.; Remigy, J.-C. Application of nanofiltration hollow fibre membranes, developed by photografting, to treatment of anionic dye solutions. J. Membr. Sci. 2007, 297, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, J.; Zhang, Z. Application Progress of Nanofiltration Combination Technology in the Treatment of Landfill Leachate. Meteorol. Environ. Res. 2020, 11, 85–89. [Google Scholar]
- Ang, M.B.M.Y.; Wu, Y.-L.; Chu, M.-Y.; Wu, P.-H.; Chiao, Y.-H.; Millare, J.C.; Huang, S.-H.; Tsai, H.-A.; Lee, K.-R. Nanofiltration Membranes Formed through Interfacial Polymerization Involving Cycloalkane Amine Monomer and Trimesoyl Chloride Showing Some Tolerance to Chlorine during Dye Desalination. Membranes 2022, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Darvishmanesh, S.; Tasselli, F.; Jansen, J.; Tocci, E.; Bazzarelli, F.; Bernardo, P.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B. Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes. J. Membr. Sci. 2011, 384, 89–96. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Cheng, Q.; Lü, Z.; Liu, M.; Gao, C. Application of thin-film composite hollow fiber membrane to submerged nanofiltration of anionic dye aqueous solutions. Sep. Purif. Technol. 2012, 88, 121–129. [Google Scholar] [CrossRef]
- Liu, L.; Qu, S.; Yang, Z.; Chen, Y. Fractionation of dye/NaCl mixture using a loose nanofiltration membrane based on the incorporation of WS2 in self-assembled layer-by-layer polymeric electrolytes. Ind. Eng. Chem. Res. 2020, 59, 18160–18169. [Google Scholar] [CrossRef]
- Deng, L.; Li, S.; Qin, Y.; Zhang, L.; Chen, H.; Chang, Z.; Hu, Y. Fabrication of antifouling thin-film composite nanofiltration membrane via surface grafting of polyethyleneimine followed by zwitterionic modification. J. Membr. Sci. 2020, 619, 118564. [Google Scholar] [CrossRef]
- Zhu, W.-P.; Sun, S.-P.; Gao, J.; Fu, F.-J.; Chung, T.-S. Dual-layer polybenzimidazole/polyethersulfone (PBI/PES) nanofiltration (NF) hollow fiber membranes for heavy metals removal from wastewater. J. Membr. Sci. 2014, 456, 117–127. [Google Scholar] [CrossRef]
- Li, Q.; Liao, Z.; Xie, J.; Ni, L.; Wang, C.; Qi, J.; Sun, X.; Wang, L.; Li, J. Enhancing nanofiltration performance by incorporating tannic acid modified metal-organic frameworks into thin-film nanocomposite membrane. Environ. Res. 2020, 191, 110215. [Google Scholar] [CrossRef]
- Ramon, G.; Wong, M.C.; Hoek, E.M. Transport through composite membrane, Part 1: Is there an optimal support membrane? J. Membr. Sci. 2012, 415–416, 298–305. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.; Ismail, A.; Matsuura, T.; Rana, D. Study on the thin film composite poly(piperazine-amide) nanofiltration membrane: Impacts of physicochemical properties of substrate on interfacial polymerization formation. Desalination 2014, 344, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhao, S.; Wang, Z.; Tian, X.; Shi, M.; Wang, J.; Wang, S. Improved performance of polyamide thin-film composite nanofiltration membrane by using polyetersulfone/polyaniline membrane as the substrate. J. Membr. Sci. 2015, 493, 263–274. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Yang, F.; Kang, J.; Cao, Y.; Xiang, M. Probing influences of support layer on the morphology of polyamide selective layer of thin film composite membrane. J. Membr. Sci. 2018, 556, 374–383. [Google Scholar] [CrossRef]
- Jin, J.-B.; Liu, D.-Q.; Zhang, D.-D.; Yin, Y.-H.; Zhao, X.-Y.; Zhang, Y.-F. Preparation of thin-film composite nanofiltration membranes with improved antifouling property and flux using 2,2′-oxybis-ethylamine. Desalination 2015, 355, 141–146. [Google Scholar] [CrossRef]
- Weng, X.-D.; Ji, Y.-L.; Ma, R.; Zhao, F.-Y.; An, Q.-F.; Gao, C.-J. Superhydrophilic and antibacterial zwitterionic polyamide nanofiltration membranes for antibiotics separation. J. Membr. Sci. 2016, 510, 122–130. [Google Scholar] [CrossRef]
- Ma, T.; Su, Y.; Li, Y.; Zhang, R.; Liu, Y.; He, M.; Li, Y.; Dong, N.; Wu, H.; Jiang, Z. Fabrication of electro-neutral nanofiltration membranes at neutral pH with antifouling surface via interfacial polymerization from a novel zwitterionic amine monomer. J. Membr. Sci. 2016, 503, 101–109. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Liu, Y.; Guo, Z.; Shao, L. Nanofiltration membrane achieving dual resistance to fouling and chlorine for “green” separation of antibiotics. J. Membr. Sci. 2015, 493, 156–166. [Google Scholar] [CrossRef]
- Hu, J.; Lv, Z.; Xu, Y.; Zhang, X.; Wang, L. Fabrication of a High-flux Sulfonated Polyamide Nanofiltration Membrane: Experimental and Dissipative Particle Dynamics Studies. J. Membr. Sci. 2016, 505, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Wang, L.; Zhang, L.; Yu, Q.J. An acid resistant nanofiltration membrane prepared from a precursor of poly(s-triazine-amine) by interfacial polymerization. J. Membr. Sci. 2018, 546, 225–233. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Xu, Z.-L.; Xue, S.-M.; Wei, Y.-M.; Yang, H. A chlorine-tolerant nanofiltration membrane prepared by the mixed diamine monomers of PIP and BHTTM. J. Membr. Sci. 2016, 498, 374–384. [Google Scholar] [CrossRef]
- Wen, P.; Chen, Y.; Hu, X.; Cheng, B.; Liu, D.; Zhang, Y.; Nair, S. Polyamide thin film composite nanofiltration membrane modified with acyl chlorided graphene oxide. J. Membr. Sci. 2017, 535, 208–220. [Google Scholar] [CrossRef]
- Zhao, F.-Y.; Ji, Y.-L.; Weng, X.-D.; Mi, Y.-F.; Ye, C.-C.; An, Q.-F.; Gao, C.-J. High-flux positively charged nanocomposite nanofiltration membranes filled with poly (dopamine) modified multiwall carbon nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 6693–6700. [Google Scholar] [CrossRef]
- Xue, S.; Xu, Z.-L.; Tang, Y.-J.; Ji, C.-H. Polypiperazine-amide nanofiltration membrane modified by different functionalized multiwalled carbon nanotubes (MWCNTs). ACS Appl. Mater. Interfaces 2016, 8, 19135–19144. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Mahmood, A.; Kim, S.-J.; Lee, K.-H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal–organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hou, J.; Yuan, S.; Zhao, Y.; Li, Y.; Zhang, R.; Tian, M.; Li, J.; Wang, J.; Van der Bruggen, B. MOF-positioned polyamide membranes with a fishnet-like structure for elevated nanofiltration performance. J. Mater. Chem. A 2019, 7, 16313–16322. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, J.; Li, Z.; Yin, Y.; Cao, L.; Zhong, Y.; Wu, H. Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J. Membr. Sci. 2017, 523, 273–281. [Google Scholar] [CrossRef]
- Batool, M.; Shafeeq, A.; Haider, B.; Ahmad, N. TiO2 nanoparticle filler-based mixed-matrix PES/CA nanofiltration membranes for enhanced desalination. Membranes 2021, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, B.; Wu, P. Optimization, characterization and nanofiltration properties test of MWNTs/polyester thin film nanocomposite membrane. J. Membr. Sci. 2013, 428, 425–433. [Google Scholar] [CrossRef]
- Wu, M.; Lv, Y.; Yang, H.-C.; Liu, L.-F.; Zhang, X.; Xu, Z.-K. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Gao, S.; Zhang, F.; Zhang, W.; Liu, Z.; Jin, J. Single-walled carbon nanotube film supported nanofiltration membrane with a nearly 10 nm thick polyamide selective layer for high-flux and high-rejection desalination. Small 2016, 12, 5034–5041. [Google Scholar] [CrossRef]
- Cheng, S.; Oatley, D.L.; Williams, P.M.; Wright, C.J. Positively charged nanofiltration membranes: Review of current fabrication methods and introduction of a novel approach. Adv. Colloid Interface Sci. 2011, 164, 12–20. [Google Scholar] [CrossRef]
- Wang, J.-J.; Yang, H.-C.; Wu, M.-B.; Zhang, X.; Xu, Z.-K. Nanofiltration membranes with cellulose nanocrystals as an interlayer for unprecedented performance. J. Mater. Chem. A 2017, 5, 16289–16295. [Google Scholar] [CrossRef]
- Soyekwo, F.; Zhang, Q.; Gao, R.; Qu, Y.; Lin, C.; Huang, X.; Zhu, A.; Liu, Q. Cellulose nanofiber intermediary to fabricate highly-permeable ultrathin nanofiltration membranes for fast water purification. J. Membr. Sci. 2017, 524, 174–185. [Google Scholar] [CrossRef]
- Wu, M.; Yuan, J.; Wu, H.; Su, Y.; Yang, H.; You, X.; Zhang, R.; He, X.; Khan, N.A.; Kasher, R.; et al. Ultrathin nanofiltration membrane with polydopamine-covalent organic framework interlayer for enhanced permeability and structural stability. J. Membr. Sci. 2019, 576, 131–141. [Google Scholar] [CrossRef]
- Park, M.J.; Wang, C.; Seo, D.H.; Gonzales, R.R.; Matsuyama, H.; Shon, H.K. Inkjet printed single walled carbon nanotube as an interlayer for high performance thin film composite nanofiltration membrane. J. Membr. Sci. 2021, 620, 118901. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Chen, B.-Z.; Lu, T.-D.; Wu, H.-L.; Fan, Y.-Q.; Xing, W.; Sun, S.-P. Designing high-performance nanofiltration membranes for high-salinity separation of sulfate and chloride in the chlor-alkali process. Ind. Eng. Chem. Res. 2019, 58, 12280–12290. [Google Scholar] [CrossRef]
- Oldani, M.; Schock, G. Characterization of ultrafiltration membranes by infrared spectroscopy, ESCA, and contact angle measurements. J. Membr. Sci. 1989, 43, 243–258. [Google Scholar] [CrossRef]
- Tang, C.; Kwon, Y.-N.; Leckie, J. Probing the nano-and micro-scales of reverse osmosis membranes—A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Membr. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]
- Song, Y.; Liu, F.; Sun, B. Preparation, characterization, and application of thin film composite nanofiltration membranes. Appl. Polym. Sci. 2005, 95, 1251–1261. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A novel approach toward fabrication of high performance thin film composite polyamide membranes. Sci. Rep. 2016, 6, 22069. [Google Scholar] [CrossRef] [Green Version]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. Thin film composite polyamide membranes: Parametric study on the influence of synthesis conditions. RSC Adv. 2015, 5, 54985–54997. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Zhang, H.; Cao, Z.; Zhao, M.; Guan, Y.; Zhang, Y. Establishment of transport channels with carriers for water in reverse osmosis membrane by incorporating hydrotalcite into the polyamide layer. RSC Adv. 2018, 8, 12439–12448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.S.; Joshi, S.; Trivedi, J.; Devmurari, C.; Rao, A.P.; Ghosh, P. Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Membr. Sci. 2006, 278, 19–25. [Google Scholar] [CrossRef]
- Ng, Z.; Lau, W.; Kartohardjono, S.; Ismail, A.F. Comprehensive studies of membrane rinsing on the physicochemical properties and separation performance of TFC RO membranes. Desalination 2020, 491, 114345. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Al Mayyahi, A. Important approaches to enhance reverse osmosis (RO) thin film composite (TFC) membranes performance. Membranes 2018, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Kwak, S.-Y.; Jung, S.G.; Kim, S.H. Structure-motion-performance relationship of flux-enhanced reverse osmosis (RO) membranes composed of aromatic polyamide thin films. Environ. Sci. Technol. 2001, 35, 4334–4340. [Google Scholar] [CrossRef]
- Schatzberg, P. Molecular diameter of water from solubility and diffusion measurements. J. Phys. Chem. 1967, 71, 4569–4570. [Google Scholar] [CrossRef]
- Jamil, T.S.; Mansor, E.S.; Abdallah, H.; Shaban, A. Innovative high flux/low pressure blend thin film composite membranes for water softening. React. Funct. Polym. 2018, 131, 384–399. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.J.; Ong, C.S.; Ismail, A.F.; Matsuura, T. Study on the thin film composite poly (piperazine-amide) nanofiltration membranes made of different polymeric substrates: Effect of operating conditions. Korean J. Chem. Eng. 2015, 32, 753–760. [Google Scholar] [CrossRef]



| Specific Surface Area (S/P) | Roughness | ||
|---|---|---|---|
| Ra/nm | Rms/nm | ||
| NF-0 | 1.68 | 76.3 | 97.2 |
| NF-1 | 1.74 | 79.6 | 101.1 |
| NF-3 | 1.82 | 85.9 | 109.9 |
| NF-5 | 1.95 | 94.1 | 120.1 |
| NF-7 | 2.06 | 103.5 | 132.6 |
| NF-9 | 2.11 | 105.4 | 135.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, X.; Zheng, T.; Mo, B.; Xu, H.; Huang, Y.; Wang, J.; Gao, C.; Gao, X. High Flux Nanofiltration Membranes with Double-Walled Carbon Nanotube (DWCNT) as the Interlayer. Membranes 2022, 12, 1011. https://doi.org/10.3390/membranes12101011
Wang Z, Wang X, Zheng T, Mo B, Xu H, Huang Y, Wang J, Gao C, Gao X. High Flux Nanofiltration Membranes with Double-Walled Carbon Nanotube (DWCNT) as the Interlayer. Membranes. 2022; 12(10):1011. https://doi.org/10.3390/membranes12101011
Chicago/Turabian StyleWang, Zhen, Xiaojuan Wang, Tao Zheng, Bing Mo, Huacheng Xu, Yijun Huang, Jian Wang, Congjie Gao, and Xueli Gao. 2022. "High Flux Nanofiltration Membranes with Double-Walled Carbon Nanotube (DWCNT) as the Interlayer" Membranes 12, no. 10: 1011. https://doi.org/10.3390/membranes12101011




