Preparation and Desalination Performance of PA/UiO-66/PES Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of UiO-66 Nanoparticles
2.3. Membrane Preparation
2.4. Characterizations
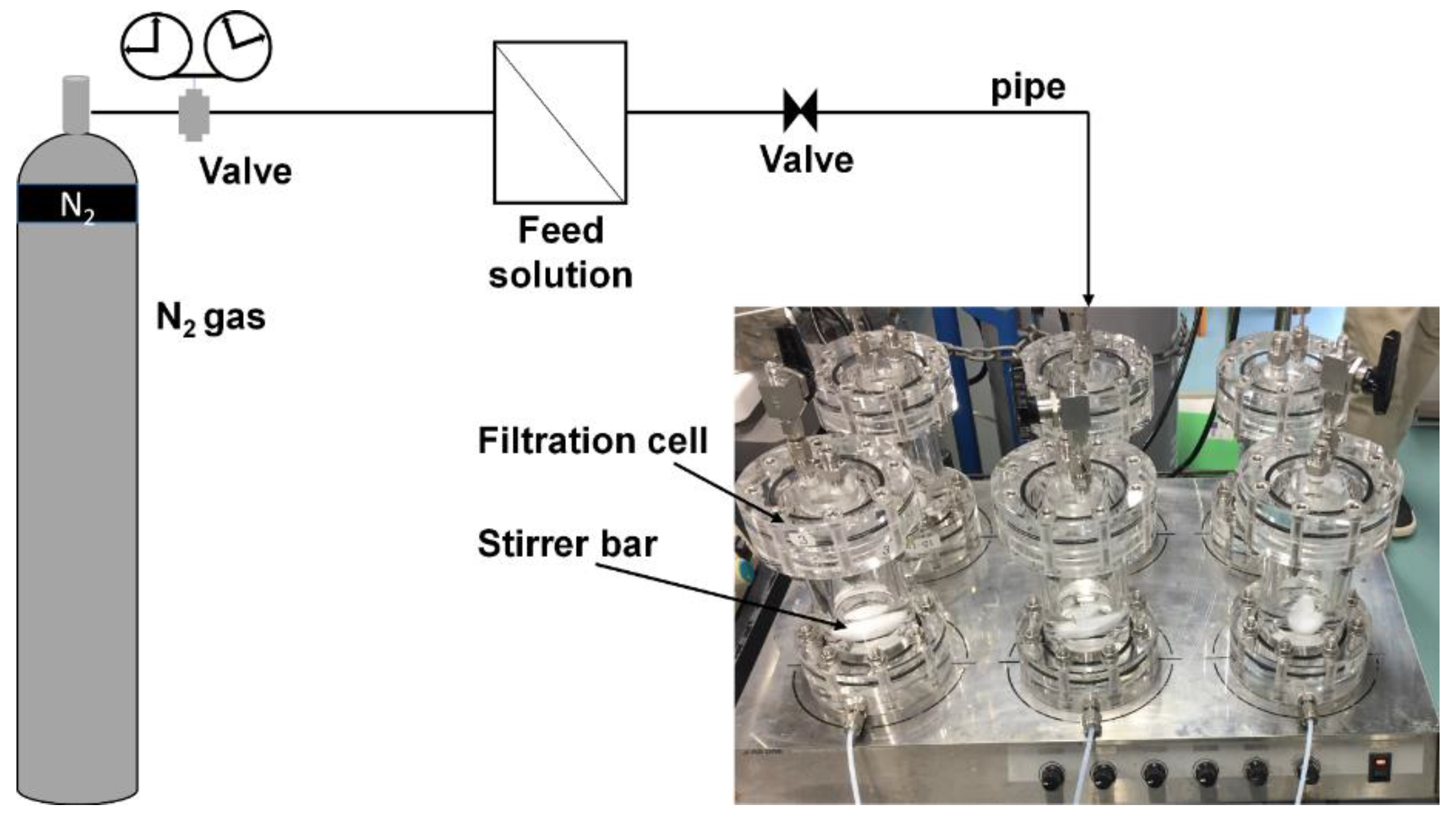
2.5. Desalination Tests
3. Results and Discussions
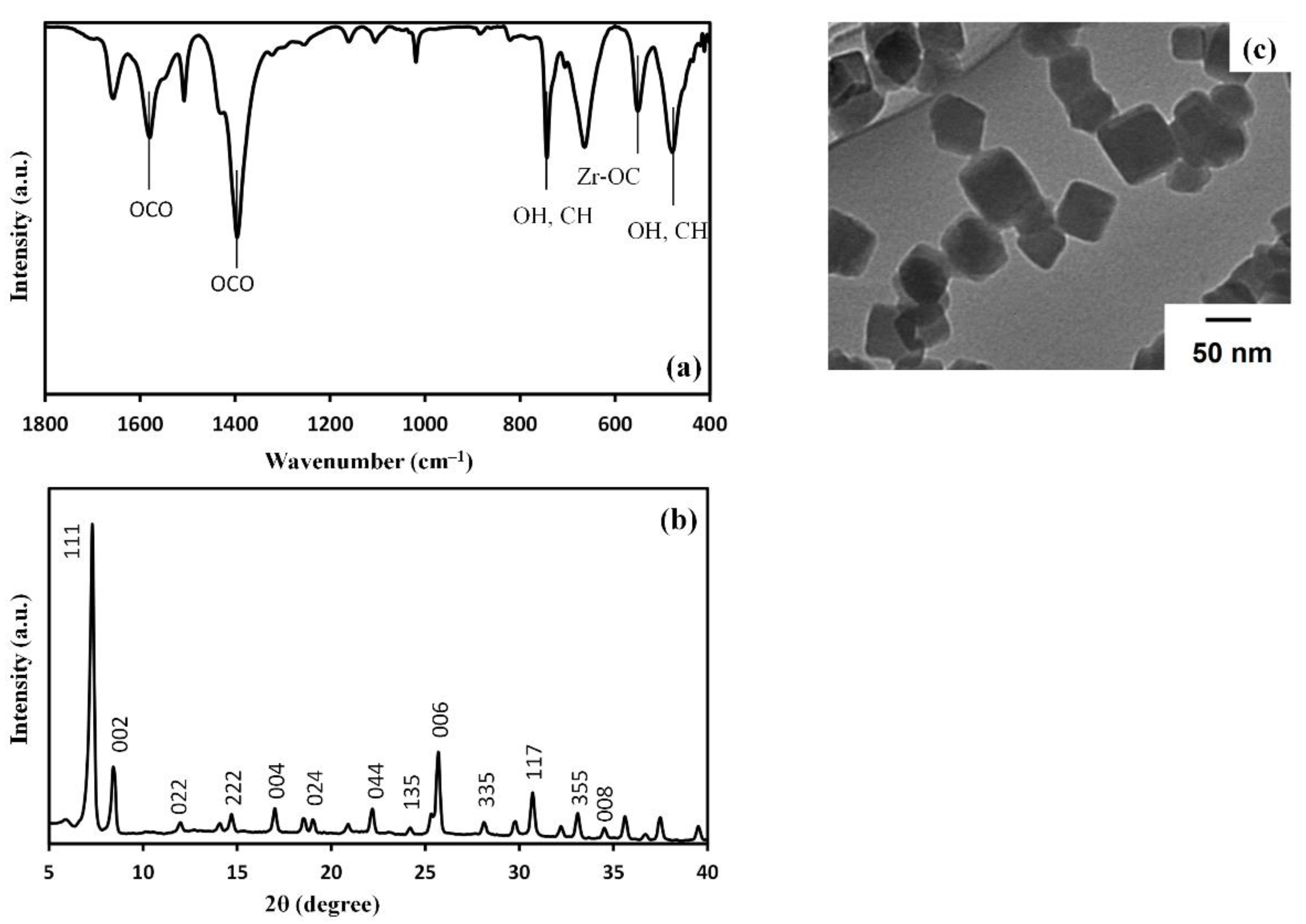
3.1. Characterization of UiO-66 Nanoparticles
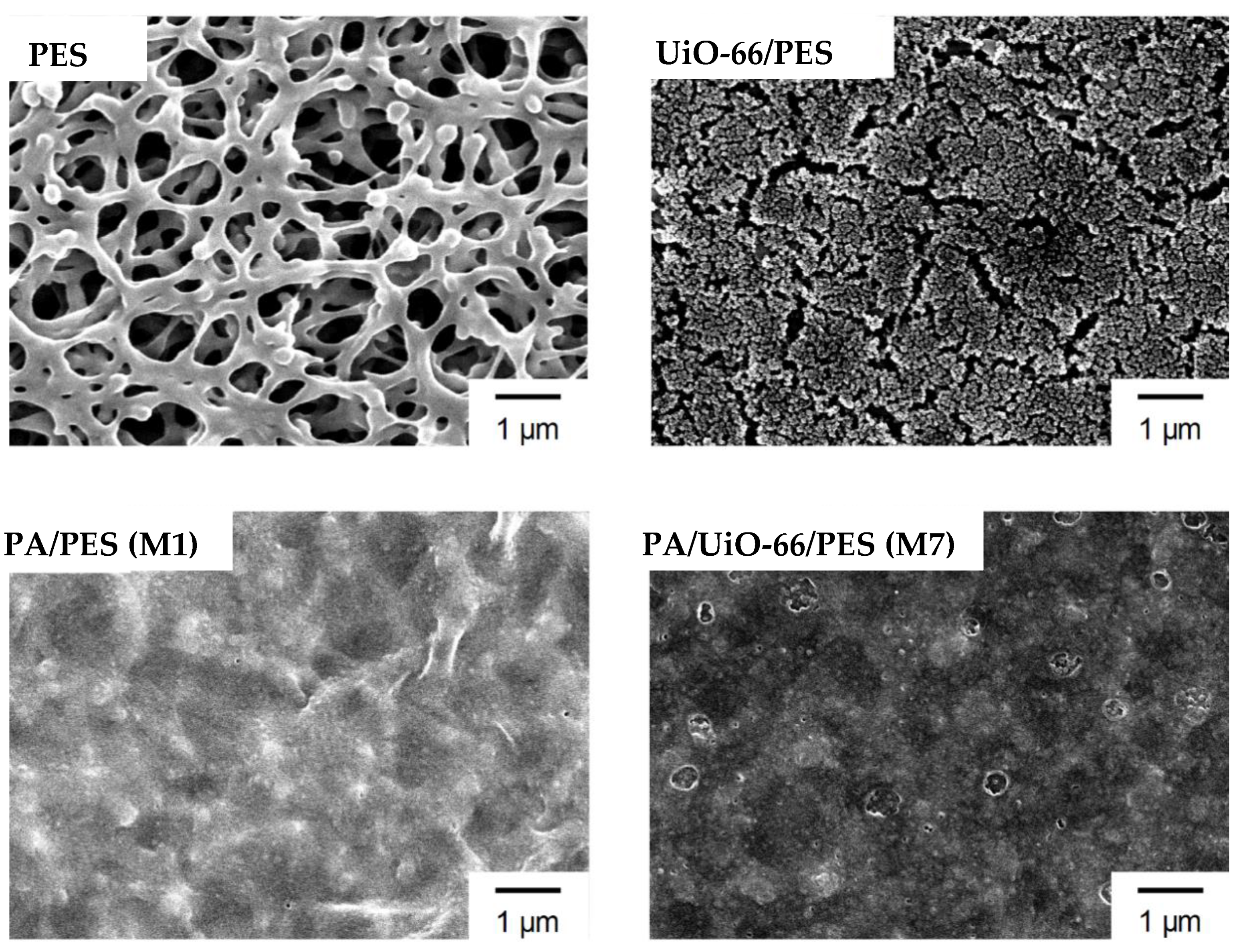
3.2. Characterization of the Prepared Membranes
3.3. Desalination Performance of the Prepared Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Shao, C.; Zhao, Y.; Qu, L. Tunable Graphene Systems for Water Desalination. ChemNanoMat 2020, 6, 1028–1048. [Google Scholar] [CrossRef] [Green Version]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast Mass Transport Through Sub-2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Peng, X. Mass transport through metal organic framework membranes. Sci. China Mater. 2019, 62, 25–42. [Google Scholar] [CrossRef] [Green Version]
- Escobar, I.C.; Van Der Bruggen, B. Microfiltration and ultrafiltration membrane science and technology. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Volodin, A.; Martens, J.; Vankelecom, I.F.J. Polymer supported ZIF-8 membranes prepared via an interfacial synthesis method. Chem. Commun. (Camb). 2015, 51, 918–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite ultrafiltration membrane tailored by MOF @ GO with highly improved water purification performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly Water-Stable Zirconium Metal-Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, D.X.; Phuong, T.; Tran, N.; Taniike, T. Fabrication of new composite membrane filled with UiO-66 nanoparticles and its application to nanofiltration. Sep. Purif. Technol. 2017, 177, 249–256. [Google Scholar] [CrossRef]
- Shangkum, G.Y.; Chammingkwan, P.; Trinh, D.X.; Taniike, T. Design of a semi-continuous selective layer based on deposition of UiO-66 nanoparticles for nanofiltration. Membranes 2018, 8, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.H.; Han, J.; Han, S.; Guiver, M.D.; Park, H.B. Polyamide Thin-Film Composite Membranes Based on Carboxylated Polysulfone Microporous Support Membranes for Forward Osmosis; Elsevier: Amsterdam, The Netherlands, 2013; Volume 445, ISBN 8222220233. [Google Scholar]
- Lee, J.; Hill, A.; Kentish, S. Formation of a thick aromatic polyamide membrane by interfacial polymerization. Sep. Purif. Technol. 2013, 104, 276–283. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; Li, S.; Jin, B.; Yue, Q.; Wang, Z. Cerium oxide doped nanocomposite membranes for reverse osmosis desalination. Chemosphere 2019, 218, 974–983. [Google Scholar] [CrossRef]
- Aljundi, I.H. Desalination characteristics of TFN-RO membrane incorporated with ZIF-8 nanoparticles. Desalination 2017, 420, 12–20. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, K.; Baek, Y.; Kim, D.G.; Shim, J.; Yoon, J.; Lee, J.C. High-performance reverse osmosis CNT/polyamide nanocomposite membrane by controlled interfacial interactions. ACS Appl. Mater. Interfaces 2014, 6, 2819–2829. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Yao, Z.K.; Mei, Y.; Tang, C.Y. Hydrophilic Silver Nanoparticles Induce Selective Nanochannels in Thin Film Nanocomposite Polyamide Membranes. Environ. Sci. Technol. 2019, 53, 5301–5308. [Google Scholar] [CrossRef]
- Bao, M.; Zhu, G.; Wang, L.; Wang, M.; Gao, C. Preparation of monodispersed spherical mesoporous nanosilica-polyamide thin film composite reverse osmosis membranes via interfacial polymerization. Desalination 2013, 309, 261–266. [Google Scholar] [CrossRef]
- Online, V.A.; Huang, H.; Qu, X.; Dong, H.; Zhang, L.; Chen, H. RSC Advances Role of NaA zeolites in the interfacial polymerization process towards a polyamide nanocomposite reverse osmosis membrane. RSC Adv. 2013, 3, 8203–8207. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahimi, A. Zwitterion functionalized graphene oxide/polyamide thin film nanocomposite membrane: Towards improved anti-fouling performance for reverse osmosis. Desalination 2018, 433, 94–107. [Google Scholar] [CrossRef]
- Donato, L.; Garofalo, A.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Improved performance of vacuum membrane distillation in desalination with zeolite membranes. Sep. Purif. Technol. 2020, 237, 116376. [Google Scholar] [CrossRef]
- Garofalo, A.; Carnevale, M.C.; Donato, L.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Scale-up of MFI zeolite membranes for desalination by vacuum membrane distillation. Desalination 2016, 397, 205–212. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal-organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Schelling, M.; Kim, M.; Otal, E.; Hinestroza, J. Decoration of cotton fibers with a water-stable metal–organic framework (UiO-66) for the decomposition and enhanced adsorption of micropollutants in water. Bioengineering 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, H.T.; Tran, N.T.; Trinh, D.X. Investigation into the Adsorption of Methylene Blue and Methyl Orange by UiO-66-NO 2 Nanoparticles. J. Anal. Methods Chem. 2021, 2021, 5512174. [Google Scholar] [CrossRef]
- Miao, A.; Wei, M.; Xu, F.; Wang, Y. Influence of membrane hydrophilicity on water permeability: An experimental study bridging simulations. J. Memb. Sci. 2020, 604, 118087. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, P.; Hou, J.; Lu, J.; Easton, C.D.; Zhang, X.; Hill, M.R.; Thornton, A.W.; Liu, J.Z.; et al. Fast and selective fluoride ion conduction in sub-1-nanometer metal-organic framework channels. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Yu, S.; Zhu, J.; Li, P.; Gao, X.; Zhang, R. Incorporation of lysine-modified UiO-66 for the construction of thin-film nanocomposite nanofiltration membrane with enhanced water flux and salt selectivity. Desalination 2020, 493, 114661. [Google Scholar] [CrossRef]
- Xu, T.; Shehzad, M.A.; Wang, X.; Wu, B.; Ge, L.; Xu, T. Engineering Leaf-Like UiO-66-SO3H Membranes for Selective Transport of Cations. Nano-Micro Lett. 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Hou, J.; Hu, Y.; Wang, P.; Ou, R.; Jiang, L.; Zhe Liu, J.; Freeman, B.D.; Hill, A.J.; Wang, H. Ultrafast selective transport of alkali metal ions in metal organic frameworks with subnanometer pores. Sci. Adv. 2018, 4, eaaq0066. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Lin, X.; Tong, Y.; Du, H.; Gu, L.; Yuan, Y. UiO-66 MOFs as electron transport channel to short circuit dye photosensitizer and NiS2 co-catalyst for increased hydrogen generation. Mater. Lett. 2019, 255, 126593. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.Q.; Tsai, F.C.; Xie, L.; Zhang, K.D.; Liu, H.L.; Ma, N.; Shi, D.; Jiang, T. Ligands-coordinated Zr-based MOF for wastewater treatment. Nanomaterials 2018, 8, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination-Development to date and future potential. J. Memb. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van Der Bruggen, B. Metal-organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticles | Intraparticle Channels | Feed Solution mg·L−1 | Permeability Increase | NaCl Rejection (%) c | Ref. |
|---|---|---|---|---|---|
| CeO2 NPs a | without | 2000 | 1.50 | 98.7–98.0 | [17] |
| ZIF-8 a | with | 2000 | 2.07 | 98.1–99.5 | [18] |
| CNTs a | with | 2000 | 1.18 | 97.5–95.4 | [19] |
| Ag NPs b | with | 2000 | 2.68 | 97.4–99.1 | [20] |
| SiO2 a | with | 2000 | 2.24 | 90.7–91.1 | [21] |
| NaA zeolite a | with | 2000 | 1.63 | 91.4–98.0 | [22] |
| GO a | with | 1000 | 1.76 | 94.0–94.8 | [23] |
| UiO-66 b | with | 1000 | 3.02 | 91.9–94.3 | this work |
| Entry | Membrane | MPD Concentration (wt%) | UiO-66 Loading (mg) | Contact Time in Aqueous Phase (s) | Contact Time in Organic Phase (s) |
|---|---|---|---|---|---|
| 0 | PA/PES (M0) | 3 | 0 | 120 | 15 |
| 1 | PA/PES (M1) | 1 | 0 | 120 | 15 |
| 2 | PA/UiO-66/PES (M2) | 3 | 0.5 | 300 | 60 |
| 3 | PA/UiO-66/PES (M3) | 3 | 0.5 | 300 | 15 |
| 4 | PA/UiO-66/PES (M4) | 3 | 0.5 | 120 | 15 |
| 5 | PA/UiO-66/PES (M5) | 1 | 0.5 | 120 | 5 |
| 6 | PA/UiO-66/PES (M6) | 1 | 0.5 | 120 | 10 |
| 7 | PA/UiO-66/PES (M7) | 1 | 0.5 | 120 | 15 |
| 8 | PA/UiO-66/PES (M8) | 1 | 1.0 | 120 | 15 |
| 9 | PA/ZrO2/PES (M9) | 1 | 0.5 (ZrO2) | 120 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, D.X.; Pham, N.N.; Chammingkwan, P.; Taniike, T. Preparation and Desalination Performance of PA/UiO-66/PES Composite Membranes. Membranes 2021, 11, 628. https://doi.org/10.3390/membranes11080628
Trinh DX, Pham NN, Chammingkwan P, Taniike T. Preparation and Desalination Performance of PA/UiO-66/PES Composite Membranes. Membranes. 2021; 11(8):628. https://doi.org/10.3390/membranes11080628
Chicago/Turabian StyleTrinh, Dai Xuan, Ngo Nghia Pham, Patchanee Chammingkwan, and Toshiaki Taniike. 2021. "Preparation and Desalination Performance of PA/UiO-66/PES Composite Membranes" Membranes 11, no. 8: 628. https://doi.org/10.3390/membranes11080628






