Control Over the Morphology of Electrospun Microfibrous Mats of a Polymer of Intrinsic Microporosity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning Technology
2.3. Characterisation Methods
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Measurement of Pore Size Diameter (r), Hydrostatic Pressure (PH2O) and Air Flow Resistance (Rair)
2.3.3. Conductivity Measurement
3. Results
3.1. Effect of the Process Conditions
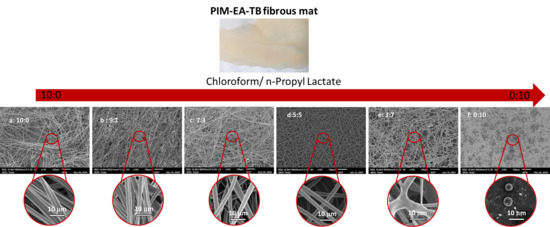
3.1.1. Effect of Solvent
- Morphology
- Fibre diameter
3.1.2. Effect of the Electrospinning Process Conditions
- Effect of flow rate
- Effect of gap
- Effect of voltage
3.2. Characterisation
3.2.1. Thermal Analysis
3.2.2. Pore Size
3.2.3. Air Resistance
3.2.4. Water Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khayet, M. Membranes and theoritical modelling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Satilmis, B.; Uyar, T. Development of superhydrophobic electrospun fibrous membrane of polymers of intrinsic microporosity (PIM-2). Eur. Polym. J. 2019, 112, 87–94. [Google Scholar] [CrossRef]
- Hou, L.; Wang, N.; Wu, J.; Cui, Z.; Jiang, L.; Zhao, Y. Bioinspired Superwettability Electrospun Micro/Nanofibers and Their Applications. Adv. Funct. Mater. 2018, 28, 180114. [Google Scholar] [CrossRef]
- Wang, S.; Shi, K.; Tripathi, A.; Chakraborty, U.; Parsons, G.N.; Khan, S.A. Designing Intrinsically Microporous Polymer (PIM-1) Microfibers with Tunable Morphology and Porosity via Controlling Solvent/Nonsolvent/Polymer Interactions. ACS Appl. Polym. Mater. 2020, 2, 2434–2443. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(E-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tabata, Y. Effect of the fiber diameter and porosity of non-woven PET fabrics on the osteogenic differentiation of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2004, 15, 41–57. [Google Scholar] [CrossRef]
- Long, Y.-Z.; Yan, X.; Wang, X.-X.; Zhang, J.; Yu, M. Electrospinning: The Set-Up and Procedure. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–52. [Google Scholar]
- Nitanan, T.; Opanasopit, P.; Akkaramongkolporn, P.; Rojanarata, T.; Ngawhirunpat, T.; Supaphol, P. Effects of processing parameters on morphology of electrospun polystyrene nanofibers. Korean J. Chem. Eng. 2011, 29, 173–181. [Google Scholar] [CrossRef]
- E Teo, W.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef]
- Yousefzadeh, M. Modeling and simulation of electrospinning process. In Electrospun Nanofibers; Woodhead Publishing: Duxford, UK, 2017; pp. 277–301. [Google Scholar]
- Lasseuguette, E.; Malpass-Evans, R.; Casalini, S.; McKeown, N.B.; Ferrari, M.-C. Optimization of the fabrication of amidoxime modified PIM-1 electrospun fibres for use as breathable and reactive materials. Polymer 2012, 213, 123205. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Tonkins, A.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. A Spirobifluorene-Based Polymer of Intrinsic Microporosity with Improved Performance for Gas Separation. Adv. Mater. 2012, 24, 5930–5933. [Google Scholar] [CrossRef] [PubMed]
- Lasseuguette, E.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Ferrari, M.-C. Temperature and Pressure Dependence of Gas Permeation in a Microporous Tröger’s Base Polymer. Membranes 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, N.B. Polymers of Intrinsic Microporosity (PIMs). Polymer 2020, 202, 122736. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Rose, I.; Bezzu, C.G.; Carta, M.; Comesaña-Gándara, B.; Lasseuguette, E.; Ferrari, M.-C.; Bernardo, P.; Clarizia, G.; Fuoco, A.; Jansen, J.C.; et al. Polymer ultrapermeability from the inefficient packing of 2D chains. Nat. Mater. 2017, 16, 932–937. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; MacAgray, E.; Webb, C. Review of polymers of intrinsic microporosity for hydrogen storage applications. Int. J. Hydrog. Energy 2016, 41, 16944–16965. [Google Scholar] [CrossRef]
- McKeown, N.; Budd, P. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef]
- Carturan, S.; Antonaci, A.; Maggioni, G.; Quaranta, A.; Tonezzer, M.; Milan, R.; della Mea, G. Optical vapors sensing capabilities of polymers of intrinsic microporosity. In Sensors and Microsystems; Springer: Dordrecht, The Netherlands, 2009; pp. 55–68. [Google Scholar]
- Fritsch, D.; Merten, P.; Heinrich, K.; Lazar, M.; Priske, M. High performance organic solvent nanofiltration membranes: Development and thorough testing of thin film composite membranes made of polymers of intrinsic microporosity (PIMs). J. Membr. Sci. 2012, 401–402, 222–231. [Google Scholar] [CrossRef]
- Satilmis, B.; Uyar, T. Amine modified electrospun PIM-1 ultrafine fibers for an efficient removal of methyl orange from an aqueous system. Appl. Surf. Sci. 2018, 453, 220–229. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Ferrari, M.-C. Development of microporous electrospun PIM-1 fibres. Mater. Lett. 2016, 177, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Satilmis, B.; Uyar, T. Electrospinning of uniform nanofibers of Polymers of Intrinsic Microporosity (PIM-1): The influence of solution conductivity and relative humidity. Polymer 2019, 178, 121610. [Google Scholar] [CrossRef]
- Wang, S.; Pomerantz, N.; Dai, Z.; Xie, W.; Anderson, E.; Miller, T.; Khan, S.; Parsons, G. Polymer of intrinsic microporosity (PIM) based fibrous mat: Combining particle filtration and rapid catalytic hydrolysis of chemical warfare agent simulants into a highly sorptive, breathable, and mechanically robust fiber matrix. Mater. Today Adv. 2020, 8, 100085. [Google Scholar] [CrossRef]
- Bernardo, P.; Scorzafave, V.; Clarizia, G.; Tocci, E.; Jansen, J.; Borgogno, A.; Malpass-Evans, R.; McKeown, N.; Carta, M.; Tasselli, F. Thin film composite membranes based on a polymer of intrinsic microporosity derived from Tröger’s base: A combined experimental and computational investigation of the role of residual casting solvent. J. Membr. Sci. 2019, 569, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Marken, F.; Carta, M.; McKeown, N.B. Polymers of Intrinsic Microporosity in the Design of Electrochemical Multicomponent and Multiphase Interfaces. Anal. Chem. 2021, 93, 1213–1220. [Google Scholar] [CrossRef]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibres. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 469–481. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun porous cellulose acetate fibers from volatile solvent mixture. Mater. Lett. 2011, 65, 2291–2294. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of molecular weight of atactic poly(vinyl alcohol) (PVA) in the structure and properties of PVA nanofabric prepared by electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Dong, C.; Sheng, J. Morphology of ultrafine polysulfone fibers prepared by electrospinning. Polym. Int. 2004, 53, 1704–1710. [Google Scholar] [CrossRef]








| Solvent | Chloroform | n-Propyl Lactate |
|---|---|---|
| Boiling point (°C) | 61 (a) | 170 (a) |
| Viscosity at 20 °C (10−3 Pa·s) | 0.56 (a) | 3.3 (a) |
| Electrical conductivity at 20 °C (S·cm−1) | 1 × 10−11 (a) | 2 × 10−6 (b) |
| Ratio Chloroform/n-PL | 10:0 | 9:1 | 7:3 | 5:5 |
|---|---|---|---|---|
| Fibre diameter (μm) ± 0.3 μm | 7.9 | 7.2 | 5.3 | 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasseuguette, E.; Malpass-Evans, R.; Tobin, J.M.; McKeown, N.B.; Ferrari, M.-C. Control Over the Morphology of Electrospun Microfibrous Mats of a Polymer of Intrinsic Microporosity. Membranes 2021, 11, 422. https://doi.org/10.3390/membranes11060422
Lasseuguette E, Malpass-Evans R, Tobin JM, McKeown NB, Ferrari M-C. Control Over the Morphology of Electrospun Microfibrous Mats of a Polymer of Intrinsic Microporosity. Membranes. 2021; 11(6):422. https://doi.org/10.3390/membranes11060422
Chicago/Turabian StyleLasseuguette, Elsa, Richard Malpass-Evans, John M. Tobin, Neil B. McKeown, and Maria-Chiara Ferrari. 2021. "Control Over the Morphology of Electrospun Microfibrous Mats of a Polymer of Intrinsic Microporosity" Membranes 11, no. 6: 422. https://doi.org/10.3390/membranes11060422