Infusion of Silver–Polydopamine Particles into Polyethersulfone Matrix to Improve the Membrane’s Dye Desalination Performance and Antibacterial Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ag-PDA Nanoparticles
2.3. Preparation of Mixed-Matrix Membranes
2.4. Characterization of Ag-PDA Nanoparticles and Membranes
2.5. Evaluation of Membrane Performance
2.6. Bacterial Attachment Test
3. Results and Discussion
3.1. Characterization of Particle
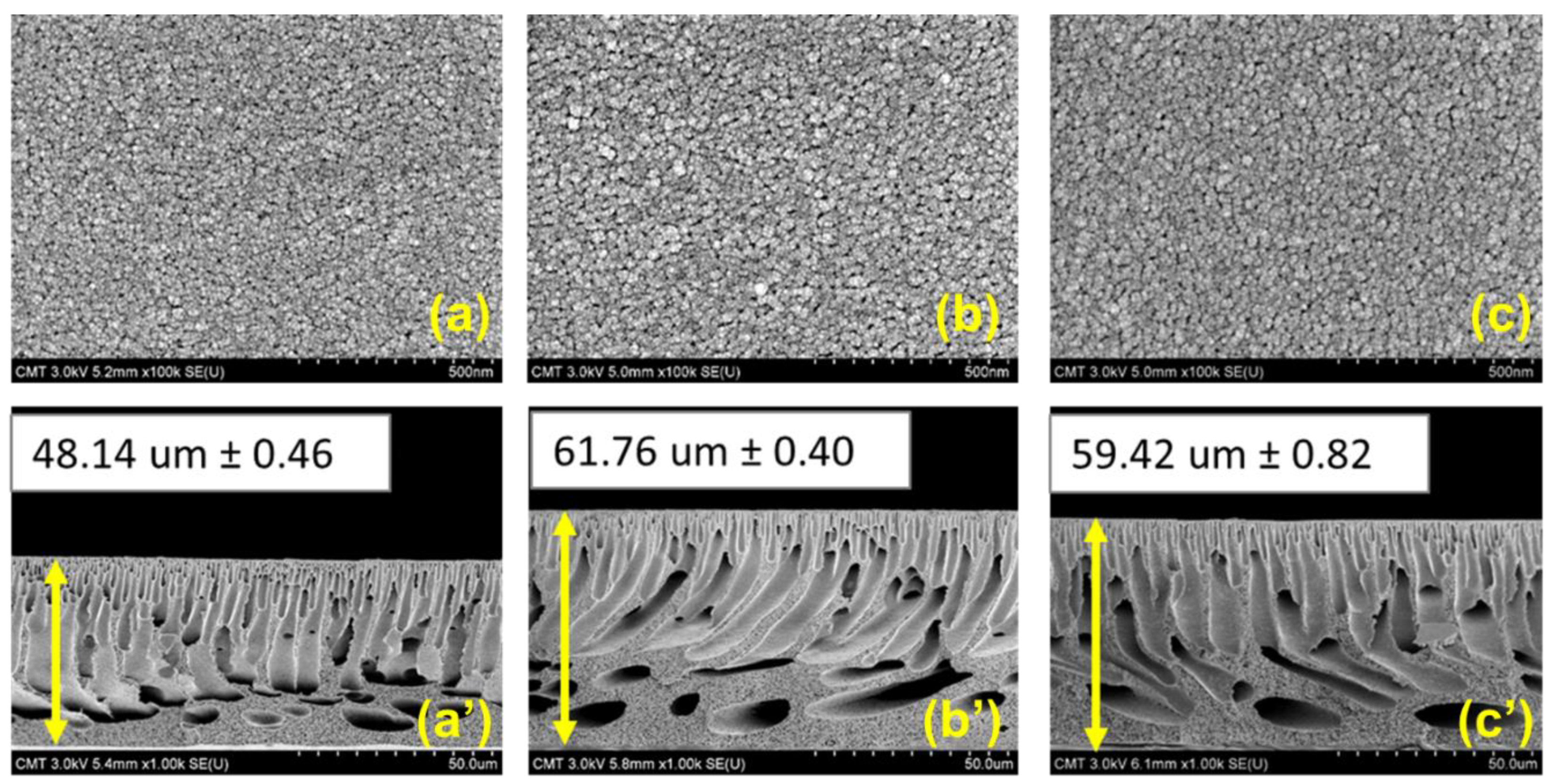
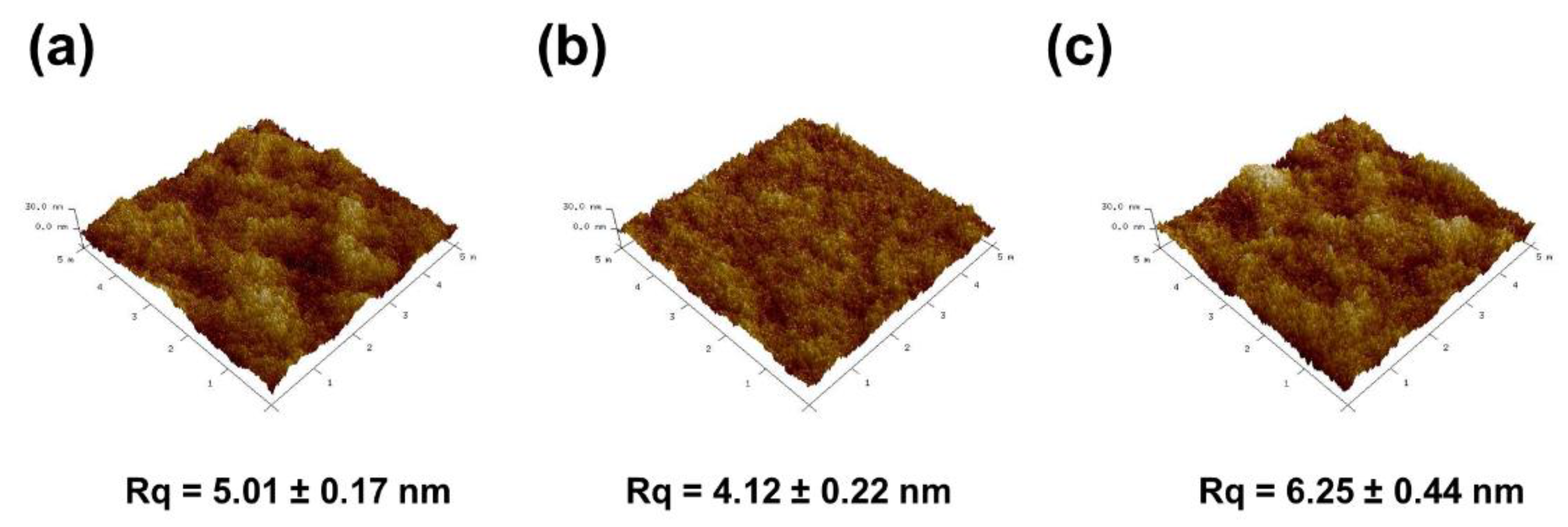
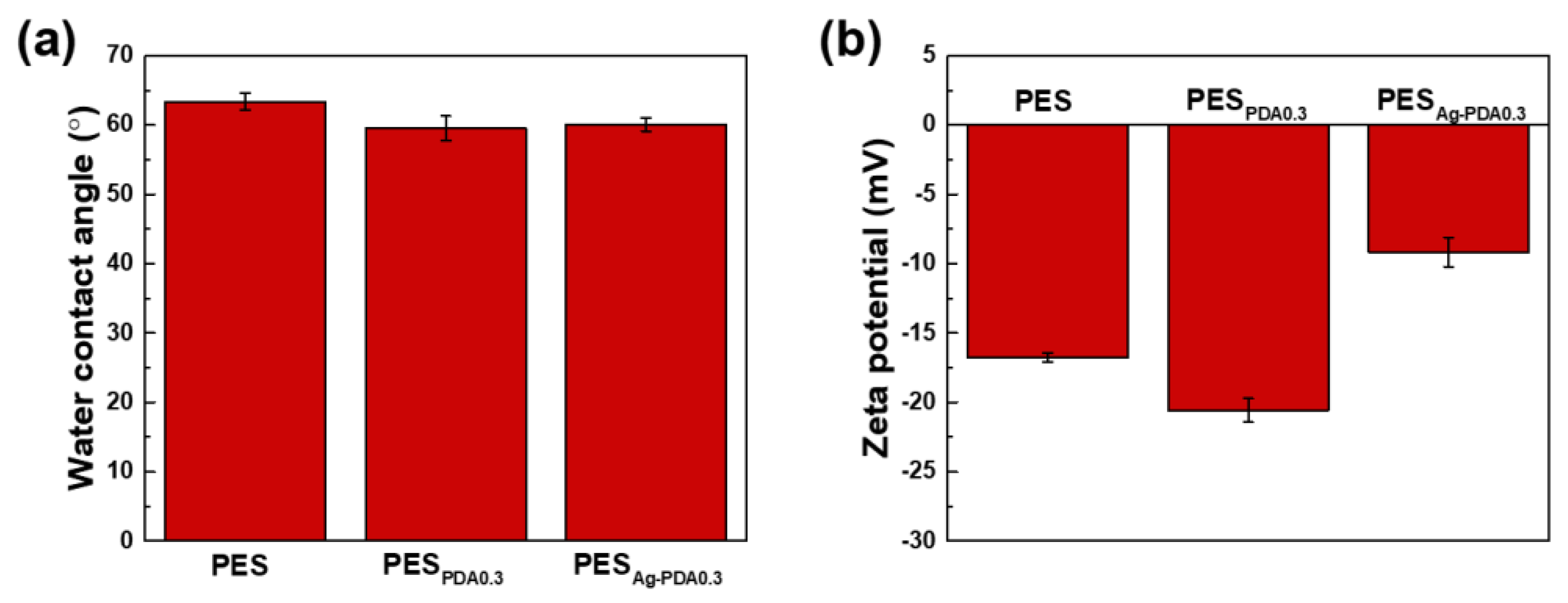
3.2. Characterization of the Membranes
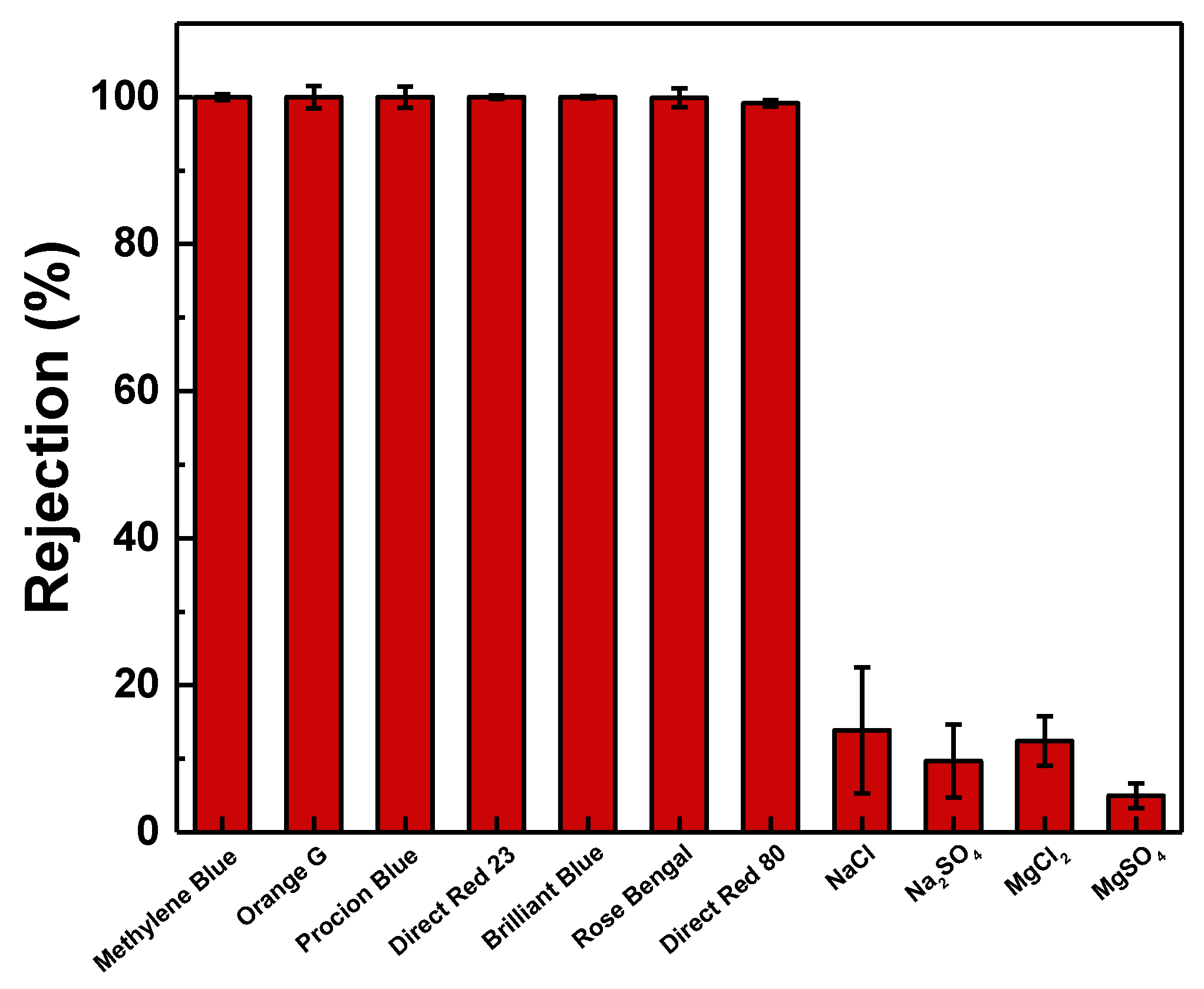
3.3. Performance Evaluation
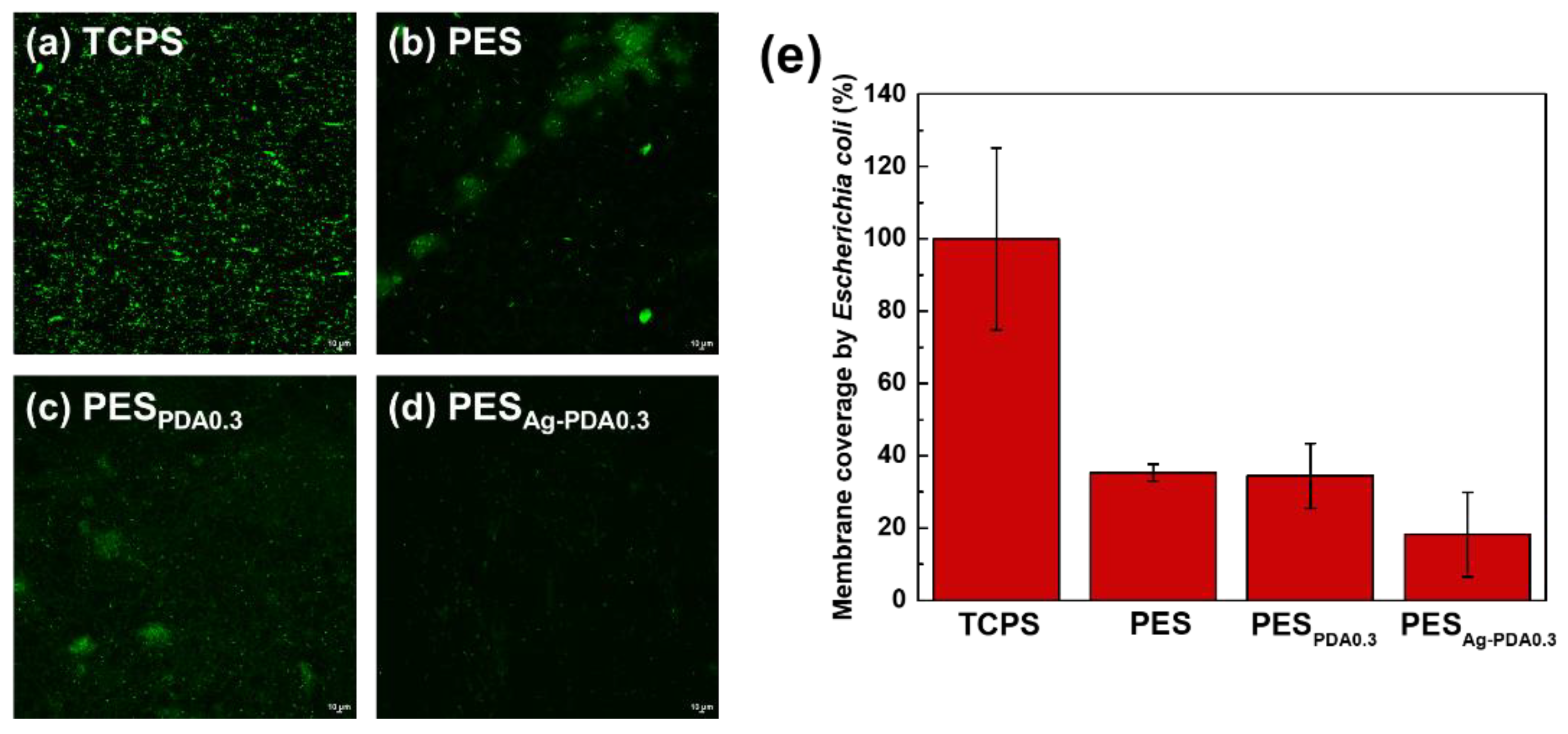
3.4. Bacterial Attachment Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xie, Y.; Tang, C.; Wang, Z.; Xu, Y.; Zhao, W.; Sun, S.; Zhao, C. Co-deposition towards mussel-inspired antifouling and antibacterial membranes by using zwitterionic polymers and silver nanoparticles. J. Mater. Chem. B 2017, 5, 7186–7193. [Google Scholar] [CrossRef]
- Myint, A.A.; Lee, W.; Mun, S.; Ahn, C.H.; Lee, S.; Yoon, J. Influence of membrane surface properties on the behavior of initial bacterial adhesion and biofilm development onto nanofiltration membranes. Biofouling 2010, 26, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chiao, Y.H.; Sengupta, A.; Chen, S.T.; Huang, S.H.; Hu, C.C.; Hung, W.S.; Chang, Y.; Qian, X.H.; Wickramasinghe, S.R.; Lee, K.R.; et al. Zwitterion augmented polyamide membrane for improved forward osmosis performance with significant antifouling characteristics. Sep. Purif. Technol. 2019, 212, 316–325. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Polymers. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [Green Version]
- Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling resistance of reverse osmosis membrane modified with polydopamine. Desalination 2014, 336, 87–96. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Flemming, H.-C. Reverse osmosis membrane biofouling. Exp. Therm. Fluid Sci. 1997, 14, 382–391. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Munoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Xu, Z.-L.; Chen, C. Polypiperazine-amide nanofiltration membrane containing silica nanoparticles prepared by interfacial polymerization. Desalination 2012, 301, 75–81. [Google Scholar] [CrossRef]
- García-Ivars, J.; Corbatón-Báguena, M.-J.; Iborra-Clar, M.-I. Development of mixed matrix membranes: Incorporation of metal nanoparticles in polymeric membranes. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–178. [Google Scholar]
- Banerjee, P.; Mukhopadhyay, A.; Das, P. Graphene oxide–nanobentonite composite sieves for enhanced desalination and dye removal. Desalination 2019, 451, 231–240. [Google Scholar] [CrossRef]
- Abid, M.F.; Zablouk, M.A.; Abid-Alameer, A.M. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran. J. Environ. Health Sci. Eng. 2012, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Y.; Hu, X.; Zhang, Y.; Hu, L. Desalination of dye solution utilizing pva/pvdf hollow fiber composite membrane modified with tio2 nanoparticles. J. Membr. Sci. 2014, 471, 118–129. [Google Scholar] [CrossRef]
- Rozelle, L.; Cadotte, J.; Corneliussen, R.; Erickson, E.; Cobian, K.; Kopp, C., Jr. Phase inversion membranes. In Encyclopedia of Separation Science; Mulder, M., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 3331–3346. [Google Scholar]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A comprehensive review on polymeric nano-composite membranes for water treatment. J. Membr. Sci. Technol. 2018, 8, 1000179. [Google Scholar] [CrossRef]
- Li, D.; Wu, J.; Yang, S.; Zhang, W.; Ran, F. Hydrophilicity and anti-fouling modification of polyethersulfone membrane by grafting copolymer chains via surface initiated electrochemically mediated atom transfer radical polymerization. New J. Chem. 2017, 41, 9918–9930. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Iborra-Clar, M.-I.; Alcaina-Miranda, M.-I.; Mendoza-Roca, J.-A.; Pastor-Alcañiz, L. Surface photomodification of flat-sheet pes membranes with improved antifouling properties by varying uv irradiation time and additive solution ph. Chem. Eng. J. 2016, 283, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, R.; Anton, E.; Ulbricht, M. Uv-photo graft functionalization of polyethersulfone membrane with strong polyelectrolyte hydrogel and its application for nanofiltration. ACS Appl. Mater. Interfaces 2012, 4, 3438–3446. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Zinadini, S.; Rostami, S.; Vatanpour, V.; Jalilian, E. Preparation of antibiofouling polyethersulfone mixed matrix nf membrane using photocatalytic activity of zno/mwcnts nanocomposite. J. Membr. Sci. 2017, 529, 133–141. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Tavangar, T.; Karimi, M.; Rezakazemi, M.; Reddy, K.R.; Aminabhavi, T.M. Textile waste, dyes/inorganic salts separation of cerium oxide-loaded loose nanofiltration polyethersulfone membranes. Chem. Eng. J. 2020, 385, 123787. [Google Scholar] [CrossRef]
- Ghaemi, N.; Daraei, P.; Akhlaghi, F.S. Polyethersulfone nanofiltration membrane embedded by chitosan nanoparticles: Fabrication, characterization and performance in nitrate removal from water. Carbohydr. Polym. 2018, 191, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, R.; Han, X.; Jiao, D.; Zhu, J.; Lai, F.; Liu, X.; Liu, J.; Zhang, Y.; Van der Bruggen, B. High performance loose nanofiltration membranes obtained by a catechol-based route for efficient dye/salt separation. Chem. Eng. J. 2019, 375, 121982. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.R.; Parvizian, F.; Hosseini, S.M.; Van der Bruggen, B. Tailoring the separation performance and fouling reduction of pes based nanofiltration membrane by using a pva/fe3o4 coating layer. Chem. Eng. Res. Des. 2019, 144, 418–428. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Nguyen, N.C.; Nguyen, H.T. Electrospinning: A versatile fabrication technique for nanofibrous membranes for use in desalination. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–273. [Google Scholar]
- Huang, J.; Arthanareeswaran, G.; Zhang, K. Effect of silver loaded sodium zirconium phosphate (nanoagz) nanoparticles incorporation on pes membrane performance. Desalination 2012, 285, 100–107. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Yu, D. Characterization and inhibitory effect of antibacterial pan-based hollow fiber loaded with silver nitrate. J. Membr. Sci. 2003, 225, 115–123. [Google Scholar] [CrossRef]
- Veerapandian, M.; Sadhasivam, S.; Choi, J.; Yun, K. Glucosamine functionalized copper nanoparticles: Preparation, characterization and enhancement of anti-bacterial activity by ultraviolet irradiation. Chem. Eng. J. 2012, 209, 558–567. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Bhakya, S.; Kumar, T.S.; Rao, M.V. Biosynthesis, characterization and antibacterial effect of plant-mediated silver nanoparticles using Ceropegia thwaitesii—An endemic species. Ind. Crop. Prod. 2015, 63, 119–124. [Google Scholar] [CrossRef]
- Annamalai, A.; Christina, V.; Sudha, D.; Kalpana, M.; Lakshmi, P. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surfaces B Biointerfaces 2013, 108, 60–65. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Lee, D.K.; Gil Cha, H.; Kim, A.C.W.; Kang, Y.S. Synthesis and Characterization of Antibacterial Ag−SiO2 Nanocomposite. J. Phys. Chem. C 2007, 111, 3629–3635. [Google Scholar] [CrossRef]
- Chou, W.-L.; Yu, D.-G.; Yang, M.-C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005, 16, 600–607. [Google Scholar] [CrossRef]
- Toroghi, M.; Raisi, A.; Aroujalian, A. Preparation and characterization of polyethersulfone/silver nanocomposite ultrafiltration membrane for antibacterial applications. Polym. Adv. Technol. 2014, 25, 711–722. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.; Lee, J.; Perreault, F.; Elimelech, M. Controlled architecture of dual-functional block copolymer brushes on thin-film composite membranes for integrated “defending” and “attacking” strategies against biofouling. ACS Appl. Mater. Interfaces 2015, 7, 23069–23079. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Fei, F.; Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Tailoring the performance of organic solvent nanofiltration membranes with biophenol coatings. ACS Appl. Polym. Mater. 2019, 1, 452–460. [Google Scholar] [CrossRef]
- Derami, H.G.; Gupta, P.; Gupta, R.; Rathi, P.; Morrissey, J.J.; Singamaneni, S. Palladium Nanoparticle-Decorated Mesoporous Polydopamine/Bacterial Nanocellulose as a Catalytically Active Universal Dye Removal Ultrafiltration Membrane. ACS Appl. Nano Mater. 2020, 3, 5437–5448. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Ji, Y.L.; Huang, S.H.; Lee, K.R.; Lai, J.Y. A facile and versatile strategy for fabricating thin-film nanocomposite membranes with polydopamine-piperazine nanoparticles generated in situ. J. Membr. Sci. 2019, 579, 79–89. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Andra, C.K.A.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Huang, S.-H.; Lee, K.-R. Increased performance and antifouling of mixed-matrix membranes of cellulose acetate with hydrophilic nanoparticles of polydopamine-sulfobetaine methacrylate for oil-water separation. J. Membr. Sci. 2020, 620, 118881. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Zhan, Y.Q.; He, S.J.; Zhang, L.H.; Zeng, G.Y.; Chiao, Y.H. Construction of superhydrophilic/underwater superoleophobic polydopamine-modified h-bn/poly(arylene ether nitrile) composite membrane for stable oil-water emulsions separation. Polym. Adv. Technol. 2020, 31, 1007–1018. [Google Scholar] [CrossRef]
- Zeng, G.; Wei, K.; Yang, D.; Yan, J.; Zhou, K.; Patra, T.; Sengupta, A.; Chiao, Y.-H. Improvement in performance of pvdf ultrafiltration membranes by co-incorporation of dopamine and halloysite nanotubes. Colloids Surf. A 2020, 586, 124142. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-inspired surface engineering for water-remediation materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhang, G.; Xia, T.; Li, Z.; Zhao, K.; Deng, Z.; Guo, D.; Peng, B. Bioinspired synthesis of polydopamine/ag nanocomposite particles with antibacterial activities. Mater. Sci. Eng. C 2015, 55, 155–165. [Google Scholar] [CrossRef]
- Black, K.C.L.; Sileika, T.S.; Yi, J.; Zhang, R.; Rivera, J.G.; Messersmith, P.B. Bacterial killing by light-triggered release of silver from biomimetic metal nanorods. Small 2014, 10, 169–178. [Google Scholar] [CrossRef]
- Li, S.K.; Yan, Y.X.; Wang, J.L.; Yu, S.H. Bio-inspired in situ growth of monolayer silver nanoparticles on graphene oxide paper as multifunctional substrate. Nanoscale 2013, 5, 12616–12623. [Google Scholar] [CrossRef]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. Engl. 2009, 48, 3914–3921. [Google Scholar] [CrossRef]
- Orooji, Y.; Liang, F.; Razmjou, A.; Liu, G.; Jin, W. Preparation of anti-adhesion and bacterial destructive polymeric ultrafiltration membranes using modified mesoporous carbon. Sep. Purif. Technol. 2018, 205, 273–283. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, M. Polyethersulfone (pes)–silver composite uf membrane: Effect of silver loading and pvp molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Devanadera, K.P.O.; Duena, A.N.R.; Luo, Z.-Y.; Chiao, Y.-H.; Millare, J.C.; Aquino, R.R.; Huang, S.-H.; Lee, K.-R. Modifying cellulose acetate mixed-matrix membranes for improved oil–water separation: Comparison between sodium and organo-montmorillonite as particle additives. Membranes 2021, 11, 80. [Google Scholar] [CrossRef]
- Hsiao, S.W.; Venault, A.; Yang, H.S.; Chang, Y. Bacterial resistance of self-assembled surfaces using ppom-b-psbman zwitterionic copolymer—concomitant effects of surface topography and surface chemistry on attachment of live bacteria. Colloids Surf. B Biointerfaces 2014, 118, 254–260. [Google Scholar] [CrossRef]
- Son, E.J.; Kim, J.H.; Kim, K.; Park, C.B. Quinone and its derivatives for energy harvesting and storage materials. J. Mater. Chem. A 2016, 4, 11179–11202. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Wang, J.; Cao, B.; Tang, C.Y. In situ reduction of silver by polydopamine: A novel antimicrobial modification of a thin-film composite polyamide membrane. Environ. Sci. Technol. 2016, 50, 9543–9550. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of polydopamine–scope, variation, and limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Choi, G.H.; do Rhee, K.; Park, A.R.; Oh, M.J.; Hong, S.; Richardson, J.J.; Guo, J.; Caruso, F.; Yoo, P.J. Ag nanoparticle/polydopamine-coated inverse opals as highly efficient catalytic membranes. ACS Appl. Mater. Interfaces 2016, 8, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Afshari, M.; Fazlali, A.R.; Koudzari Farahani, S.; Bandehali, S.; Van der Bruggen, B.; Bagheripour, E. Mixed matrix pes-based nanofiltration membrane decorated by (fe3o4–polyvinylpyrrolidone) composite nanoparticles with intensified antifouling and separation characteristics. Chem. Eng. Res. Des. 2019, 147, 390–398. [Google Scholar] [CrossRef]
- Balkanloo, P.G.; Mahmoudian, M.; Hosseinzadeh, M.T. A comparative study between mmt-fe3o4/pes, mmt-hbe/pes, and mmtacid activated/pes mixed matrix membranes. Chem. Eng. J. 2020, 396, 125188. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Construction of tio 2 @graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Membr. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, H.; Zhang, N.; Jiang, B.; Sun, Y.; Yang, N. A loose nf membrane by grafting tio2-hmdi nanoparticles on pes/β-cd substrate for dye/salt separation. Sep. Purif. Technol. 2019, 218, 8–19. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Baltaru, M.-C.; Tang, Y.P.; Bernstein, N.J.; Gao, P.; Balta, S.; Vlad, M.; Volodin, A.; Sotto, A.; et al. Tight ultrafiltration membranes for enhanced separation of dyes and na2so4 during textile wastewater treatment. J. Membr. Sci. 2016, 514, 217–228. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Dong, G.; Zhang, Y.; Guo, N.; Liu, J. Sulfonated halloysite nanotubes/polyethersulfone nanocomposite membrane for efficient dye purification. Sep. Purif. Technol. 2015, 150, 243–251. [Google Scholar] [CrossRef]
- Lessan, F.; Karimi, M.; Arami, M. Tailoring the hierarchical porous structure within polyethersulfone/cellulose nanosheets mixed matrix membrane to achieve efficient dye/salt mixture fractionation. J. Polym. Res. 2016, 23, 1–10. [Google Scholar] [CrossRef]
- Rahimi, M.; Dadari, S.; Zeinaddini, S.; Mohamadian, E. Flux, antifouling and separation characteristics enhancement of nanocomposite polyethersulfone mixed-matrix membrane by embedding synthesized hydrophilic adipate ferroxane nanoparticles. Korean J. Chem. Eng. 2017, 34, 1444–1455. [Google Scholar] [CrossRef]
- Saniei, N.; Ghasemi, N.; Zinatizadeh, A.A.; Zinadini, S.; Ramezani, M.; Derakhshan, A.A. Preparation and characterization of a novel antifouling nano filtration poly ethersulfone (pes) membrane by embedding goethite-tannic acid nanoparticles. Sep. Purif. Technol. 2020, 241, 116646. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/tio2 blended pes nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Effective treatment of dye wastewater via positively charged teta-mwcnt/pes hybrid nanofiltration membranes. Sep. Purif. Technol. 2018, 194, 488–502. [Google Scholar] [CrossRef]
- Low, Z.-X.; Ji, J.; Blumenstock, D.; Chew, Y.-M.; Wolverson, D.; Mattia, D. Fouling resistant 2d boron nitride nanosheet—Pes nanofiltration membranes. J. Membr. Sci. 2018, 563, 949–956. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Wang, J.; Zhang, Y.; Wu, W.; Liu, J.; Van der Bruggen, B. Zwitterionic functionalized mos2 nanosheets for a novel composite membrane with effective salt/dye separation performance. J. Membr. Sci. 2019, 573, 270–279. [Google Scholar] [CrossRef]





| Solution | PES (wt %) | DMAc (wt %) | Particle (wt %) a | Viscosity (cp) |
|---|---|---|---|---|
| PES | 19 | 81 | 0 | 424.47 ± 2.10 |
| PESPDA0.3 | 19 | 81 | 0.3 | 438.93 ± 0.49 |
| PESAg-PDA0.1 | 19 | 81 | 0.1 | 456.60 ± 1.56 |
| PESAg-PDA0.3 | 19 | 81 | 0.3 | 462.73 ± 0.51 |
| PESAg-PDA0.5 | 19 | 81 | 0.5 | 502.47 ± 1.50 |
| Membrane | C (%) | O (%) | S (%) | N (%) | Ag (%) |
|---|---|---|---|---|---|
| PES | 77.70 ± 1.30 | 15.43 ± 0.95 | 6.88 ± 0.37 | - | - |
| PESPDA0.3 | 74.35 ± 0.42 | 18.32 ± 0.28 | 6.21 ± 0.24 | 1.12 ± 0.13 | - |
| PESAg-PDA0.3 | 69.98 ± 1.12 | 22.01 ± 1.44 | 5.39 ± 0.28 | 2.40 ± 0.28 | 0.22 ± 0.27 |
| Different Dyes Dye Concentration: 50 ppm | Molecular Weights (g∙mol−1) |
|---|---|
| Methylene Blue | 319.85 |
| Orange G | 452.38 |
| Procion Blue | 637.44 |
| Direct Red 23 | 813.73 |
| Brilliant Blue | 826.98 |
| Rose Bengal | 1017.65 |
| Direct Red 80 | 1373.08 |
| Membrane | Operating Pressure (bar) | Permeability (L∙m−2∙h−1∙bar−1) | Dye Concentration (ppm) | Molecular Weight of Dye (g/mol) | Dye Rejection (%) | NaCl Concentration (ppm) | NaCl Rejection (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| PES | 4.00 | 0.61 | 50.00 | 973.67 | 99.00 | - | - | This study |
| PESPDA0.3 | 4.00 | 1.35 | 50.00 | 973.67 | 99.51 | 1000.00 | - | This study |
| PESAg-PDA0.3 | 4.00 | 3.33 | 50.00 | 319.85 | 99.99 | 1000.00 | 12.40 | This study |
| PES + HNTs-SO3H | 4.00 | 18.25 | 1000.00 | 576.49 | 80.00 | 1000.00 | 3.00 | [69] |
| PES + CNs | 5.00 | 18.00 | 984.20 | 813.73 | 98.00 | 2000.00 | 8.00 | [70] |
| PES + AFNPs | 4.00 | 4.19 | 30.00 | 637.55 | 98.00 | 200.00 | 18.00 | [71] |
| PES + G-TA | 4.00 | 8.00 | 50.00 | 637.55 | 92.61 | - | - | [72] |
| PES-CDs | 3.00 | 25.5 | 100.00 | 983.5 | 98.9 | 200.00 | 20.1 | [49] |
| PES + rGO/TiO2 | 5.00 | 9.00 | 100.00 | 1079.60 | 85.00 | - | - | [73] |
| PES + TETA-MWCNT | 10.00 | 8.30 | 50.00 | 407.98 | 98.00 | 1000.00 | 23.00 | [74] |
| PES + ZnO/MWCNTs | 4.00 | 4.18 | 30.00 | 637.55 | 95.00 | - | - | [20] |
| PES + BNNS | 4.00 | 8.00 | 10.00 | 973.67 | 88.00 | - | - | [75] |
| PES + MoS2-PSBMA | 6.00 | 18.10 | 400.00 | 991.8 | 98.2 | 1000.00 | 1.1 | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maganto, H.L.C.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Aquino, R.R.; Huang, S.-H.; Tsai, H.-A.; Lee, K.-R. Infusion of Silver–Polydopamine Particles into Polyethersulfone Matrix to Improve the Membrane’s Dye Desalination Performance and Antibacterial Property. Membranes 2021, 11, 216. https://doi.org/10.3390/membranes11030216
Maganto HLC, Ang MBMY, Dizon GVC, Caparanga AR, Aquino RR, Huang S-H, Tsai H-A, Lee K-R. Infusion of Silver–Polydopamine Particles into Polyethersulfone Matrix to Improve the Membrane’s Dye Desalination Performance and Antibacterial Property. Membranes. 2021; 11(3):216. https://doi.org/10.3390/membranes11030216
Chicago/Turabian StyleMaganto, Hazel Lynn C., Micah Belle Marie Yap Ang, Gian Vincent C. Dizon, Alvin R. Caparanga, Ruth R. Aquino, Shu-Hsien Huang, Hui-An Tsai, and Kueir-Rarn Lee. 2021. "Infusion of Silver–Polydopamine Particles into Polyethersulfone Matrix to Improve the Membrane’s Dye Desalination Performance and Antibacterial Property" Membranes 11, no. 3: 216. https://doi.org/10.3390/membranes11030216








