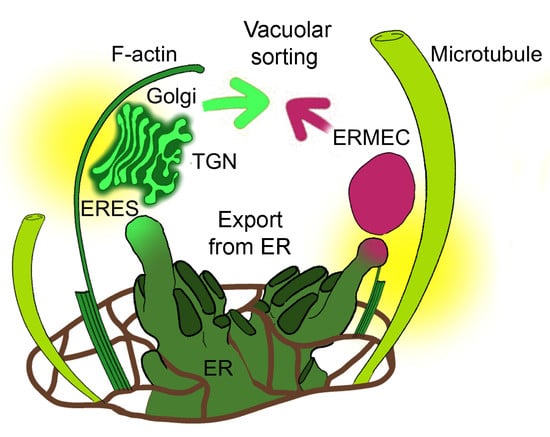
Actin and Microtubules Differently Contribute to Vacuolar Targeting Specificity during the Export from the ER
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Genetic Constructs
2.2. Plant Material: Protoplast Preparation and Transformation
2.3. Drug Treatments
2.4. Confocal Laser Scanning Microscopy
2.5. Data Analysis
2.6. Network Analysis of In Silico Interactions
3. Results
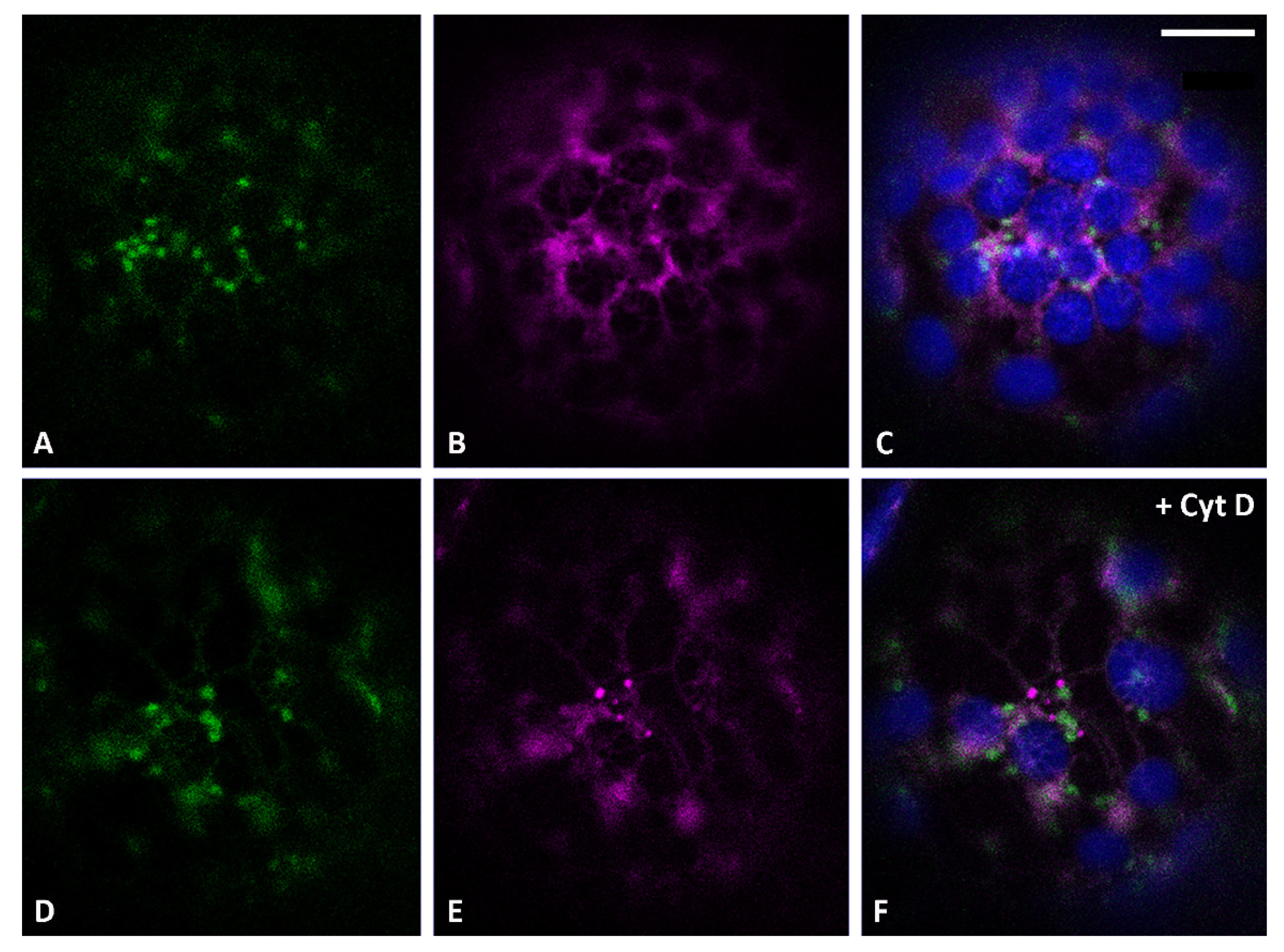
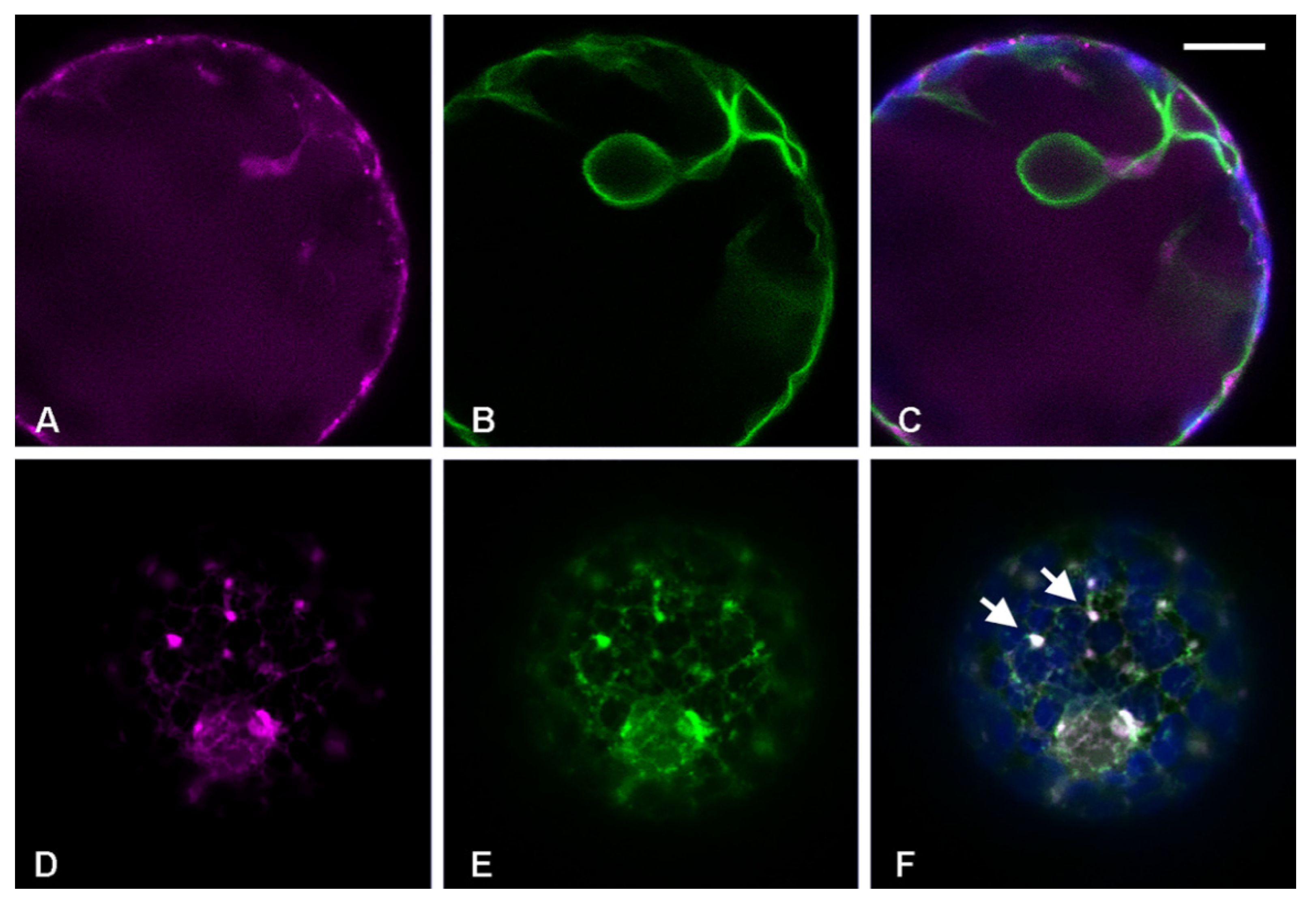
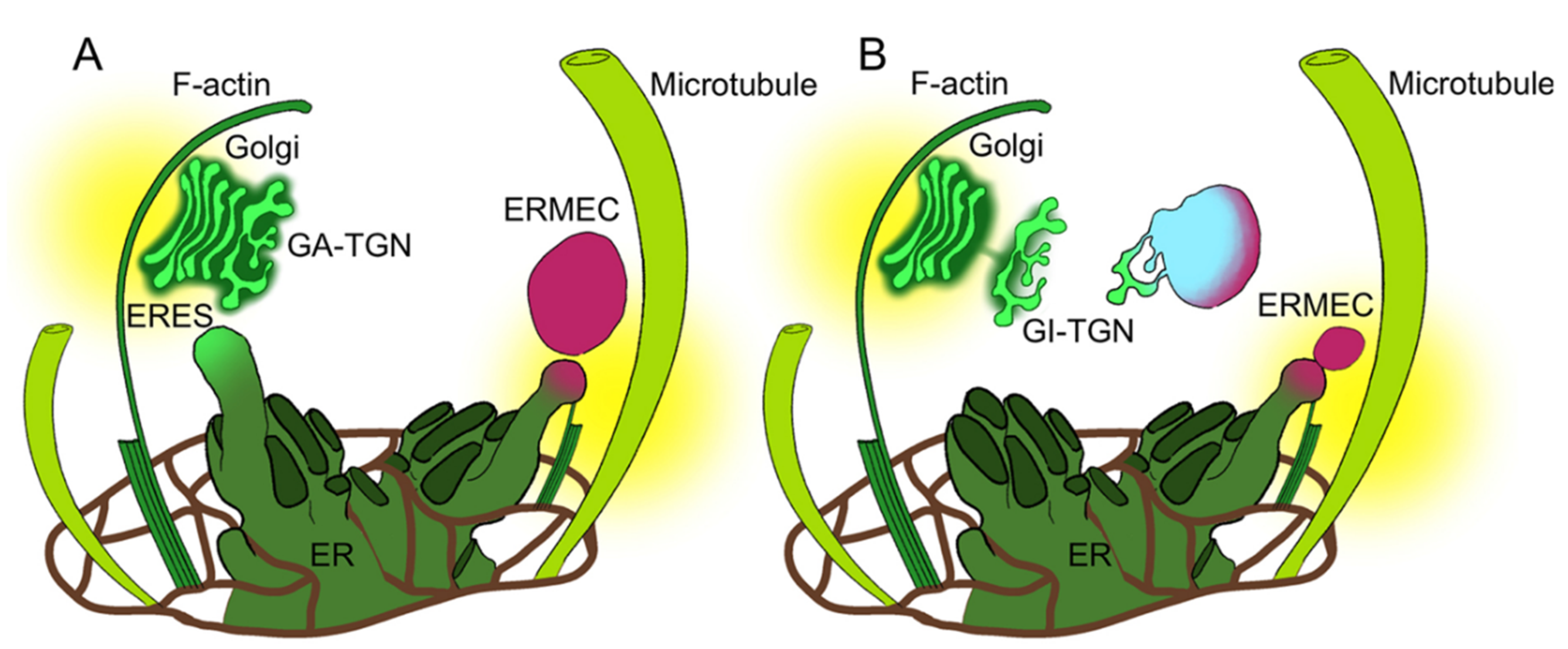
3.1. Golgi-Independent ER Export Leads to an Uncharacterized Intermediate Compartment
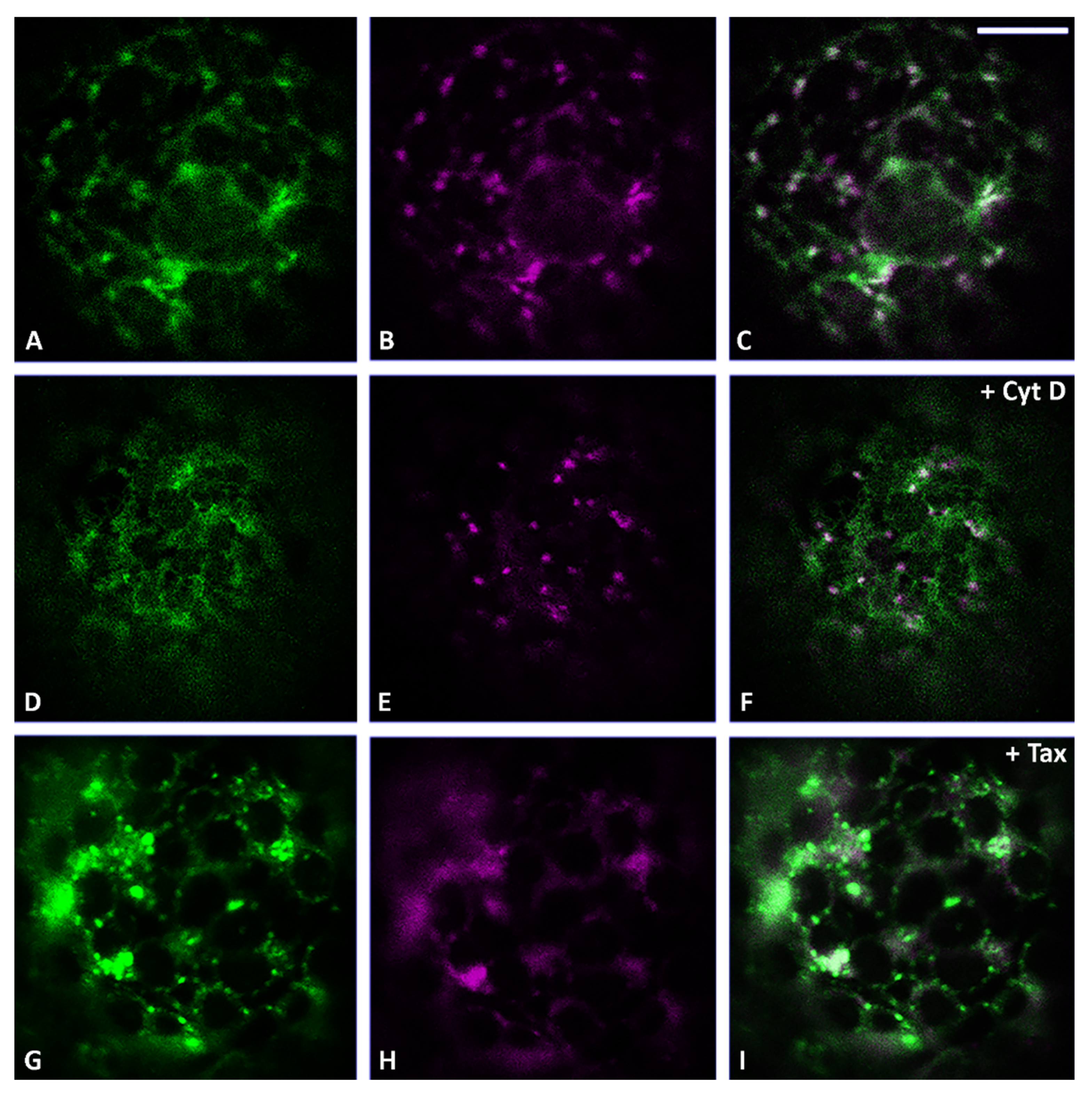
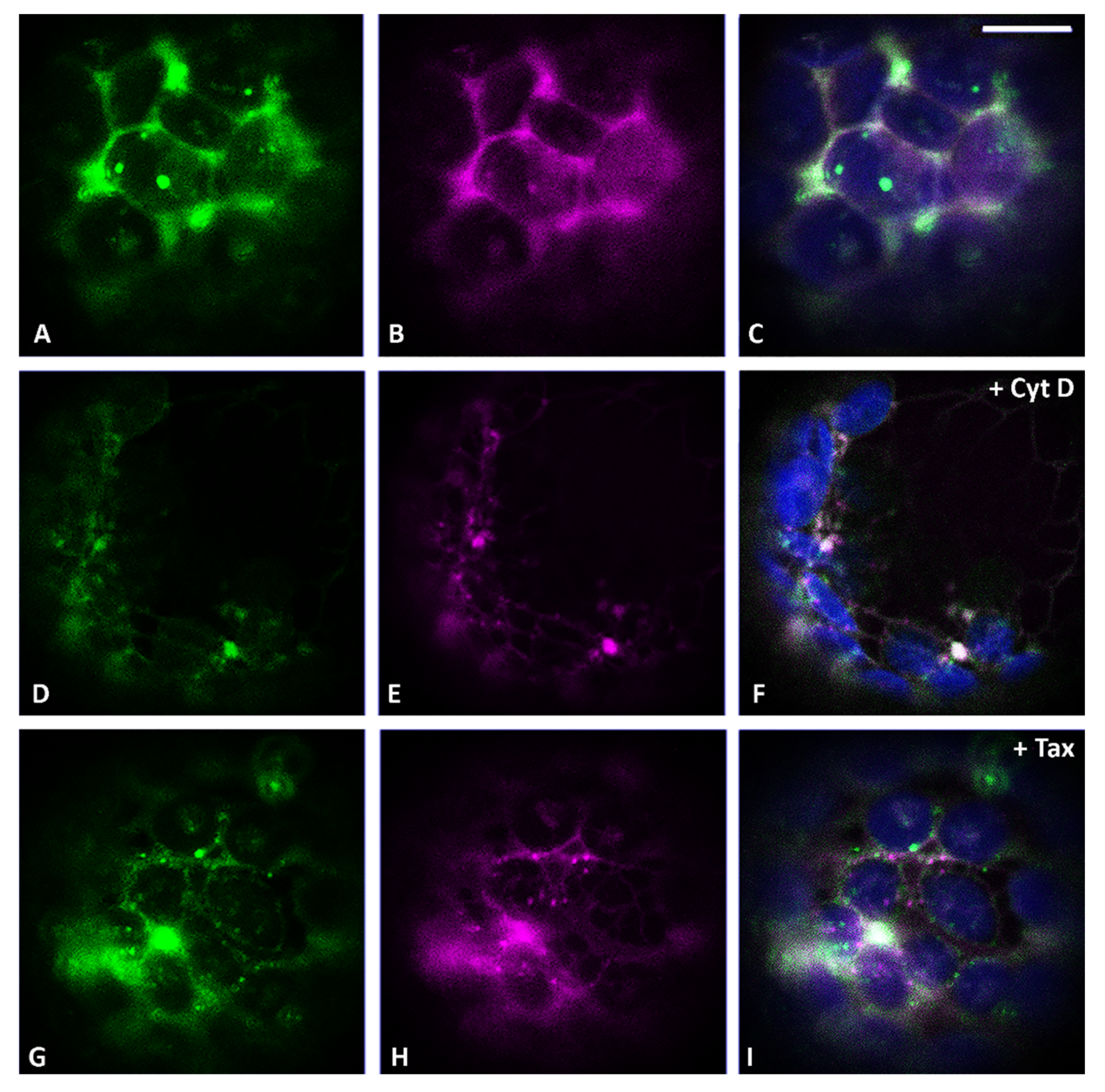
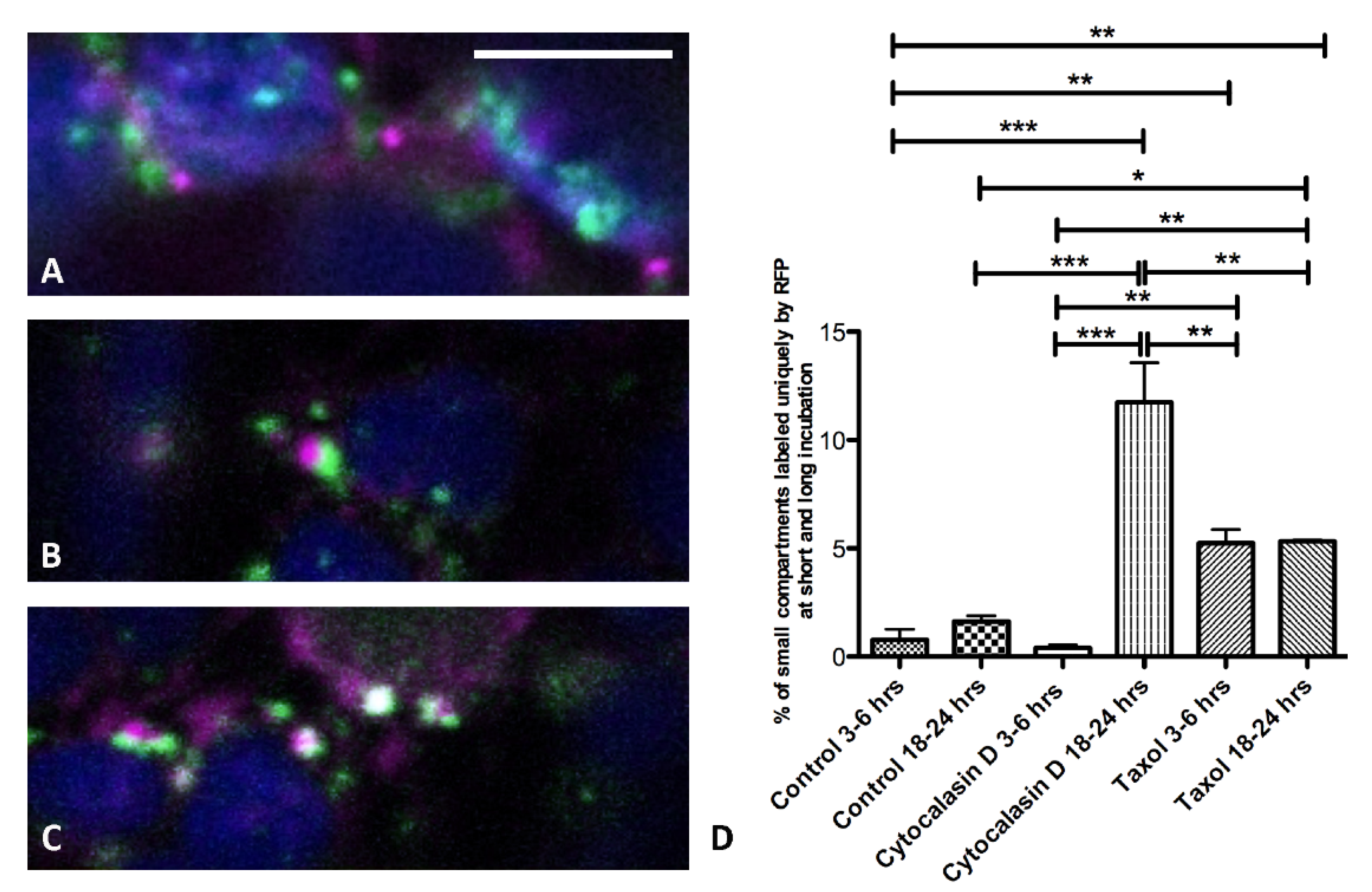
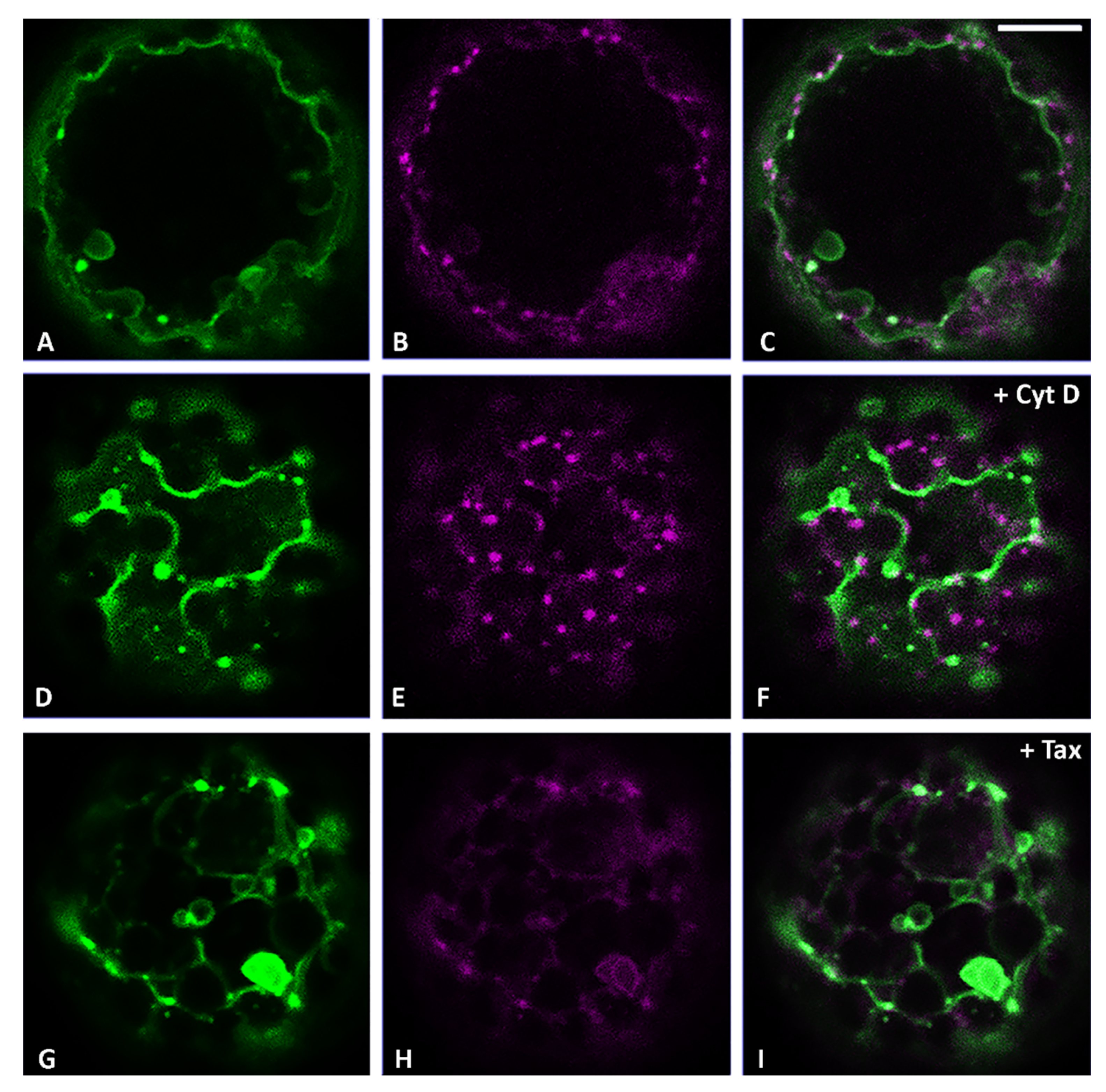
3.2. Actin Filaments and Microtubules Differently Affect Golgi-Dependent and Golgi-Independent Traffic
3.3. RFP-Chi Transits through Intermediate Compartments Different from Those Highlighted by Aleu-GFP
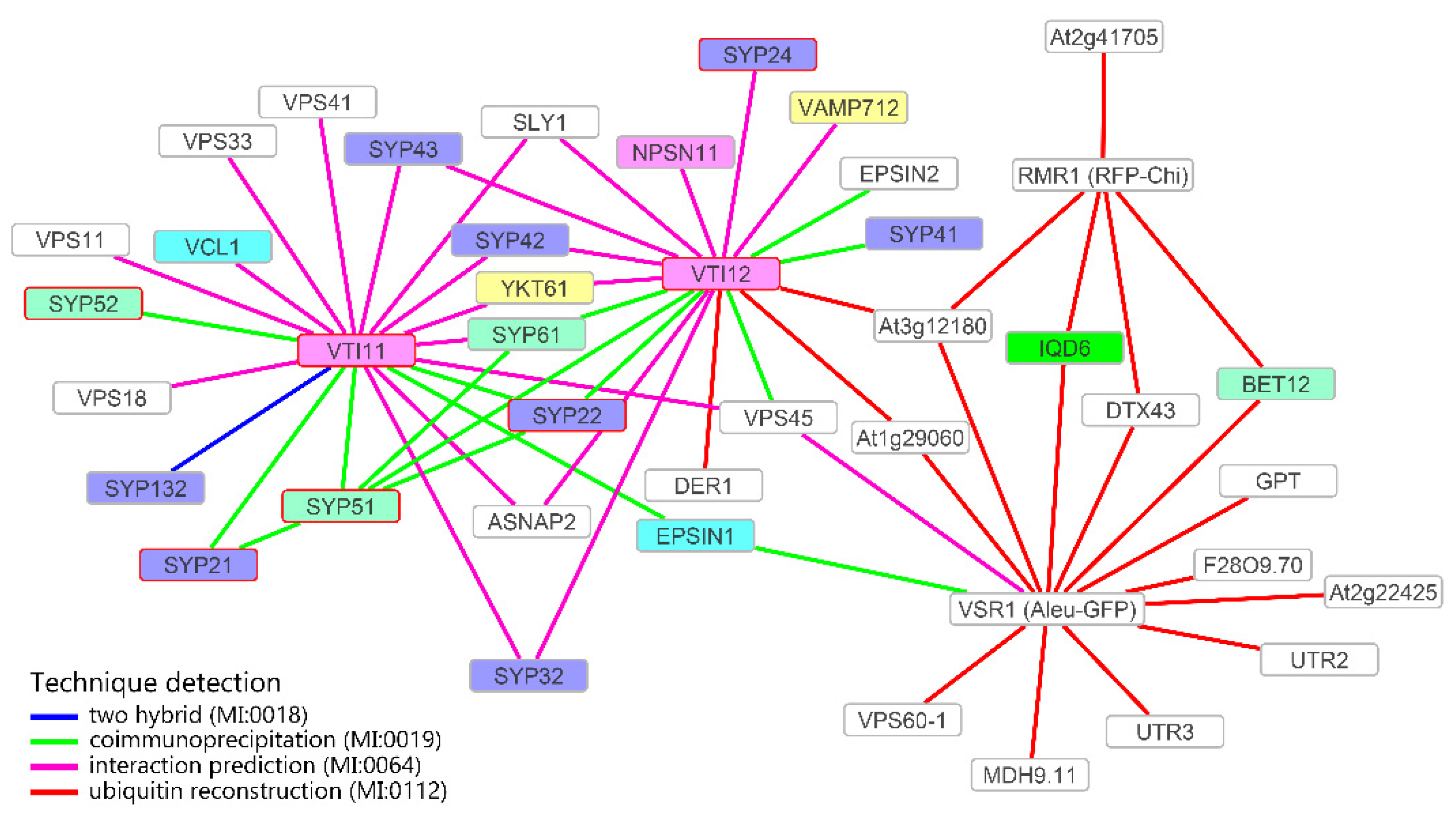
3.4. Definition of the Protein Interaction Network Leading to Alternative Vacuolar Sorting Pathways
3.5. Both Actin Filaments and Microtubules Are Required for the Correct Organization of TGN
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Furt, F.; Lemoi, K.; Tüzel, E.; Vidali, L. Quantitative analysis of organelle distribution and dynamics in Physcomitrella patens protonemal cells. BMC Plant Biol. 2012, 12, 70. [Google Scholar] [CrossRef] [Green Version]
- Elliott, L.; Moore, I.; Kirchhelle, C. Spatio-temporal control of post-Golgi exocytic trafficking in plants. J. Cell Sci. 2020, 133, jcs237065. [Google Scholar] [CrossRef] [PubMed]
- Schuh, M. An actin-dependent mechanism for long-range vesicle transport. Nat. Cell Biol. 2011, 13, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Nebenführ, A.; Dixit, R. Kinesins and Myosins: Molecular Motors that Coordinate Cellular Functions in Plants. Annu. Rev. Plant Biol. 2018, 69, 329–361. [Google Scholar] [CrossRef] [PubMed]
- Brandizzi, F.; Wasteneys, G.O. Cytoskeleton-dependent endomembrane organization in plant cells: An emerging role for microtubules. Plant J. 2013, 75, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Renna, L.; Stefano, G.; Slabaugh, E.; Wormsbaecher, C.; Sulpizio, A.; Zienkiewicz, K.; Brandizzi, F. TGNap1 is required for microtubule-dependent homeostasis of a subpopulation of the plant trans-Golgi network. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- De Caroli, M.; Manno, E.; Perrotta, C.; De Lorenzo, G.; Di Sansebastiano, G.-P.; Piro, G. CesA6 and PGIP2 Endocytosis Involves Different Subpopulations of TGN-Related Endosomes. Front. Plant Sci. 2020, 11, 350. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, R.; Kalbfuß, N.; Gendre, D.; Steiner, A.; Altmann, M.; Altmann, S.; Rybak, K.; Edelmann, H.; Stephan, F.; Lampe, M.; et al. Independent yet overlapping pathways ensure the robustness and responsiveness of trans-Golgi network functions in Arabidopsis. Development 2018, 145, dev169201. [Google Scholar] [CrossRef] [Green Version]
- Uemura, T.; Suda, Y.; Ueda, T.; Nakano, A. Dynamic Behavior of the trans-Golgi Network in Root Tissues of Arabidopsis Revealed by Super-Resolution Live Imaging. Plant Cell Physiol. 2014, 55, 694–703. [Google Scholar] [CrossRef] [Green Version]
- Crowell, E.F.; Bischoff, V.; Desprez, T.; Rolland, A.; Stierhof, Y.-D.; Schumacher, K.; Gonneau, M.; Höfte, H.; Vernhettes, S. Pausing of Golgi Bodies on Microtubules Regulates Secretion of Cellulose Synthase Complexes in Arabidopsis. Plant Cell 2009, 21, 1141–1154. [Google Scholar] [CrossRef] [Green Version]
- Occhialini, A.; Gouzerh, G.; Di Sansebastiano, G.-P.; Neuhaus, J.-M. Dimerization of the Vacuolar Receptors AtRMR1 and -2 from Arabidopsis thaliana Contributes to Their Localization in the trans-Golgi Network. Int. J. Mol. Sci. 2016, 17, 1661. [Google Scholar] [CrossRef] [Green Version]
- Goring, D.R.; Di Sansebastiano, G.P. Protein and membrane trafficking routes in plants: Conventional or unconventional? J. Exp. Bot. 2017, 69, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, K.; Dutta, T.; Saha, A.; Sarkar, D.; Sil, A.; Ray, K.; Sengupta, M. Mapping the TYR gene reveals novel and previously reported variants in Eastern Indian patients highlighting preponderance of the same changes in multiple unrelated ethnicities. Ann. Hum. Genet. 2020, 84, 303–312. [Google Scholar] [CrossRef]
- Müller, S. Plant cell division — defining and finding the sweet spot for cell plate insertion. Curr. Opin. Cell Biol. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Di Sansebastiano, G.P.; Paris, N.; Marc-Martin, S.; Neuhaus, J.-M. Regeneration of a Lytic Central Vacuole and of Neutral Peripheral Vacuoles Can Be Visualized by Green Fluorescent Proteins Targeted to Either Type of Vacuoles. Plant Physiol. 2001, 126, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stigliano, E.; Faraco, M.; Neuhaus, J.-M.; Montefusco, A.; Dalessandro, G.; Piro, G.; Di Sansebastiano, G.-P. Two glycosylated vacuolar GFPs are new markers for ER-to-vacuole sorting. Plant Physiol. Biochem. 2013, 73, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, M.; Ordóñez, A.; Sohn, E.J.; Robert, S.; Sánchez-Serrano, J.J.; Surpin, M.A.; Raikhel, N.V.; Rojo, E. Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 3645–3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barozzi, F.; Papadia, P.; Stefano, G.; Renna, L.; Brandizzi, F.; Migoni, D.; Fanizzi, F.P.; Piro, G.; Di Sansebastiano, G.-P. Variation in Membrane Trafficking Linked to SNARE AtSYP51 Interaction With Aquaporin NIP1;1. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y.; Uemura, T.; Shoda, K.; Fujimoto, M.; Ueda, T.; Nakano, A. cis-Golgi proteins accumulate near the ER exit sites and act as the scaffold for Golgi regeneration after brefeldin A treatment in tobacco BY-2 cells. Mol. Biol. Cell 2012, 23, 3203–3214. [Google Scholar] [CrossRef]
- De Caroli, M.; Lenucci, M.S.; Di Sansebastiano, G.-P.; Tunno, M.; Montefusco, A.; Dalessandro, G.; Piro, G. Cellular localization and biochemical characterization of a chimeric fluorescent protein fusion of Arabidopsis Cellulose Synthase-Like A2 inserted into Golgi membrane. Sci. World J. 2014, 792420. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; Bonsegna, S.; Santino, A.; Lenucci, M.S.; Poltronieri, P.; Di Sansebastiano, G.-P. Localization of Seed Oil Body Proteins in Tobacco Protoplasts Reveals Specific Mechanisms of Protein Targeting to Leaf Lipid Droplets. J. Integr. Plant Biol. 2011, 53, 858–868. [Google Scholar] [CrossRef]
- De Caroli, M.; Lenucci, M.S.; Manualdi, F.; Dalessandro, G.; De Lorenzo, G.; Piro, G. Molecular dissection of Phaseolus vulgaris polygalacturonase-inhibiting protein 2 reveals the presence of hold/release domains affecting protein trafficking toward the cell wall. Front. Plant Sci. 2015, 6, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leucci, M.R.; Di Sansebastiano, G.-P.; Gigante, M.; Dalessandro, G.; Piro, G. Secretion marker proteins and cell-wall polysaccharides move through different secretory pathways. Planta 2006, 225, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; O’Toole, N.; Ammar, R.; Provart, N.J.; Millar, A.H.; Geisler, M. A Predicted Interactome for Arabidopsis. Plant Physiol. 2007, 145, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stigliano, E.; Di Sansebastiano, G.-P.; Neuhaus, J.-M. Contribution of Chitinase A’s C-Terminal Vacuolar Sorting Determinant to the Study of Soluble Protein Compartmentation. Int. J. Mol. Sci. 2014, 15, 11030–11039. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.E.; Tour, O.; Palmer, A.E.; Steinbach, P.A.; Baird, G.S.; Zacharias, D.A.; Tsien, R.Y. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 2002, 99, 7877–7882. [Google Scholar] [CrossRef] [Green Version]
- Shinoda, H.; Ma, Y.; Nakashima, R.; Sakurai, K.; Matsuda, T.; Nagai, T. Acid-Tolerant Monomeric GFP from Olindias formosa. Cell Chem. Biol. 2018, 25, 330–338.e7. [Google Scholar] [CrossRef] [Green Version]
- Holzinger, A.; Blaas, K. Actin-Dynamics in Plant Cells: The Function of Actin-Perturbing Substances: Jasplakinolide, Chondramides, Phalloidin, Cytochalasins, and Latrunculins. Methods Mol. Biol. 2016, 1365, 243–261. [Google Scholar] [CrossRef] [Green Version]
- Di Sansebastiano, G.-P.; Rehman, R.U.; Neuhaus, J.-M. Rat β-glucuronidase as a reporter protein for the analysis of the plant secretory pathway. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2007, 141, 329–336. [Google Scholar] [CrossRef]
- Saint-Jore-Dupas, C.; Nebenführ, A.; Boulaflous, A.; Follet-Gueye, M.-L.; Plasson, C.; Hawes, C.; Driouich, A.; Faye, L.; Gomord, V. Plant N-Glycan Processing Enzymes Employ Different Targeting Mechanisms for Their Spatial Arrangement along the Secretory Pathway. Plant Cell 2006, 18, 3182–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naaz, F.; Haider, R.; Shafi, S.; Yar, M.S. Anti-tubulin agents of natural origin: Targeting taxol, vinca, and colchicine binding domains. Eur. J. Med. Chem. 2019, 171, 310–331. [Google Scholar] [CrossRef] [PubMed]
- De Caroli, M.; Lenucci, M.S.; Di Sansebastiano, G.P.; Dalessandro, G.; De Lorenzo, G.; Piro, G. Dynamic protein trafficking to the cell wall. Plant Signal. Behav. 2011, 6, 1012–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, L.; Ende, W.V.D. Trafficking of Plant Vacuolar Invertases: From a Membrane-Anchored to a Soluble Status. Understanding Sorting Information in Their Complex N-Terminal Motifs. Plant Cell Physiol. 2013, 54, 1263–1277. [Google Scholar] [CrossRef] [Green Version]
- Rojo, E.; Gillmor, C.; Kovaleva, V.; Somerville, C.R.; Raikhel, N.V. VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis. Dev. Cell 2001, 1, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.A.; LaMontagne, E.D.; Anderson, J.C.; Ekanayake, G.; Clarke, A.S.; Bond, L.N.; Salamango, D.J.; Cornish, P.V.; Peck, S.C.; Heese, A. EPSIN1 Modulates the Plasma Membrane Abundance of FLAGELLIN SENSING2 for Effective Immune Responses. Plant Physiol. 2020, 182, 1762–1775. [Google Scholar] [CrossRef] [Green Version]
- Bassham, D.C.; Sanderfoot, A.A.; Kovaleva, V.; Zheng, H.; Raikhel, N.V. AtVPS45 Complex Formation at thetrans-Golgi Network. Mol. Biol. Cell 2000, 11, 2251–2265. [Google Scholar] [CrossRef] [Green Version]
- Di Sansebastiano, G.P.; Barozzi, F.; Piro, G.; Denecke, J.; Lousa, C.D.M. Trafficking routes to the plant vacuole: Connecting alternative and classical pathways. J. Exp. Bot. 2017, 69, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Zhao, Q.; Hu, S.; Jiang, L. Vacuole Biogenesis in Plants: How Many Vacuoles, How Many Models? Trends Plant Sci. 2020, 25, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Scheuring, D.; Löfke, C.; Krüger, F.; Kittelmann, M.; Eisa, A.; Hughes, L.; Smith, R.S.; Hawes, C.; Schumacher, K.; Kleine-Vehn, J. Actin-dependent vacuolar occupancy of the cell determines auxin-induced growth repression. Proc. Natl. Acad. Sci. USA 2016, 113, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.C.W.; Winistorfer, S.C.; Poupon, V.; Luzio, J.P.; Piper, R.C. Mammalian Late Vacuole Protein Sorting Orthologues Participate in Early Endosomal Fusion and Interact with the Cytoskeleton. Mol. Biol. Cell 2004, 15, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Ebine, K.; Askani, J.C.; Krüger, F.; Gonzalez, Z.A.; Ito, E.; Goh, T.; Schumacher, K.; Nakano, A.; Ueda, T. Distinct sets of tethering complexes, SNARE complexes, and Rab GTPases mediate membrane fusion at the vacuole in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E2457–E2466. [Google Scholar] [CrossRef] [Green Version]
- Kutsuna, N.; Kumagai, F.; Sato, M.H.; Hasezawa, S. Three-dimensional reconstruction of tubular structure of vacuolar membrane throughout mitosis in living tobacco cells. Plant Cell Physiol. 2003, 44, 1045–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, M.J.; Calcutt, J.R.; Ingle, E.K.; Hawkins, T.J.; Chapman, S.; Richardson, A.C.; Mentlak, D.A.; Dixon, M.R.; Cartwright, F.; Smertenko, A.P.; et al. A Superfamily of Actin-Binding Proteins at the Actin-Membrane Nexus of Higher Plants. Curr. Biol. 2012, 22, 1595–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgadillo, M.O.; Ruano, G.; Zouhar, J.; Sauer, M.; Shen, J.; Lazarova, A.; Sanmartín, M.; Lai, L.T.F.; Deng, C.; Wang, P.; et al. MTV proteins unveil ER- and microtubule-associated compartments in the plant vacuolar trafficking pathway. Proc. Natl. Acad. Sci. USA 2020, 117, 9884–9895. [Google Scholar] [CrossRef]
- Ambrose, C.; Ruan, Y.; Gardiner, J.; Tamblyn, L.M.; Catching, A.; Kirik, V.; Marc, J.; Overall, R.; Wasteneys, G.O. CLASP Interacts with Sorting Nexin 1 to Link Microtubules and Auxin Transport via PIN2 Recycling in Arabidopsis thaliana. Dev. Cell 2013, 24, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Heucken, N.; Ivanov, R. The retromer, sorting nexins and the plant endomembrane protein trafficking. J. Cell Sci. 2017, 131, jcs203695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livanos, P.; Müller, S. Division Plane Establishment and Cytokinesis. Annu. Rev. Plant Biol. 2019, 70, 239–267. [Google Scholar] [CrossRef]
- Müller, S.; Jürgens, G.; Mueller, S. Plant cytokinesis—No ring, no constriction but centrifugal construction of the partitioning membrane. Semin. Cell Dev. Biol. 2016, 53, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, M.; Li, E.; Sampathkumar, A.; Kocabek, T.; Hauser, M.-T.; Persson, S. POM-POM2/CELLULOSE SYNTHASE INTERACTING1 Is Essential for the Functional Association of Cellulose Synthase and Microtubules in Arabidopsis. Plant Cell 2012, 24, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Li, S.; Pan, S.; Xin, X.; Gu, Y. CSI1, PATROL1, and exocyst complex cooperate in delivery of cellulose synthase complexes to the plasma membrane. Proc. Natl. Acad. Sci. USA 2018, 115, E3578–E3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, T.I.; Wilson, J.E.; Cork, A.; Williamson, R.E. Morphology and Microtubule Organization in Arabidopsis Roots Exposed to Oryzalin or Taxol. Plant Cell Physiol. 1994, 35, 935–942. [Google Scholar] [CrossRef]
- Vergara, D.; Ferraro, M.M.; Cascione, M.; Del Mercato, L.L.; Leporatti, S.; Ferretta, A.; Tanzarella, P.; Pacelli, C.; Santino, A.; Maffia, M.; et al. Cytoskeletal Alterations and Biomechanical Properties of parkin-Mutant Human Primary Fibroblasts. Cell Biophys. 2014, 71, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caroli, M.; Barozzi, F.; Renna, L.; Piro, G.; Di Sansebastiano, G.-P. Actin and Microtubules Differently Contribute to Vacuolar Targeting Specificity during the Export from the ER. Membranes 2021, 11, 299. https://doi.org/10.3390/membranes11040299
De Caroli M, Barozzi F, Renna L, Piro G, Di Sansebastiano G-P. Actin and Microtubules Differently Contribute to Vacuolar Targeting Specificity during the Export from the ER. Membranes. 2021; 11(4):299. https://doi.org/10.3390/membranes11040299
Chicago/Turabian StyleDe Caroli, Monica, Fabrizio Barozzi, Luciana Renna, Gabriella Piro, and Gian-Pietro Di Sansebastiano. 2021. "Actin and Microtubules Differently Contribute to Vacuolar Targeting Specificity during the Export from the ER" Membranes 11, no. 4: 299. https://doi.org/10.3390/membranes11040299
APA StyleDe Caroli, M., Barozzi, F., Renna, L., Piro, G., & Di Sansebastiano, G.-P. (2021). Actin and Microtubules Differently Contribute to Vacuolar Targeting Specificity during the Export from the ER. Membranes, 11(4), 299. https://doi.org/10.3390/membranes11040299