Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review
Abstract
:| Table of Contents | ||
| 1. | Introduction | 2 |
| 2. | Commercial membranes for VRFB Work | 5 |
| 3. | Polymer membrane Development | 9 |
| 3.1 Membrane Structures | 9 | |
| 3.2 Membrane Polymers | 10 | |
| 4. | Polymer membrane Development | 11 |
| 4.1 Membrane Properties | 15 | |
| 4.2 Membrane Impact on VRFB Cell Performance | 32 | |
| 5. | Cycle Stability | 46 |
| 6. | Membrane Costs | 47 |
| 7. | Conclusions | 48 |
| 8. | Acknowledgements | 50 |
| 9. | Abbreviations | 51 |
| 10. | References | 52 |
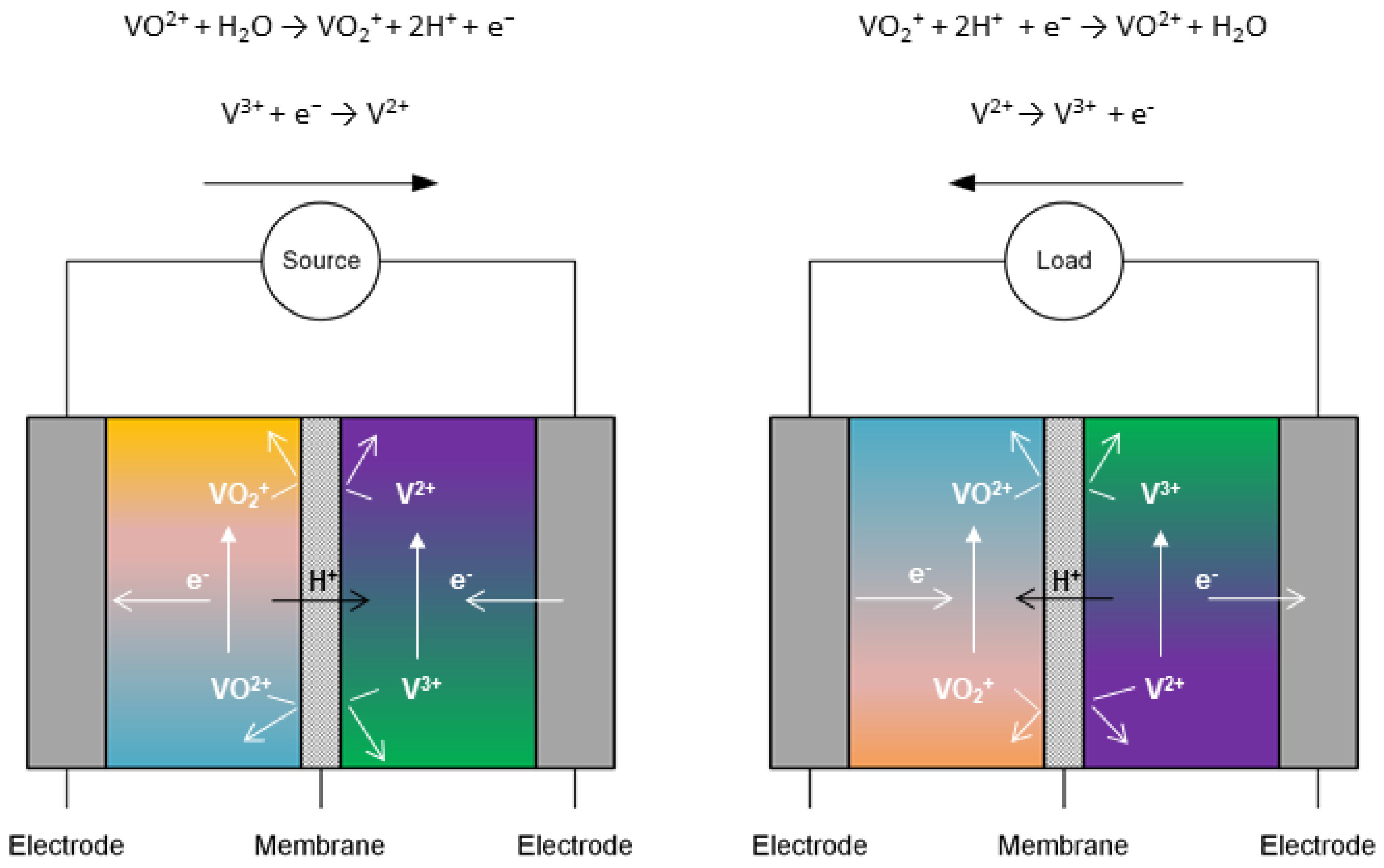
1. Introduction
2. Commercial Membranes for VRFB
3. Polymer Membrane Development
3.1. Membrane Structures
3.2. Membrane Polymers
4. Membrane Developments in Recent Years
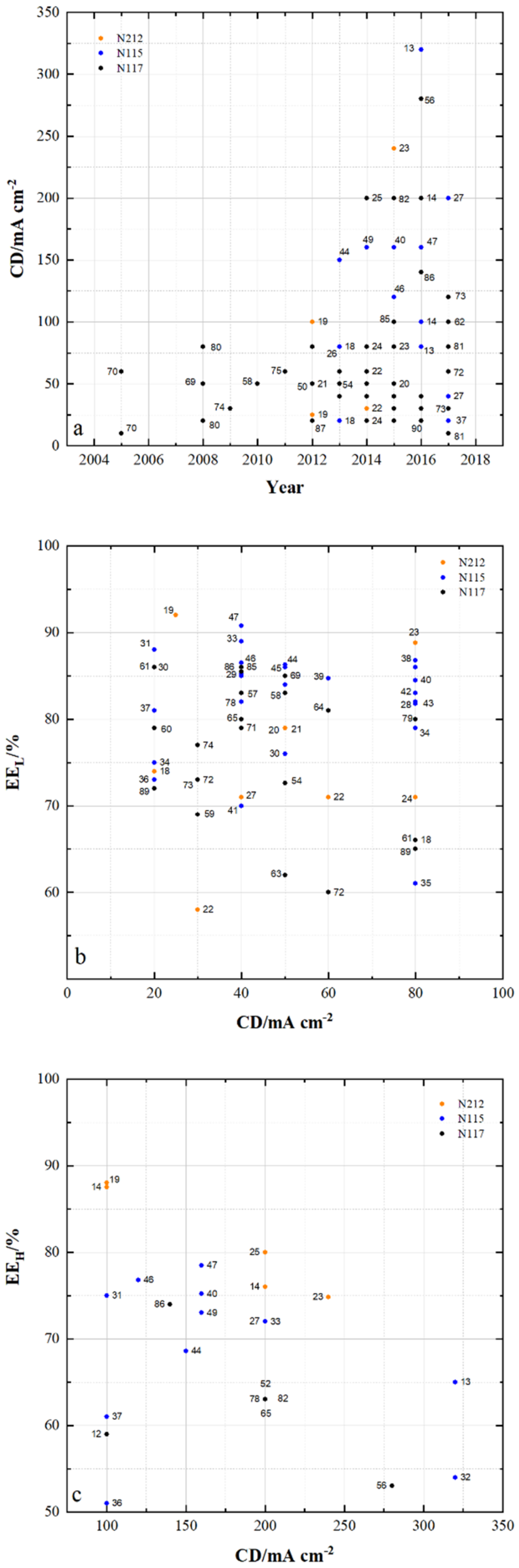
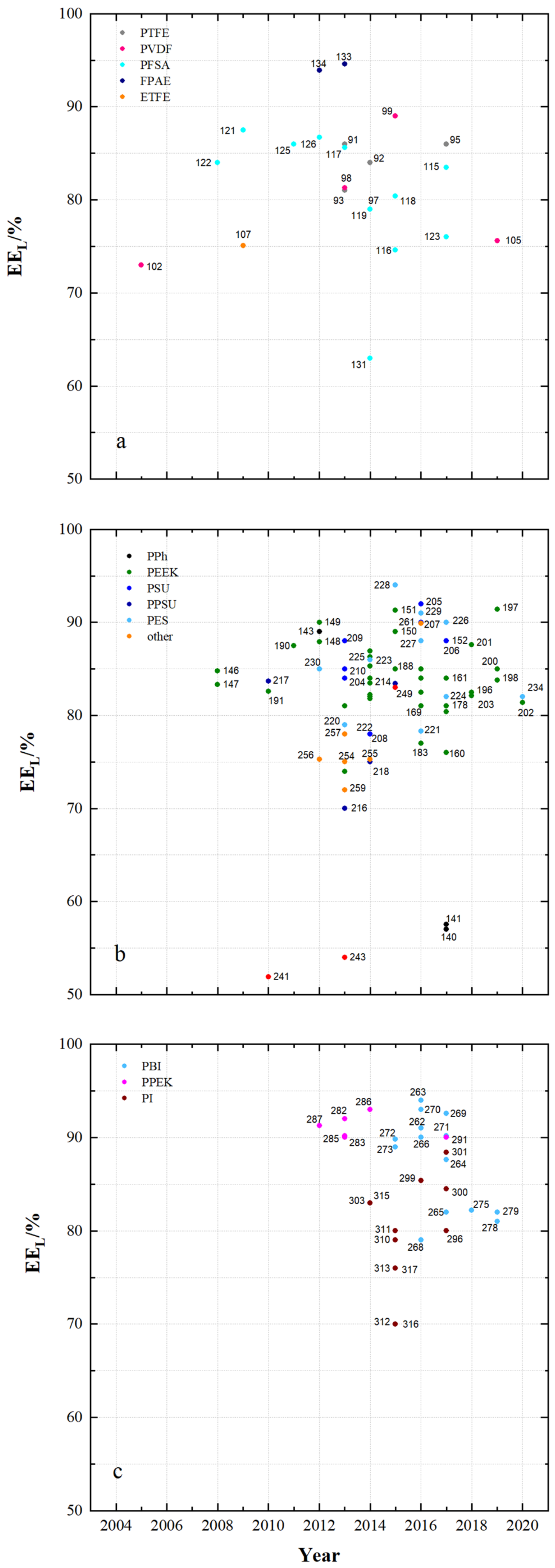
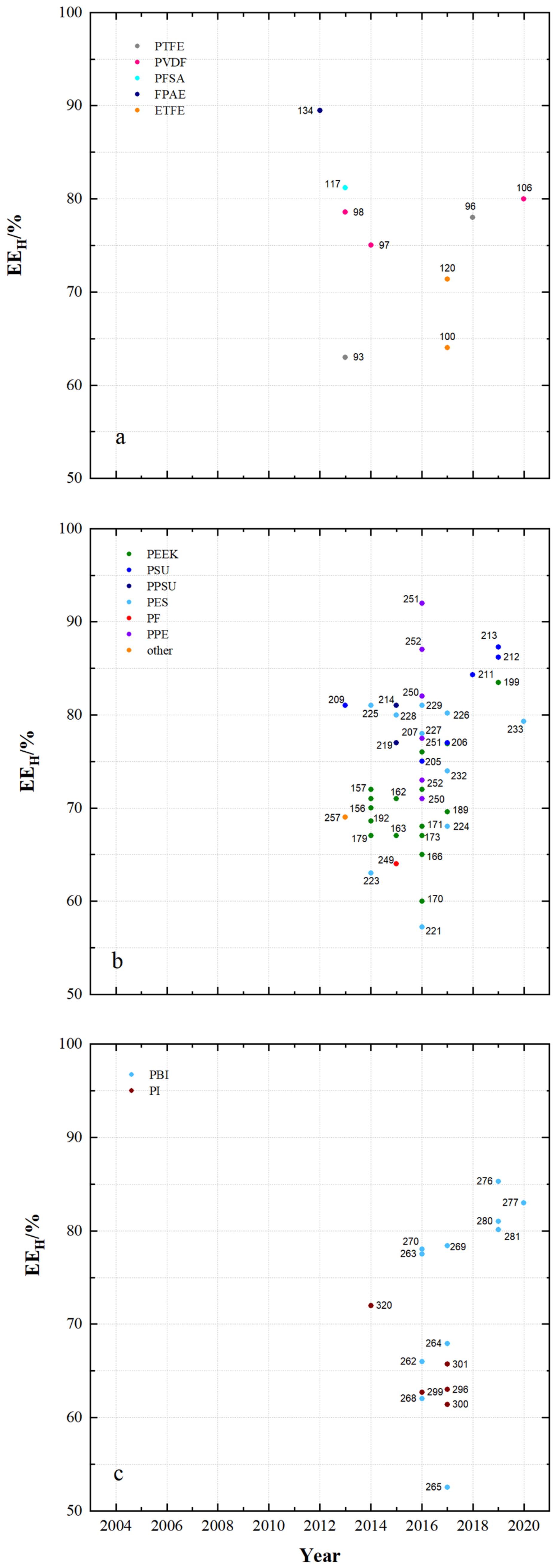
4.1. Membrane Properties
- PTFE 4.62 × 10−8 to 7.1 × 10−7 cm2 min−1
- PVDF 6.7 × 10−8 to 7.9 × 10−7 cm2 min−1
- ETFE 2.9 × 10−9 to 3.9 × 10−8 cm2 min−1
- PFSA 3.6 × 10−9 to 6.72 × 10−6 cm2 min−1
- FPAE (MS134) 1.16 × 10−8 cm2 min−1
- PPh 3.3 × 10−9 to 1.4 × 10−6 cm2 min−1
- PEEK 1.05 × 10−9 to 4.2 × 10−6 cm2 min−1
- PSU 1.5 × 10−8 to 2.94 × 10−6 cm2 min−1
- PPSU 1.6 × 10−9 to 2.07 × 10−7 cm2 min−1
- PES 1.41 × 10−8 to 4 × 10−6 cm2 min−1
- PF 8.8 × 10−8 to 9.85 × 10−7 cm2 min−1
- PPE 1.1 × 10−8 to 3.6 × 10−8 cm2 min−1
- Other 6.9 × 10−8 to 1.56 × 10−7 cm2 min−1
- PBI 1.28 × 10−11 to 5.74 × 10−7 cm2 min−1
- PPEK 1.24 × 10−7 to 5.75 × 10−7 cm2 min−1
- PI 4.8 × 10−8 to 2.6 × 10−6 cm2 min−1.
4.2. Membrane Impact on VRFB Cell Performance
5. Cycle Stability
6. Membrane Costs
7. Conclusions
- Dimensional stability after soaking the dry membrane in battery electrolyte or water is very important to keep the ion channels diameters as small as possible.
- For sulfonated polymers as a proton conductor in the membranes, it should be taken into account that its acidity is dependent on the polymer used and influences the proton conductivity.
- The thickness of the membrane (length of ion channels) should be optimized for high selectivity.
- As many ion channels as possible should be aimed for good conduction between the two half-cells.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEM | anion exchange membrane |
| AEM* | anion exchange membrane (acidic environment) |
| AIEM | amphoteric ion exchange membrane |
| AIEM* | amphoteric ion exchange membrane (acidic environment) |
| asym | asymmetric |
| CD | current density |
| CEH | coulombic efficiency (≥ 100 mA cm−2) |
| CEL | coulombic efficiency (< 100 mA cm−2) |
| CEM | cation exchange membrane |
| chem | chemistry |
| CL | cross-linked |
| d | membrane thickness |
| Dc | diffusion coefficient |
| Dr | diffusion coefficient ratio |
| DAPP | diels-Alder Poly(phenylene) |
| dhe | dense and heterogeneous |
| dho | dense and homogeneous |
| DOE | U.S. Department of Energy |
| EE | energy efficiency (charge-discharge) |
| EEr | energy efficiency ratio |
| EEH | energy efficiency (≥ 100 mA cm−2) |
| EEL | energy efficiency (< 100 mA cm−2) |
| ETFE | poly(ethylene-tetrafluoroethylene) |
| FPAE | fluorinated poly(arylene ether) |
| IEC | ion exchange capacity |
| Mem | membrane |
| MS | membrane sample |
| PA | poly(amide) |
| PBI | poly(benzimidazole) |
| PEEK | poly(ether ether ketone) |
| PEK | poly(ether ketones) |
| PES | poly(ether sulfone) |
| PF | poly(fluorenyle) |
| PFSA | perfluorosulfonic acid |
| PI | poly(imide) |
| PPE | poly(phenylene ether) |
| PPEK | poly(phthalazinone ether ketones) |
| PPh | poly(phenylene) |
| PPSU | poly(phenyl sulfones) |
| PS | poly(styrene) |
| PSU | poly(sulfones) |
| PTFE | poly(tetrafluoroethylene) |
| pub | publication |
| PVA | poly(vinyl alcohol) |
| PVC | poly(vinyl chloride) |
| PVDF | poly(vinylidene fluoride) |
| Ref | reference |
| struc | structure |
| sym | symmertric |
| V | vanadium |
| VEH | voltage efficiency (≥ 100 mA cm−2) |
| VEL | voltage efficiency (< 100 mA cm−2) |
| VRFB | all vanadium redox flow battery |
| WU | water uptake |
References
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- REN21, Renewables 2020—Global Status Report. 2020. Available online: https://www.ren21.net/reports/global-status-report/ (accessed on 25 February 2021).
- CellCube. CellCube Reference Project. Potential for improvement of the VRFB. 2020. Available online: https://www.cellcube.com/pellworm (accessed on 25 February 2021).
- Colthorpe, A. China’s Biggest Flow Battery Project so Far Is Underway with Hundreds More Megawatts to Come. 2018. Available online: https://www.energy-storage.news/news/chinas-biggest-flow-battery-project-so-far-is-underway-with-hundreds-more-m (accessed on 25 February 2021).
- Vanadiumcorp, Sumitomo Electric 60 Megawatt Hour Vanadium Redox Battery for Hokkaido. 2020. Available online: https://www.vanadiumcorp.com/news/industry/sumitomo-electric-60-megawatt-hour-vanadium-redox-battery-for-hokkaido/ (accessed on 25 February 2021).
- Fraunhofer ICT, Großprojekt RedoxWind. 2020. Available online: https://www.ict.fraunhofer.de/de/komp/ae/RFBWind.html (accessed on 25 February 2021).
- Storion Energy GmbH, Products. 2020. Available online: http://storion-energy.de/produkte/ (accessed on 25 February 2021).
- Voltstorage, Vanadium Redox Flow Technology. 2020. Available online: https://voltstorage.com/ (accessed on 25 February 2021).
- Volterion, Systems. 2020. Available online: https://www.volterion.com/systeme-2/ (accessed on 25 February 2021).
- Minke, C.; Turek, T. Materials, system designs and modelling approaches in techno-economic assessment of all-vanadium redox flow batteries—A review. J. Power Sources 2018, 376, 66–81. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane Development for Vanadium Redox Flow Batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, S.-H. Recent Development of Nanocomposite Membranes for Vanadium Redox Flow Batteries. J. Nanomater. 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.N.L.; Hoang, T.K.A.; Chen, P. Recent development of polymer membranes as separators for all-vanadium redox flow batteries. RSC Adv. 2015, 5, 72805–72815. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.-H.; Kim, Y.; Moon, S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, Z.; Zhao, Y.; Zhang, H.; Zhang, H.; Li, X. Porous membranes in secondary battery technologies. Chem. Soc. Rev. 2017, 46, 2199–2236. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, H.; Li, X. Ion conducting membranes for aqueous flow battery systems. Chem. Commun. 2018, 54, 7570–7588. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.M.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Gubler, L. Membranes and separators for redox flow batteries. Curr. Opin. Electrochem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Tempelman, C.; Jacobs, J.F.; Balzer, R.M.; Degirmenci, V. Membranes for all vanadium redox flow batteries. J. Energy Storage 2020, 32, 101754. [Google Scholar] [CrossRef]
- FumaTech GmbH, VRFB Membranes. 2019. Available online: https://www.fumatech.com/EN/Membranes/Batteries/index.html (accessed on 30 November 2019).
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Zhu, X.B. Performance of a vanadium redox flow battery with a VANADion membrane. Appl. Energy 2016, 180, 353–359. [Google Scholar] [CrossRef]
- Mohammadi, T.; Kazacos, M. Modification of anion-exchange membranes for vanadium redox flow battery applications. J. Power Sources 1996, 63, 179–186. [Google Scholar] [CrossRef]
- Hwang, G.-J.; Ohya, H. Crosslinking of anion exchange membrane by accelerated electron radiation as a separator for the all-vanadium redox flow battery. J. Membr. Sci. 1997, 132, 55–61. [Google Scholar] [CrossRef]
- AGC, Selemion. 2019. Available online: https://www.amp-ionex.com/products/selemion/pdf/selemion.pdf (accessed on 30 November 2019).
- Ding, C.; Zhang, H.; Li, X.; Zhang, H.; Yao, C.; Shi, D. Morphology and Electrochemical Properties of Perfluorosulfonic Acid Ionomers for Vanadium Flow Battery Applications: Effect of Side-Chain Length. ChemSusChem 2013, 6, 1262–1269. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Xi, X.; Lai, Q.; Liu, T.; Zhang, H. The transfer behavior of different ions across anion and cation exchange membranes under vanadium flow battery medium. J. Power Sources 2014, 271, 1–7. [Google Scholar] [CrossRef]
- David, O.; Percin, K.; Luo, T.; Gendel, Y.; Wessling, M. Proton-exchange membranes based on sulfonated poly(ether ether ketone)/polyaniline blends for all- and air-vanadium redox flow battery applications. J. Energy Storage 2015, 1, 65–71. [Google Scholar] [CrossRef]
- Chromik, A.; Santos, A.R.d.; Turek, T.; Kunz, U.; Häring, T.; Kerres, J. Stability of acid-excess acid–base blend membranes in all-vanadium redox-flow batteries. J. Membr. Sci. 2015, 476, 148–155. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; Wei, L.; Zeng, Y.K.; Zhang, Z.H. Highly stable pyridinium-functionalized cross-linked anion exchange membranes for all vanadium redox flow batteries. J. Power Sources 2016, 331, 452–461. [Google Scholar] [CrossRef]
- Jiang, B.; Yu, L.; Wu, L.; Mu, D.; Liu, L.; Xi, J.; Qiu, X. Insights into the Impact of the Nafion Membrane Pretreatment Process on Vanadium Flow Battery Performance. ACS Appl. Mater. Interfaces 2016, 8, 12228–12238. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries. Electrochem. Commun. 2013, 26, 37–40. [Google Scholar] [CrossRef]
- Dai, W.; Shen, Y.; Li, Z.; Yu, L.; Xi, J.; Qiu, X. SPEEK/Graphene oxide nanocomposite membranes with superior cyclability for highly efficient vanadium redox flow battery. J. Mater. Chem. A 2014, 2, 12423–12432. [Google Scholar] [CrossRef]
- Chen, D.; Kim, S.; Li, L.; Yang, G.; Hickner, M.A. Stable fluorinated sulfonated poly(arylene ether) membranes for vanadium redox flow batteries. RSC Adv. 2012, 2, 8087. [Google Scholar] [CrossRef]
- Jia, C.; Cheng, Y.; Ling, X.; Wei, G.; Liu, J.; Yan, C. Sulfonated Poly(Ether Ether Ketone)/Functionalized Carbon Nanotube Composite Membrane for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2015, 153, 44–48. [Google Scholar] [CrossRef]
- Fang, J.; Xu, H.; Wei, X.; Guo, M.; Lu, X.; Lan, C.; Zhang, Y.; Liu, Y.; Peng, T. Preparation and characterization of quaternized poly (2,2,2-trifluoroethyl methacrylate-co-N-vinylimidazole) membrane for vanadium redox flow battery. Polym. Adv. Technol. 2013, 24, 168–173. [Google Scholar] [CrossRef]
- Jia, C.; Liu, J.; Yan, C. A multilayered membrane for vanadium redox flow battery. J. Power Sources 2012, 203, 190–194. [Google Scholar] [CrossRef]
- Fujimoto, C.; Kim, S.; Stains, R.; Wei, X.; Li, L.; Yang, Z.G. Vanadium redox flow battery efficiency and durability studies of sulfonated Diels Alder poly(phenylene)s. Electrochem. Commun. 2012, 20, 48–51. [Google Scholar] [CrossRef]
- Lu, S.; Wu, C.; Liang, D.; Tan, Q.; Xiang, Y. Layer-by-layer self-assembly of Nafion–[CS–PWA] composite membranes with suppressed vanadium ion crossover for vanadium redox flow battery applications. RSC Adv. 2014, 4, 24831–24837. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Reed, D.; Thomsen, E.; Wang, W.; Nie, Z.; Li, B.; Wei, X.; Koeppel, B.; Sprenkle, V. Performance of Nafion® N115, Nafion® NR-212, and Nafion® NR-211 in a 1 kW class all vanadium mixed acid redox flow battery. J. Power Sources 2015, 285, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jeon, J.-D.; Kwak, S.-Y. Nafion-based composite membrane with a permselective layered silicate layer for vanadium redox flow battery. Electrochem. Commun. 2014, 38, 68–70. [Google Scholar] [CrossRef]
- Kim, S.; Yan, J.; Schwenzer, B.; Zhang, J.; Li, L.; Liu, J.; Yang, Z.; Hickner, M.A. Cycling performance and efficiency of sulfonated poly(sulfone) membranes in vanadium redox flow batteries. Electrochem. Commun. 2010, 12, 1650–1653. [Google Scholar] [CrossRef]
- Semiz, L.; Sankir, N.D.; Sankir, M. Influence of the basic membrane properties of the disulfonated poly(arylene ether sulfone) copolymer membranes on the vanadium redox flow battery performance. J. Membr. Sci. 2014, 468, 209–215. [Google Scholar] [CrossRef]
- Kong, L.; Zheng, L.; Niu, R.; Wang, H.; Shi, H. A sulfonated poly(ether ether ketone)/amine-functionalized graphene oxide hybrid membrane for vanadium redox flow batteries. RSC Adv. 2016, 6, 100262–100270. [Google Scholar] [CrossRef]
- Lee, K.J.; Chu, Y.H. Preparation of the graphene oxide (GO)/Nafion composite membrane for the vanadium redox flow battery (VRB) system. Vacuum 2014, 107, 269–276. [Google Scholar] [CrossRef]
- Sun, C.-N.; Tang, Z.; Belcher, C.; Zawodzinski, T.A.; Fujimoto, C. Evaluation of Diels–Alder poly(phenylene) anion exchange membranes in all-vanadium redox flow batteries. Electrochem. Commun. 2014, 43, 63–66. [Google Scholar] [CrossRef]
- Teng, X.; Dai, J.; Su, J.; Zhu, Y.; Liu, H.; Song, Z. A high performance polytetrafluoroethene/Nafion composite membrane for vanadium redox flow battery application. J. Power Sources 2013, 240, 131–139. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, L. Preparation and characterization of sulfonated polyimide/TiO2 composite membrane for vanadium redox flow battery. J. Solid State Electrochem. 2014, 18, 729–737. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, C.; Dai, Y.; Zheng, W.; Ruan, X.; He, G. A novel imidazolium-based amphoteric membrane for high-performance vanadium redox flow battery. J. Membr. Sci. 2017, 544, 98–107. [Google Scholar] [CrossRef]
- Li, X.; Santos, A.R.d.; Drache, M.; Ke, X.; Gohs, U.; Turek, T.; Becker, M.; Kunz, U.; Beuermann, S. Polymer electrolyte membranes prepared by pre-irradiation induced graft copolymerization on ETFE for vanadium redox flow battery applications. J. Membr. Sci. 2017, 524, 419–427. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X.; Wu, L.; Jiang, C.; Hossain, M.M.; Xu, T. Quaternized membranes bearing zwitterionic groups for vanadium redox flow battery through a green route. J. Membr. Sci. 2015, 483, 60–69. [Google Scholar] [CrossRef]
- Li, Z.; Dai, W.; Yu, L.; Xi, J.; Qiu, X.; Chen, L. Sulfonated poly(ether ether ketone)/mesoporous silica hybrid membrane for high performance vanadium redox flow battery. J. Power Sources 2014, 257, 221–229. [Google Scholar] [CrossRef]
- Chen, D.; Li, X. Sulfonated poly(ether ether ketone) membranes containing pendent carboxylic acid groups and their application in vanadium flow battery. J. Power Sources 2014, 247, 629–635. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Yu, L.; Wang, L.; Xi, J.; Qiu, X.; Chen, L. Characterization of sulfonated poly(ether ether ketone)/poly(vinylidene fluoride-co-hexafluoropropylene) composite membrane for vanadium redox flow battery application. J. Power Sources 2014, 272, 427–435. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Chen, Y.; Jian, X. Low vanadium ion permeabilities of sulfonated poly(phthalazinone ether ketone)s provide high efficiency and stability for vanadium redox flow batteries. J. Power Sources 2017, 355, 23–30. [Google Scholar] [CrossRef]
- Choi, E.M.; Kim, M.K.; Kang, E.T.; Kang, K.B.; Kim, D.S. Perfluorinated polymer for vanadium flow battery. Desalination Water Treat. 2013, 51, 5172–5178. [Google Scholar] [CrossRef]
- Liao, J.B.; Lu, M.Z.; Chu, Y.Q.; Wang, J.L. Ultra-low vanadium ion diffusion amphoteric ion-exchange membranes for all-vanadium redox flow batteries. J. Power Sources 2015, 282, 241–247. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, M.-C.; Wei, H.-J. Amino-silica modified Nafion membrane for vanadium redox flow battery. J. Power Sources 2015, 282, 562–571. [Google Scholar] [CrossRef]
- Jang, J.-K.; Kim, T.-H.; Yoon, S.J.; Lee, J.Y.; Lee, J.-C.; Hong, Y.T. Highly proton conductive, dense polybenzimidazole membranes with low permeability to vanadium and enhanced H2SO4 absorption capability for use in vanadium redox flow batteries. J. Mater. Chem. A 2016, 4, 14342–14355. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Ding, Y.; Liu, B.; Han, X.; Song, Y. Novel sulfonated poly (ether ether keton)/polyetherimide acid-base blend membranes for vanadium redox flow battery applications. Electrochim. Acta 2014, 130, 90–96. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Chen, J.; Qian, P.; Zhai, Y. Modification of Nafion membrane using interfacial polymerization for vanadium redox flow battery applications. J. Membr. Sci. 2008, 311, 98–103. [Google Scholar] [CrossRef]
- Luo, X.; Lu, Z.; Xi, J.; Wu, Z.; Zhu, W.; Chen, L.; Qiu, X. Influences of permeation of vanadium ions through PVDF-g-PSSA membranes on performances of vanadium redox flow batteries. J. Phys. Chem. B 2005, 109, 20310–20314. [Google Scholar] [CrossRef]
- Kim, S.; Yuk, S.; Kim, H.G.; Choi, C.; Kim, R.; Lee, J.Y.; Hong, Y.T.; Kim, H.-T. A hydrocarbon/Nafion bilayer membrane with a mechanical nano-fastener for vanadium redox flow batteries. J. Mater. Chem. A 2017, 5, 17279–17286. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.-H.; Lee, J.-Y.; Kim, Y.; Moon, S.-H. Amphoteric nanoporous polybenzimidazole membrane with extremely low crossover for a vanadium redox flow battery. RSC Adv. 2016, 6, 5198–5204. [Google Scholar] [CrossRef]
- Kondratenko, M.S.; Karpushkin, E.A.; Gvozdik, N.A.; Gallyamov, M.O.; Stevenson, K.J.; Sergeyev, V.G. Influence of aminosilane precursor concentration on physicochemical properties of composite Nafion membranes for vanadium redox flow battery applications. J. Power Sources 2017, 340, 32–39. [Google Scholar] [CrossRef]
- Niu, R.; Kong, L.; Zheng, L.; Wang, H.; Shi, H. Novel graphitic carbon nitride nanosheets/sulfonated poly(ether ether ketone) acid-base hybrid membrane for vanadium redox flow battery. J. Membr. Sci. 2017, 525, 220–228. [Google Scholar] [CrossRef]
- Leung, P.K.; Xu, Q.; Zhao, T.S.; Zeng, L.; Zhang, C. Preparation of silica nanocomposite anion-exchange membranes with low vanadium-ion crossover for vanadium redox flow batteries. Electrochim. Acta 2013, 105, 584–592. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, X.; Yang, P.; Zhou, Y.; Xuan, S.; Zhang, Y. Effect of non-sulfonated diamine monomer on branched sulfonated polyimide membrane for vanadium redox flow battery application. Electrochim. Acta 2017, 241, 50–62. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; He, Z.; Zhou, Z. Semi-fluorinated sulfonated polyimide membranes with enhanced proton selectivity and stability for vanadium redox flow batteries. Electrochim. Acta 2016, 216, 320–331. [Google Scholar] [CrossRef]
- Teng, X.; Zhao, Y.; Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/organic silica modified TiO2 composite membrane for vanadium redox flow battery via in situ sol–gel reactions. J. Membr. Sci. 2009, 341, 149–154. [Google Scholar] [CrossRef]
- Wang, N.; Peng, S.; Lu, D.; Liu, S.; Liu, Y.; Huang, K. Nafion/TiO2 hybrid membrane fabricated via hydrothermal method for vanadium redox battery. J. Solid State Electrochem. 2012, 16, 1577–1584. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; He, Z.; Zhou, Z. A novel branched side-chain-type sulfonated polyimide membrane with flexible sulfoalkyl pendants and trifluoromethyl groups for vanadium redox flow batteries. J. Power Sources 2017, 347, 114–126. [Google Scholar] [CrossRef]
- Wang, N.; Peng, S.; Wang, H.; Li, Y.; Liu, S.; Liu, Y. SPPEK/WO3 hybrid membrane fabricated via hydrothermal method for vanadium redox flow battery. Electrochem. Commun. 2012, 17, 30–33. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Zhou, Z.; Fang, D.; Liu, S.; Liu, Y. SPPEK/TPA composite membrane as a separator of vanadium redox flow battery. J. Membr. Sci. 2013, 437, 114–121. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Cao, J.; Xu, W.; Zhang, H. Composite porous membranes with an ultrathin selective layer for vanadium flow batteries. Chem. Commun. 2014, 50, 4596–4599. [Google Scholar] [CrossRef]
- Xi, J.; Dai, W.; Yu, L. Polydopamine coated SPEEK membrane for a vanadium redox flow battery. RSC Adv. 2015, 5, 33400–33406. [Google Scholar] [CrossRef]
- Mai, Z.; Zhang, H.; Li, X.; Bi, C.; Dai, H. Sulfonated poly(tetramethydiphenyl ether ether ketone) membranes for vanadium redox flow battery application. J. Power Sources 2011, 196, 482–487. [Google Scholar] [CrossRef]
- Xi, J.; Li, Z.; Yu, L.; Yin, B.; Wang, L.; Liu, L.; Qiu, X.; Chen, L. Effect of degree of sulfonation and casting solvent on sulfonated poly(ether ether ketone) membrane for vanadium redox flow battery. J. Power Sources 2015, 285, 195–204. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Teng, X.; Zhao, Y.; Chen, L.; Qiu, X. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232. [Google Scholar] [CrossRef]
- Seepana, M.M.; Pandey, J.; Shukla, A. Design and synthesis of highly stable poly(tetrafluoroethylene)-zirconium phosphate (PTFE-ZrP) ion-exchange membrane for vanadium redox flow battery (VRFB). Ionics 2017, 23, 1471–1480. [Google Scholar] [CrossRef]
- Xia, Z.; Ying, L.; Fang, J.; Du, Y.-Y.; Zhang, W.-M.; Guo, X.; Yin, J. Preparation of covalently cross-linked sulfonated polybenzimidazole membranes for vanadium redox flow battery applications. J. Membr. Sci. 2017, 525, 229–239. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, H.; Li, X.; Mai, Z.; Zhang, H. Poly(tetrafluoroethylene) reinforced sulfonated poly(ether ether ketone) membranes for vanadium redox flow battery application. J. Power Sources 2012, 208, 421–425. [Google Scholar] [CrossRef]
- Xie, W.; Darling, R.M.; Perry, M.L. Processing and Pretreatment Effects on Vanadium Transport in Nafion Membranes. J. Electrochem. Soc. 2016, 163, A5084–A5089. [Google Scholar] [CrossRef]
- Yin, B.; Li, Z.; Dai, W.; Wang, L.; Yu, L.; Xi, J. Highly branched sulfonated poly(fluorenyl ether ketone sulfone)s membrane for energy efficient vanadium redox flow battery. J. Power Sources 2015, 285, 109–118. [Google Scholar] [CrossRef]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Chen, B.; Simmons, K.; Sprenkle, V.; Wang, W. Nanoporous Polytetrafluoroethylene/Silica Composite Separator as a High-Performance All-Vanadium Redox Flow Battery Membrane. Adv. Energy Mater. 2013, 3, 1215–1220. [Google Scholar] [CrossRef]
- Yin, B.; Yu, L.; Jiang, B.; Wang, L.; Xi, J. Nano oxides incorporated sulfonated poly(ether ether ketone) membranes with improved selectivity and stability for vanadium redox flow battery. J. Solid State Electrochem. 2016, 20, 1271–1283. [Google Scholar] [CrossRef]
- Xi, X.; Ding, C.; Zhang, H.; Li, X.; Cheng, Y.; Zhang, H. Solvent responsive silica composite nanofiltration membrane with controlled pores and improved ion selectivity for vanadium flow battery application. J. Power Sources 2015, 274, 1126–1134. [Google Scholar] [CrossRef]
- Yue, M.; Zhang, Y.; Wang, L. Sulfonated polyimide/chitosan composite membrane for vanadium redox flow battery: Membrane preparation, characterization, and single cell performance. J. Appl. Polym. Sci. 2013, 127, 4150–4159. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, Q.; Zhao, Y.; Lu, W.; Li, X.; Zhang, H. Polypyrrole modified porous poly(ether sulfone) membranes with high performance for vanadium flow batteries. J. Mater. Chem. A 2016, 4, 12955–12962. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, E.; Wang, G.; Yu, P.; Zhao, Q.; Yao, F. Poly(phenyl sulfone) anion exchange membranes with pyridinium groups for vanadium redox flow battery applications. J. Power Sources 2015, 282, 328–334. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Li, M.; Lu, W.; Li, X.; Zhang, H. A Highly Ion-Selective Zeolite Flake Layer on Porous Membranes for Flow Battery Applications. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 3058–3062. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Weng, Z.; Wang, G.; Zhang, E.; Yu, P.; Chen, X.; Wang, X. Quaternized adamantane-containing poly(aryl ether ketone) anion exchange membranes for vanadium redox flow battery applications. J. Power Sources 2016, 325, 801–807. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, C.; Cao, J.; Xu, W.; Li, X.; Zhang, H. A novel solvent-template method to manufacture nano-scale porous membranes for vanadium flow battery applications. J. Mater. Chem. A 2014, 2, 9524. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Xing, D.; Han, R.; Yin, C.; Jian, X. Quaternized poly(phthalazinone ether ketone ketone) anion exchange membrane with low permeability of vanadium ions for vanadium redox flow battery application. J. Power Sources 2012, 217, 296–302. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Wei, W.; Li, Y. Crosslinkable sulfonated poly (diallyl-bisphenol ether ether ketone) membranes for vanadium redox flow battery application. J. Power Sources 2012, 217, 309–315. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Yuan, Z.; Li, X.; Zhang, H.; Vankelecom, I.F.J. Advanced Charged Sponge-Like Membrane with Ultrahigh Stability and Selectivity for Vanadium Flow Batteries. Adv. Funct. Mater. 2016, 26, 210–218. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Xing, D.; Jian, X. Poly(phthalazinone ether ketone ketone) anion exchange membranes with pyridinium as ion exchange groups for vanadium redox flow battery applications. J. Mater. Chem. A 2013, 1, 12246. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, L.; Zhang, S. Sulfonated polyimide/AlOOH composite membranes with decreased vanadium permeability and increased stability for vanadium redox flow battery. J. Solid State Electrochem. 2014, 18, 3479–3490. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, J.; Wang, G.; Zhang, J.; Zhu, X.; Wang, R. Sulfonated poly(ether ether ketone)/poly(vinylidene fluoride)/tungstophosphoric acid membrane for vanadium redox flow battery application. High Perform. Polym. 2016, 28, 735–740. [Google Scholar] [CrossRef]
- Strathmann, H. Introduction to Membrane Science and Technology; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Melin, T.; Rautenbach, R. Membranverfahren: Grundlagen der Modul- und Anlagenauslegung, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nunes, S.P.; Peinemann, K.-V. Membrane Technology. In The Chemical Industry; Wiley-VCH: Hoboken, NJ, USA, 2007. [Google Scholar]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Solar Energy Mater. Solar Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Toshikatsu, S. Ion Exchange Membranes; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Mohammadi, T.; Skyllas-Kazacos, M. Preparation of sulfonated composite membrane for vanadium redox flow battery applications. J. Membr. Sci. 1995, 107, 35–45. [Google Scholar] [CrossRef]
- Teng, X.; Dai, J.; Su, J.; Yin, G. Modification of Nafion membrane using fluorocarbon surfactant for all vanadium redox flow battery. J. Membr. Sci. 2015, 476, 20–29. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Optimizing membrane thickness for vanadium redox flow batteries. J. Membr. Sci. 2013, 437, 108–113. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Optimized anion exchange membranes for vanadium redox flow batteries. ACS Appl. Mater. Interfaces 2013, 5, 7559–7566. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Dai, J.; Bi, F.; Yin, G. Ultra-thin polytetrafluoroethene/Nafion/silica composite membrane with high performance for vanadium redox flow battery. J. Power Sources 2014, 272, 113–120. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Jeon, J.-D.; Kwak, S.-Y. Ion-exchange composite membranes pore-filled with sulfonated poly(ether ether ketone) and Engelhard titanosilicate-10 for improved performance of vanadium redox flow batteries. J. Power Sources 2018, 383, 1–9. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.; Xu, W.; Li, X. Poly(vinylidene fluoride) porous membranes precipitated in water/ethanol dual-coagulation bath. J. Power Sources 2014, 249, 84–91. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, H.; Li, X.; Zhang, H.; Li, Y.; Vankelecom, I. Hydrophobic asymmetric ultrafiltration PVDF membranes: An alternative separator for VFB with excellent stability. Phys. Chem. Chem. Phys. PCCP 2013, 15, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yuan, Z.; Li, X.; Xu, W.; Zhang, H. Hydrophilic poly(vinylidene fluoride) porous membrane with well connected ion transport networks for vanadium flow battery. J. Power Sources 2015, 298, 228–235. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Peng, J.; Qiu, J.; Xu, L.; Li, J.; Zhai, M. Designing a new process to prepare amphoteric ion exchange membrane with well-distributed grafted chains for vanadium redox flow battery. J. Membr. Sci. 2012, 419–420, 1–8. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; Ma, J.; Qiu, J.; Peng, J.; Li, J.; Zhai, M. A novel amphoteric ion exchange membrane synthesized by radiation-induced grafting α-methylstyrene and N,N-dimethylaminoethyl methacrylate for vanadium redox flow battery application. J. Membr. Sci. 2012, 407–408, 184–192. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, L.; Zhai, M.; Ni, J.; Zhou, H.; Peng, J.; Li, J.; Wei, G. Pre-irradiation grafting of styrene and maleic anhydride onto PVDF membrane and subsequent sulfonation for application in vanadium redox batteries. J. Power Sources 2008, 177, 617–623. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, J.; Chen, J.; Peng, J.; Xu, L.; Zhai, M.; Li, J.; Wei, G. Amphoteric ion exchange membrane synthesized by radiation-induced graft copolymerization of styrene and dimethylaminoethyl methacrylate into PVDF film for vanadium redox flow battery applications. J. Membr. Sci. 2009, 334, 9–15. [Google Scholar] [CrossRef]
- Ling, L.; Xiao, M.; Han, D.; Ren, S.; Wang, S.; Meng, Y. Porous composite membrane of PVDF/Sulfonic silica with high ion selectivity for vanadium redox flow battery. J. Membr. Sci. 2019, 585, 230–237. [Google Scholar] [CrossRef]
- Rajput, A.; Khan, H.; Raj, S.K.; Kothandaraman, R.; Kulshrestha, V. Styrene- co -DVB grafted PVDF proton exchange membranes for vanadium redox flow battery applications. Mater. Adv. 2020, 1, 1930–1938. [Google Scholar] [CrossRef]
- Qiu, J.; Zhai, M.; Chen, J.; Wang, Y.; Peng, J.; Xu, L.; Li, J.; Wei, G. Performance of vanadium redox flow battery with a novel amphoteric ion exchange membrane synthesized by two-step grafting method. J. Membr. Sci. 2009, 342, 215–220. [Google Scholar] [CrossRef]
- Qiu, J.; Li, M.; Ni, J.; Zhai, M.; Peng, J.; Xu, L.; Zhou, H.; Li, J.; Wei, G. Preparation of ETFE-based anion exchange membrane to reduce permeability of vanadium ions in vanadium redox battery. J. Membr. Sci. 2007, 297, 174–180. [Google Scholar] [CrossRef]
- Nibel, O.; Rojek, T.; Schmidt, T.J.; Gubler, L. Amphoteric Ion-Exchange Membranes with Significantly Improved Vanadium Barrier Properties for All-Vanadium Redox Flow Batteries. ChemSusChem 2017, 10, 2767–2777. [Google Scholar] [CrossRef]
- Mallinson, S.L.; Varcoe, J.R.; Slade, R.C. Examination of Amine-Functionalised Anion-Exchange Membranes for Possible Use in the All-Vanadium Redox Flow Battery. Electrochim. Acta 2014, 140, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Zhang, H.; Liu, T.; Li, X.; Liu, Z. Cell architecture upswing based on catalyst coated membrane (CCM) for vanadium flow battery. J. Power Sources 2013, 237, 19–25. [Google Scholar] [CrossRef]
- Teng, X.; Zhao, Y.; Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/organically modified silicate hybrids membrane for vanadium redox flow battery. J. Power Sources 2009, 189, 1240–1246. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, L.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. Effectively suppressing vanadium permeation in vanadium redox flow battery application with modified Nafion membrane with nacre-like nanoarchitectures. J. Power Sources 2017, 352, 111–117. [Google Scholar] [CrossRef]
- Mai, Z.; Zhang, H.; Li, X.; Xiao, S.; Zhang, H. Nafion/polyvinylidene fluoride blend membranes with improved ion selectivity for vanadium redox flow battery application. J. Power Sources 2011, 196, 5737–5741. [Google Scholar] [CrossRef]
- Teng, X.; Lei, J.; Gu, X.; Dai, J.; Zhu, Y.; Li, F. Nafion-sulfonated organosilica composite membrane for all vanadium redox flow battery. Ionics 2012, 18, 513–521. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Schwenzer, B.; Kim, S.; Yang, Z.; Thevuthasan, S.; Liu, J.; Graff, G.L.; Hu, J. Investigation of local environments in Nafion-SiO(2) composite membranes used in vanadium redox flow batteries. Solid State Nucl. Magn. Reson. 2012, 42, 71–80. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Chen, J.; You, D.; Sun, C.; Zhang, Y. Preparation and characterization of Nafion/SPEEK layered composite membrane and its application in vanadium redox flow battery. J. Membr. Sci. 2008, 325, 553–558. [Google Scholar] [CrossRef]
- Aziz, M.A.; Shanmugam, S. Zirconium oxide nanotube–Nafion composite as high performance membrane for all vanadium redox flow battery. J. Power Sources 2017, 337, 36–44. [Google Scholar] [CrossRef]
- Chen, D.; Kim, S.; Sprenkle, V.; Hickner, M.A. Composite blend polymer membranes with increased proton selectivity and lifetime for vanadium redox flow batteries. J. Power Sources 2013, 231, 301–306. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Tang, Z.J.; Fujimoto, C.; Sun, C.-N.; Mench, M.M.; Zawodzinski, T.A. Full Cell Study of Diels Alder Poly(phenylene) Anion and Cation Exchange Membranes in Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2016, 163, A5154–A5162. [Google Scholar] [CrossRef]
- Largier, T.D.; Cornelius, C.J. Random quaternary ammonium Diels-Alder poly(phenylene) copolymers for improved vanadium redox flow batteries. J. Power Sources 2017, 352, 149–155. [Google Scholar] [CrossRef]
- Wang, T.; Jeon, J.Y.; Han, J.; Kim, J.H.; Bae, C.; Kim, S. Poly(terphenylene) anion exchange membranes with high conductivity and low vanadium permeability for vanadium redox flow batteries (VRFBs). J. Membr. Sci. 2020, 598, 117665. [Google Scholar] [CrossRef]
- Mu, D.; Yu, L.; Liu, L.; Xi, J. Rice Paper Reinforced Sulfonated Poly(ether ether ketone) as Low-Cost Membrane for Vanadium Flow Batteries. ACS Sustain. Chem. Eng. 2017, 5, 2437–2444. [Google Scholar] [CrossRef]
- Macksasitorn, S.; Changkhamchom, S.; Sirivat, A.; Siemanond, K. Sulfonated poly(ether ether ketone) and sulfonated poly(1,4-phenylene ether ether sulfone) membranes for vanadium redox flow batteries. High Perform. Polym. 2012, 24, 603–608. [Google Scholar] [CrossRef]
- Yu, L.; Xi, J. Durable and Efficient PTFE Sandwiched SPEEK Membrane for Vanadium Flow Batteries. ACS Appl. Mater. Interfaces 2016, 8, 23425–23430. [Google Scholar] [CrossRef]
- Dai, W.; Yu, L.; Li, Z.; Yan, J.; Liu, L.; Xi, J.; Qiu, X. Sulfonated Poly(Ether Ether Ketone)/Graphene composite membrane for vanadium redox flow battery. Electrochim. Acta 2014, 132, 200–207. [Google Scholar] [CrossRef]
- Wang, F.; Wang, G.; Zhang, J.; Li, B.; Zhang, J.; Deng, J.; Chen, J.; Wang, R. Novel sulfonated poly(ether ether ketone)/oxidized g-C 3 N 4 composite membrane for vanadium redox flow battery applications. J. Electroanal. Chem. 2017, 797, 107–112. [Google Scholar] [CrossRef]
- Li, Z.; Xi, J.; Zhou, H.; Liu, L.; Wu, Z.; Qiu, X.; Chen, L. Preparation and characterization of sulfonated poly(ether ether ketone)/poly(vinylidene fluoride) blend membrane for vanadium redox flow battery application. J. Power Sources 2013, 237, 132–140. [Google Scholar] [CrossRef]
- Hyeon, D.H.; Chun, J.H.; Lee, C.H.; Jung, H.C.; Kim, S.H. Composite membranes based on sulfonated poly(ether ether ketone) and SiO2 for a vanadium redox flow battery. Korean J. Chem. Eng. 2015, 32, 1554–1563. [Google Scholar] [CrossRef]
- Ji, Y.; Tay, Z.Y.; Li, S.F.Y. Highly selective sulfonated poly(ether ether ketone)/titanium oxide composite membranes for vanadium redox flow batteries. J. Membr. Sci. 2017, 539, 197–205. [Google Scholar] [CrossRef]
- Jia, C.; Liu, J.; Yan, C. A significantly improved membrane for vanadium redox flow battery. J. Power Sources 2010, 195, 4380–4383. [Google Scholar] [CrossRef]
- Li, Z.; Dai, W.; Yu, L.; Liu, L.; Xi, J.; Qiu, X.; Chen, L. Properties investigation of sulfonated poly(ether ether ketone)/polyacrylonitrile acid-base blend membrane for vanadium redox flow battery application. ACS Appl. Mater. Interfaces 2014, 6, 18885–18893. [Google Scholar] [CrossRef]
- Wang, F.; Sylvia, J.M.; Jacob, M.M.; Peramunage, D. Amphiphilic block copolymer membrane for vanadium redox flow battery. J. Power Sources 2013, 242, 575–580. [Google Scholar] [CrossRef]
- Yun, S.; Parrondo, J.; Ramani, V. Derivatized cardo-polyetherketone anion exchange membranes for all-vanadium redox flow batteries. J. Mater. Chem. A 2014, 2, 6605–6615. [Google Scholar] [CrossRef]
- Aziz, M.A.; Shanmugam, S. Sulfonated graphene oxide-decorated block copolymer as a proton-exchange membrane: Improving the ion selectivity for all-vanadium redox flow batteries. J. Mater. Chem. A 2018, 6, 17740–17750. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, B.; Shi, J.; Zhang, J.; Shi, H. An ultra-high ion selective hybrid proton exchange membrane incorporated with zwitterion-decorated graphene oxide for vanadium redox flow batteries. J. Mater. Chem. A 2019, 7, 12669–12680. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, G.; Li, A.; Wei, X.; Li, F.; Zhang, J.; Chen, J.; Wang, R. Novel sulfonated poly(ether ether ketone)/triphenylamine hybrid membrane for vanadium redox flow battery applications. RSC Adv. 2019, 9, 3838–3846. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Cheng, Y.; Sun, L.; Ding, M.; Wu, C.; Yuan, D.; Zhao, X.; Xiang, C.; Jia, C. A green SPEEK/lignin composite membrane with high ion selectivity for vanadium redox flow battery. J. Membr. Sci. 2019, 572, 110–118. [Google Scholar] [CrossRef]
- Bhushan, M.; Kumar, S.; Singh, A.K.; Shahi, V.K. High-performance membrane for vanadium redox flow batteries: Cross-linked poly(ether ether ketone) grafted with sulfonic acid groups via the spacer. J. Membr. Sci. 2019, 583, 1–8. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Guan, S.; Weng, Z.; Zhang, E.; Wang, G.; Zhang, Z.; Hu, J.; Zhang, S. High performance membranes based on new 2-adamantane containing poly(aryl ether ketone) for vanadium redox flow battery applications. J. Power Sources 2018, 399, 18–25. [Google Scholar] [CrossRef]
- Kumar, S.; Bhushan, M.; Shahi, V.K. Cross-linked amphoteric membrane: Sulphonated poly(ether ether ketone) grafted with 2,4,6-tris(dimethylaminomethyl)phenol using functionalized side chain spacers for vanadium redox flow battery. J. Power Sources 2020, 448, 227358. [Google Scholar] [CrossRef]
- Hossain, S.I.; Aziz, M.A.; Han, D.; Selvam, P.; Shanmugam, S. Fabrication of SPAEK–cerium zirconium oxide nanotube composite membrane with outstanding performance and durability for vanadium redox flow batteries. J. Mater. Chem. A 2018, 6, 20205–20213. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Qu, C. A Dication Cross-Linked Composite Anion-Exchange Membrane for All-Vanadium Flow Battery Applications. ChemSusChem 2013, 6, 2290–2298. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; Wei, L.; Zeng, Y.K.; Zhang, Z.H. Polyvinylpyrrolidone-based semi-interpenetrating polymer networks as highly selective and chemically stable membranes for all vanadium redox flow batteries. J. Power Sources 2016, 327, 374–383. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, H.; Cao, J.; Xu, W.; Li, X. Hydrophilic porous poly(sulfone) membranes modified by UV-initiated polymerization for vanadium flow battery application. J. Membr. Sci. 2014, 454, 478–487. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Zhang, F.; Li, X.; Li, Y.; Vankelecom, I. Advanced charged membranes with highly symmetric spongy structures for vanadium flow battery application. Energy Environ. Sci. 2013, 6, 776. [Google Scholar] [CrossRef]
- Jung, M.J.; Parrondo, J.; Arges, C.G.; Ramani, V. Polysulfone-based anion exchange membranes demonstrate excellent chemical stability and performance for the all-vanadium redox flow battery. J. Mater. Chem. A 2013, 1, 10458. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, L.; Wang, C.; Li, N. Side-chain-type anion exchange membranes for vanadium flow battery: Properties and degradation mechanism. J. Mater. Chem. A 2018, 6, 22778–22789. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, B.; Wang, H.; Shi, H. Sulfonated polysulfone proton exchange membrane influenced by a varied sulfonation degree for vanadium redox flow battery. J. Membr. Sci. 2019, 584, 173–180. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Jiang, Y.; Qian, P.; Shi, H. High performance acid-base composite membranes from sulfonated polysulfone containing graphitic carbon nitride nanosheets for vanadium redox flow battery. J. Membr. Sci. 2019, 591, 117332. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Anion Exchange Membranes for Vanadium Redox Flow Batteries. ECS Trans. 2013, 53, 83–89. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Li, X.; Zhang, H.; Wei, W. Porous poly (ether sulfone) membranes with tunable morphology. J. Power Sources 2013, 233, 202–208. [Google Scholar] [CrossRef]
- Chen, D.; Li, D.; Li, X. Highly symmetric spongy porous poly(ether sulfone) membranes with selective open-cells for vanadium flow battery application. RSC Adv. 2016, 6, 87104–87109. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Cao, J.; Yuan, Z.; Zhang, H. Morphology and performance of poly(ether sulfone)/sulfonated poly(ether ether ketone) blend porous membranes for vanadium flow battery application. RSC Adv. 2014, 4, 40400–40406. [Google Scholar] [CrossRef]
- Chen, D.; Li, D.; Li, X. Hierarchical porous poly (ether sulfone) membranes with excellent capacity retention for vanadium flow battery application. J. Power Sources 2017, 353, 11–18. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Z.; Lu, W.; Li, X.; Zhang, H. The porous membrane with tunable performance for vanadium flow battery. J. Power Sources 2017, 342, 327–334. [Google Scholar] [CrossRef]
- Ling, X.; Jia, C.; Liu, J.; Yan, C. Preparation and characterization of sulfonated poly(ether sulfone)/sulfonated poly(ether ether ketone) blend membrane for vanadium redox flow battery. J. Membr. Sci. 2012, 415–416, 306–312. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, R.; Zhong, Y.; Zhang, Y.; Jiang, F. Asymmetric porous membranes with ultra-high ion selectivity for vanadium redox flow batteries. J. Membr. Sci. 2020, 595, 117614. [Google Scholar] [CrossRef]
- Teng, X.; Guo, Y.; Liu, D.; Li, G.; Yu, C.; Dai, J. A polydopamine-coated polyamide thin film composite membrane with enhanced selectivity and stability for vanadium redox flow battery. J. Membr. Sci. 2020, 601, 117906. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Wang, S.; Pan, J.; Xiao, M.; Meng, Y. Directly fluorinated polyaromatic composite membranes for vanadium redox flow batteries. J. Membr. Sci. 2012, 415–416, 139–144. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Xiao, M.; Meng, Y. Preparation and properties of sulfonated poly(fluorenyl ether ketone) membrane for vanadium redox flow battery application. J. Power Sources 2010, 195, 2089–2095. [Google Scholar] [CrossRef]
- Pan, J.; Wang, S.; Xiao, M.; Hickner, M.; Meng, Y. Layered zirconium phosphate sulfophenylphosphonates reinforced sulfonated poly (fluorenyl ether ketone) hybrid membranes with high proton conductivity and low vanadium ion permeability. J. Membr. Sci. 2013, 443, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xiao, M.; Han, D.; Hickner, M.A.; Meng, Y. Layer-by-layer self-assembly of PDDA/PSS-SPFEK composite membrane with low vanadium permeability for vanadium redox flow battery. RSC Adv. 2013, 3, 15467. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. Preparation and characterization of a novel layer-by-layer porous composite membrane for vanadium redox flow battery (VRB) applications. Int. J. Hydrogen Energy 2014, 39, 16088–16095. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xiao, M.; Song, S.; Han, D.; Hickner, M.A.; Meng, Y. Amphoteric ion exchange membrane synthesized by direct polymerization for vanadium redox flow battery application. Int. J. Hydrogen Energy 2014, 39, 16123–16131. [Google Scholar] [CrossRef]
- Thong, P.T.; Sadhasivam, T.; Lim, H.; Jin, C.-S.; Ryi, S.-K.; Park, W.; Kim, H.T.; Roh, S.-H.; Jung, H.-Y. High Oxidizing Stability and Ion Selectivity of Hybrid Polymer Electrolyte Membrane for Improving Electrochemical Performance in Vanadium Redox Flow Battery. J. Electrochem. Soc. 2018, 165, A2321–A2329. [Google Scholar] [CrossRef]
- Hwang, C.W.; Park, H.-M.; Oh, C.M.; Hwang, T.S.; Shim, J.; Jin, C.-S. Synthesis and characterization of vinylimidazole-co-trifluoroethylmethacrylate-co-divinylbenzene anion-exchange membrane for all-vanadium redox flow battery. J. Membr. Sci. 2014, 468, 98–106. [Google Scholar] [CrossRef]
- Park, S.-G.; Kwak, N.-S.; Hwang, C.W.; Park, H.-M.; Hwang, T.S. Synthesis and characteristics of aminated vinylbenzyl chloride-co-styrene-co-hydroxyethyl acrylate anion-exchange membrane for redox flow battery applications. J. Membr. Sci. 2012, 423–424, 429–437. [Google Scholar] [CrossRef]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Sprenkle, V.; Wang, W. Polyvinyl Chloride/Silica Nanoporous Composite Separator for All-Vanadium Redox Flow Battery Applications. J. Electrochem. Soc. 2013, 160, A1215–A1218. [Google Scholar] [CrossRef]
- Pandey, J.; Tankal, B.R. Performance of the vanadium redox-flow battery (VRB) for Si-PWA/PVA nanocomposite membrane. J. Solid State Electrochem. 2016, 20, 2259–2265. [Google Scholar] [CrossRef]
- Kwak, N.-S.; Sim, J.B.; Koo, J.S.; Hwang, T.S.; Kim, Y.T. Synthesis and characteristics of a cross-linked DMSIP-co-HDO-co-MA ion-exchange membrane for redox flow battery applications. J. Membr. Sci. 2013, 430, 252–262. [Google Scholar] [CrossRef]
- Chae, I.S.; Luo, T.; Moon, G.H.; Ogieglo, W.; Kang, Y.S.; Wessling, M. Ultra-High Proton/Vanadium Selectivity for Hydrophobic Polymer Membranes with Intrinsic Nanopores for Redox Flow Battery. Adv. Energy Mater. 2016, 6, 1600517. [Google Scholar] [CrossRef]
- Noh, C.; Jung, M.; Henkensmeier, D.; Nam, S.W.; Kwon, Y. Vanadium Redox Flow Batteries Using meta-Polybenzimidazole-Based Membranes of Different Thicknesses. ACS Appl. Mater. Interfaces 2017, 9, 36799–36809. [Google Scholar] [CrossRef]
- Luo, T.; David, O.; Gendel, Y.; Wessling, M. Porous poly(benzimidazole) membrane for all vanadium redox flow battery. J. Power Sources 2016, 312, 45–54. [Google Scholar] [CrossRef]
- Peng, S.; Yan, X.; Wu, X.; Zhang, D.; Luo, Y.; Su, L.; He, G. Thin skinned asymmetric polybenzimidazole membranes with readily tunable morphologies for high-performance vanadium flow batteries. RSC Adv. 2017, 7, 1852–1862. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Duan, Y.; Zhang, H.; Li, X.; Zhang, H.; Vankelecom, I. Advanced porous membranes with ultra-high selectivity and stability for vanadium flow batteries. Energy Environ. Sci. 2016, 9, 441–447. [Google Scholar] [CrossRef]
- Ahn, S.M.; Jeong, H.Y.; Jang, J.-K.; Lee, J.Y.; So, S.; Kim, Y.J.; Hong, Y.T.; Kim, T.-H. Polybenzimidazole/Nafion hybrid membrane with improved chemical stability for vanadium redox flow battery application. RSC Adv. 2018, 8, 25304–25312. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Gao, L.; Yan, X.; Zheng, W.; Dai, Y.; Hao, C.; Wu, X.; He, G. Proton delivery through a dynamic 3D H-bond network constructed from dense hydroxyls for advanced ion-selective membranes. J. Mater. Chem. A 2019, 7, 15137–15144. [Google Scholar] [CrossRef]
- Gubler, L.; Vonlanthen, D.; Schneider, A.; Oldenburg, F.J. Composite Membranes Containing a Porous Separator and a Polybenzimidazole Thin Film for Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2020, 167, 100502. [Google Scholar] [CrossRef]
- Bülbül, E.; Atanasov, V.; Mehlhorn, M.; Bürger, M.; Chromik, A.; Häring, T.; Kerres, J. Highly phosphonated polypentafluorostyrene blended with polybenzimidazole: Application in vanadium redox flow battery. J. Membr. Sci. 2019, 570–571, 194–203. [Google Scholar] [CrossRef]
- Lee, W.; Jung, M.; Serhiichuk, D.; Noh, C.; Gupta, G.; Harms, C.; Kwon, Y.; Henkensmeier, D. Layered composite membranes based on porous PVDF coated with a thin, dense PBI layer for vanadium redox flow batteries. J. Membr. Sci. 2019, 591, 117333. [Google Scholar] [CrossRef]
- Wang, L.; Pingitore, A.T.; Xie, W.; Yang, Z.; Perry, M.L.; Benicewicz, B.C. Sulfonated PBI Gel Membranes for Redox Flow Batteries. J. Electrochem. Soc. 2019, 166, A1449–A1455. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Xu, W.; Liu, J.; Yan, C.; Che, X.; Yang, J.; Tong, J.; Xiao, W. Communication—Polyethylene/PBI Pore-Filling Composite Membrane for High Performance Vanadium Redox Flow Battery. J. Electrochem. Soc. 2019, 166, A3207–A3209. [Google Scholar] [CrossRef]
- Zhang, S.H.; Zhang, B.G.; Jian, X.G. Preparation and Properties of Poly (phthalazinone Ether Ketone) Based Anion Exchange Membranes for Vanadium Redox Flow Battery. AMR 2013, 773, 171–174. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Zhao, G.; Jian, X. Anion exchange membranes from brominated poly(aryl ether ketone) containing 3,5-dimethyl phthalazinone moieties for vanadium redox flow batteries. J. Mater. Chem. A 2014, 2, 3083. [Google Scholar] [CrossRef]
- Wang, N.; Peng, S.; Li, Y.; Wang, H.; Liu, S.; Liu, Y. Sulfonated poly(phthalazinone ether sulfone) membrane as a separator of vanadium redox flow battery. J. Solid State Electrochem. 2012, 16, 2169–2177. [Google Scholar] [CrossRef]
- Huang, X.; Pu, Y.; Zhou, Y.; Zhang, Y.; Zhang, H. In-situ and ex-situ degradation of sulfonated polyimide membrane for vanadium redox flow battery application. J. Membr. Sci. 2017, 526, 281–292. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Liu, S.; He, Z.; Zhou, Z.; Li, A. A Low-Cost and High-Performance Sulfonated Polyimide Proton-Conductive Membrane for Vanadium Redox Flow/Static Batteries. ACS Appl. Mater. Interfaces 2017, 9, 32643–32651. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhang, H.; Zhang, S.; Huang, X. Sulfonated polyimide membranes with different non-sulfonated diamines for vanadium redox battery applications. Electrochim. Acta 2014, 150, 114–122. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, S.; Huang, X. Sulfonated polyimide/s-MoS2 composite membrane with high proton selectivity and good stability for vanadium redox flow battery. J. Membr. Sci. 2015, 490, 179–189. [Google Scholar] [CrossRef]
- Cao, L.; Sun, Q.; Gao, Y.; Liu, L.; Shi, H. Novel acid-base hybrid membrane based on amine-functionalized reduced graphene oxide and sulfonated polyimide for vanadium redox flow battery. Electrochim. Acta 2015, 158, 24–34. [Google Scholar] [CrossRef]
- Cao, L.; Kong, L.; Kong, L.; Zhang, X.; Shi, H. Novel sulfonated polyimide/zwitterionic polymer-functionalized graphene oxide hybrid membranes for vanadium redox flow battery. J. Power Sources 2015, 299, 255–264. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, S.; Huang, X.; Wang, L. Novel sulfonated polyimide/ZrO 2 composite membrane as a separator of vanadium redox flow battery. Polym. Adv. Technol. 2014, 25, 1610–1615. [Google Scholar] [CrossRef]
- Düerkop, D.; Widdecke, H.; dos Santos, U.K. Polyimide Membrane for Vanadium Redox-Flow Battery. In Proceedings of the IFBF The International Flow Battery Forum—Conference Papers, Manchester, UK, 27–29 June 2014; pp. 70–71. [Google Scholar]
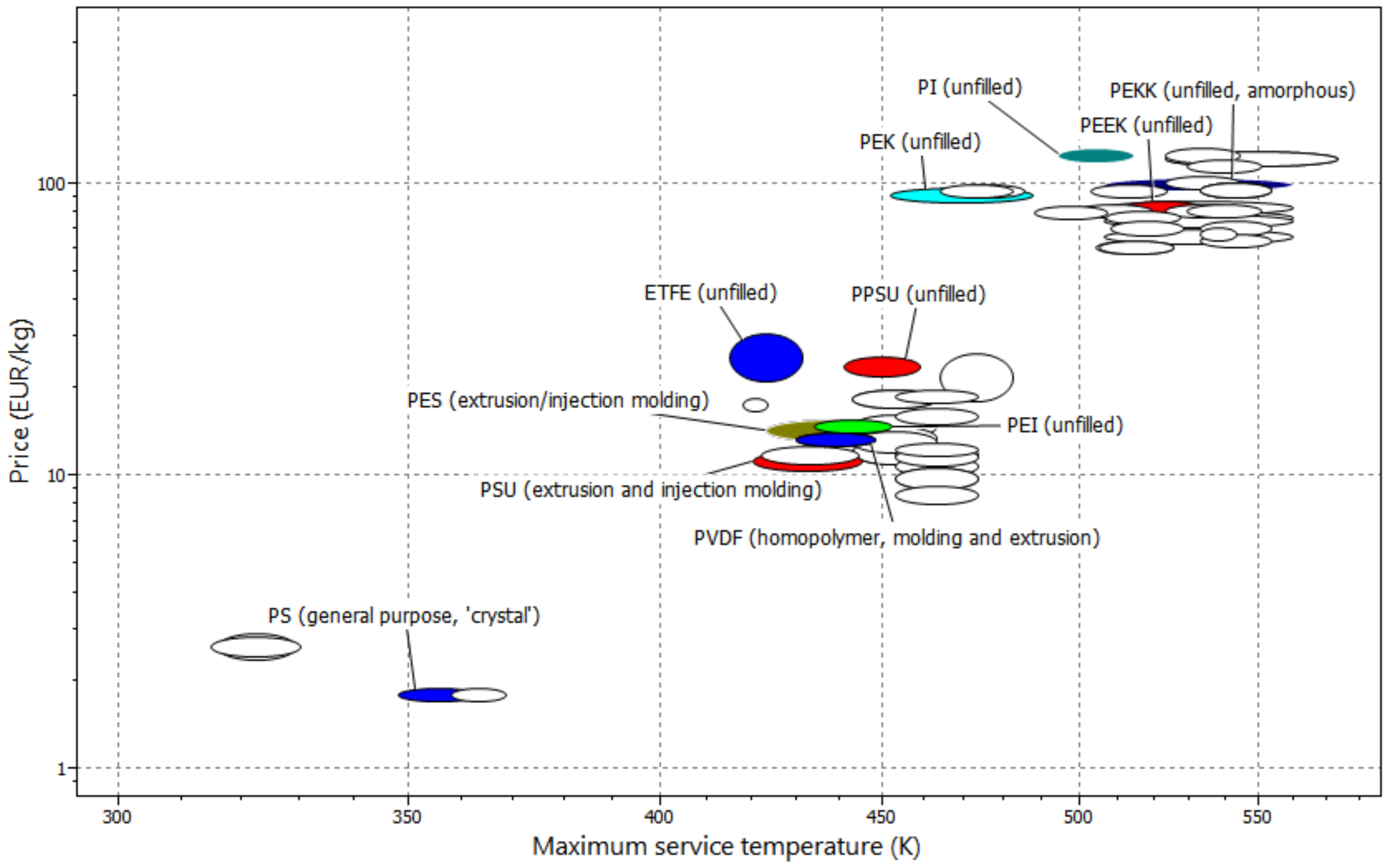
- Minke, C.; Kunz, U.; Turek, T. Techno-economic assessment of novel vanadium redox flow batteries with large-area cells. J. Power Sources 2017, 361, 105–114. [Google Scholar] [CrossRef]
- Minke, C.; Turek, T. Economics of vanadium redox flow battery membranes. J. Power Sources 2015, 286, 247–257. [Google Scholar] [CrossRef]
- CES. CES Selector Software; Granta Design Limited: Cambridge, UK, 2018; Available online: www.grantadesign.com (accessed on 17 January 2018).
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced Separators for Lithium-Ion and Lithium-Sulfur Batteries: A Review of Recent Progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef] [PubMed]




| Year | Journal | Title | Main Focus | Ref. |
|---|---|---|---|---|
| 2011 | Energy Environ. Sci. | Ion exchange membranes for vanadium redox flow battery (VRB) application | all aspects related to IEMs that are of relevance to understand IEMs for VRFB | [11] |
| 2011 | ChemSusChem | Membrane Development for Vanadium Redox Flow Batteries | basic scientific issues associated with membrane use in VRFBs | [12] |
| 2012 | Membranes | Membranes for VRFB Applications | membranes for all-vanadium redox flow battery which has received the most attention. | [13] |
| 2013 | Electrochimica Acta | Review of material research and development for vanadium redox flow battery applications | a historical overview of materials research and development | [15] |
| 2014 | Energy Environ. Sci. | Anion-exchange membranes in electrochemical energy systems | technological and scientific limitations and the future challenges related to the use of anion-exchange membranes | [16] |
| 2015 | J.o.Nanomaterials | Recent development of Nanocomposite Membranes for Vanadium redox Flow Batteries | efforts in developing nanocomposite membranes | [14] |
| 2015 | RSC Adv. | Recent development of polymer membranes as separators for all-vanadium redox flow batteries | new cation exchange membranes, anion exchange membranes, amphoteric ionexchange membranes, and non-ionic porous materials | [17] |
| 2015 | RSC Adv. | A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries | developments in the synthesis and applications of AEMs in the field of electrochemical energy conversion and storage | [18] |
| 2017 | Chem. Soc. Rev. | Porous membranes in secondary battery technologies | understanding of the preparation–structure– performance relationship | [19] |
| 2017 | Journal of Membrane Science | Ion exchange membranes: New developments and applications | new iem materials | [20] |
| 2018 | Chem. Commun. | Ion conducting membranes for aqueous flow battery systems | porous membranes, different flow batteries | [21] |
| 2018 | Energy Environ. Sci. | Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage | status and options for mechanical, thermal, electrochemical, and chemical energy storage | [1] |
| 2018 | Journal of Membrane Science | Selectivity of ion exchange membranes: A review | selectivity of ion exchange membranes | [22] |
| 2019 | Applied Energy | Recent development of membrane for vanadium redox flow battery applications: A review | research on membranes for VRFB | [23] |
| 2019 | Current Opinion in Electrochemistry | Membranes and separators for redox flow batteries | current development trends for membranes and separators for VRFB | [24] |
| 2020 | Journal of Energy Storage | Membranes for all vanadium redox flow batteries | different membrane types, membrane performance | [25] |
| 2021 | Membranes | Polymer Membranes for all-Vanadium Redox Flow Batteries: A Review | graphical overview of polymer membranes; main polymer, impact on VRFB | this paper |
| MS | Membrane | Chem | Operating Mode | CD | CE | VE | EE | Ref. |
|---|---|---|---|---|---|---|---|---|
| mA cm−2 | % | % | % | |||||
| 1 | FAP-330-PE | AEM | high current density | 20–80 | 95.9 | 94.4 | 90.5 | [26] |
| 2 | FAP-450 | AEM | high energy efficiency | 20–80 | 98 | 90.8 | 89 | [26] |
| 3 | FAP-375-PP | AEM | low self-discharging | 20–80 | 99 | 89.9 | 89 | [26] |
| 4 | FS-930 | CEM | high current density | 20–80 | 96 | 94.8 | 91 | [26] |
| 5 | F-930-RFD | CEM | high current density | 20–80 | 98.5 | 92.4 | 91 | [26] |
| 6 | F-1075-PK | CEM | low self-discharging | 20–80 | 99.5 | 90.5 | 90 | [26] |
| 7 | F-1850 | CEM | low self-discharging | 20–80 | 99.5 | 83.4 | 83 | [26] |
| 8 | VX-20 | AEM | low self-discharging | 80 | 99.99 | 84 | 84 | [32] |
| 9 | Fumapem 14,100 | CEM | - | 40 | 91.3 | 90.2 | 82.4 | [33] |
| 10 | Vanadion | CEM | high current density | 80 | 88 | 92 | 81 | [27] |
| 11 | Vanadion | CEM | high current density | 320 | 96 | 76 | 73 | [27] |
| 12 | Nafion N117 | CEM | high current density | 100 | 96 | 61 | 59 | [34] |
| 13 | Nafion N115 | CEM | high current density | 80 | 95 | 90 | 86 | [27] |
| 14 | Nafion 212 | CEM | high current density | 200 | 97.6 | 77.9 | 76 | [35] |
| 15 | New Selemion | AEM | - | 40 | 98.6 | 87.5 | 86.3 | [28] |
| 16 | New Selemion CL | AEM | - | 60 | 93.5 | 87.7 | 82 | [29] |
| 17 | Aquivion-E87 | CEM | high current density | 80 | 97 | 86 | 83 | [31] |
| MS | Membrane | CD | CE | VE | EE | Ref. | MS | Membrane | CD | CE | VE | EE | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mA cm−2 | % | % | % | mA cm−2 | % | % | % | ||||||
| 18 | N212 | 20 | 81.2 | 92 | 74 | [37] | 12 | N117 | 100 | 96 | 61 | 59 | [34] |
| 18 | N212 | 80 | 94 | 70 | 66 | [37] | 52 | N117 | 40 | 90 | 92 | 83 | [38] |
| 19 | N212 | 25 | 97 | 95 | 92 | [39] | 52 | N117 | 200 | 95 | 66 | 63 | [38] |
| 19 | N212 | 100 | 97 | 91 | 88 | [39] | 53 | N117 | 40 | 93.8 | 90.7 | 85 | [33] |
| 20 | N212 | 50 | 92 | 86 | 79 | [40] | 54 | N117 | 50 | 87.6 | 82.6 | 72.6 | [41] |
| 21 | N212 | 50 | 92 | 86 | 79 | [42] | 55 | N117 | 50 | 96.5 | 91 | 87.5 | [43] |
| 22 | N212 | 30 | 60 | 96 | 58 | [44] | 56 | N117 | 40 | 91 | 93 | 84 | [45] |
| 22 | N212 | 60 | 78 | 92.3 | 71 | [44] | 56 | N117 | 280 | 98 | 54 | 53 | [45] |
| 23 | N212 | 80 | -- | -- | 88.8 | [46] | 57 | N117 | 40 | 93 | 89 | 83 | [47] |
| 23 | N212 | 240 | -- | -- | 74.8 | [46] | 58 | N117 | 50 | 95 | 87 | 83 | [48] |
| 24 | N212 | 20 | -- | -- | 81 | [49] | 59 | N117 | 30 | 90 | 76.6 | 69 | [50] |
| 24 | N212 | 80 | -- | -- | 71 | [49] | 60 | N117 | 20 | 84 | 94.1 | 79 | [51] |
| 25 | N212 | 200 | 91 | 88 | 80 | [52] | 60 | N117 | 80 | 91 | 81 | 73.5 | [51] |
| 26 | N212 | 80 | 94 | 75 | 71 | [53] | 61 | N117 | 20 | 81 | 72 | 86 | [54] |
| 27 | N212 | 40 | 75 | 95 | 71 | [55] | 61 | N117 | 80 | 95 | 66 | 66 | [54] |
| 27 | N212 | 200 | 92 | 80 | 72 | [55] | 62 | N117 | 100 | 96 | 63 | 60.5 | [56] |
| 14 | N212 | 100 | 95.5 | 91.6 | 87.5 | [35] | 63 | N117 | 20 | 74 | 81 | 67 | [57] |
| 14 | N212 | 200 | 97.6 | 77.9 | 76 | [35] | 63 | N117 | 50 | 83 | 70 | 62 | [57] |
| 64 | N117 | 60 | 91 | 89 | 81 | [58] | |||||||
| 28 | N115 | 80 | 94.6 | 86.6 | 82 | [59] | 65 | N117 | 40 | 87 | 92 | 80 | [60] |
| 29 | N115 | 40 | 94.5 | 90.1 | 85.2 | [61] | 65 | N117 | 200 | 93 | 68 | 63 | [60] |
| 30 | N115 | 20 | 92.5 | 92.5 | 86 | [62] | 66 | N117 | 30 | 96.4 | 90.7 | 87.4 | [63] |
| 30 | N115 | 50 | 94 | 82.5 | 76 | [62] | 67 | N117 | 20 | 85 | 81 | 68.9 | [64] |
| 31 | N115 | 20 | 94 | 94 | 88 | [65] | 67 | N117 | 80 | 92 | 70 | 64.4 | [64] |
| 31 | N115 | 100 | 97.5 | 77.5 | 75 | [65] | 68 | N117 | 50 | 89.9 | 90.8 | 81.6 | [66] |
| 32 | N115 | 40 | 88 | 94 | 82 | [45] | 69 | N117 | 50 | 93.8 | 90.7 | 85 | [67] |
| 32 | N115 | 320 | 96 | 56 | 54 | [45] | 70 | N117 | 10 | 72.5 | 93.8 | 68 | [68] |
| 33 | N115 | 40 | 94 | 94.7 | 89 | [69] | 70 | N117 | 60 | 89 | 75.3 | 67 | [68] |
| 33 | N115 | 200 | 97.5 | 73.8 | 72 | [69] | 71 | N117 | 40 | 94 | 84 | 79 | [70] |
| 34 | N115 | 20 | 79 | 95 | 75 | [71] | 72 | N117 | 30 | 90 | 81 | 73 | [72] |
| 34 | N115 | 80 | 94 | 84 | 79 | [71] | 72 | N117 | 60 | 94.5 | 63 | 60 | [72] |
| 35 | N115 | 20 | 81 | 91 | 73 | [73] | 73 | N117 | 30 | 95 | 76.8 | 73 | [74] |
| 35 | N115 | 80 | 92 | 66 | 61 | [73] | 73 | N117 | 120 | 97 | 64.4 | 62.5 | [74] |
| 36 | N115 | 20 | 90 | 81 | 73 | [75] | 74 | N117 | 30 | 90.8 | 84.8 | 77 | [76] |
| 36 | N115 | 100 | 93 | 55 | 51 | [75] | 75 | N117 | 60 | 86.3 | 80.6 | 69.6 | [77] |
| 37 | N115 | 20 | 93 | 87 | 81 | [78] | 76 | N117 | 50 | 93 | 82.3 | 77 | [79] |
| 37 | N115 | 100 | 97 | 62 | 61 | [78] | 77 | N117 | 60 | 92.8 | 79.6 | 73.8 | [80] |
| 38 | N115 | 80 | 94.6 | 82.1 | 86.8 | [81] | 78 | N117 | 40 | 90 | 92 | 83 | [82] |
| 39 | N115 | 60 | 91.7 | 92.3 | 84.7 | [83] | 78 | N117 | 200 | 95 | 66 | 63 | [82] |
| 40 | N115 | 80 | -- | -- | 84.5 | [46] | 79 | N117 | 80 | 92 | 87 | 80 | [84] |
| 40 | N115 | 160 | -- | -- | 75.2 | [46] | 80 | N117 | 20 | 82 | 90 | 74 | [85] |
| 41 | N115 | 40 | 98 | 72 | 70 | [86] | 80 | N117 | 80 | 92 | 71 | 65 | [85] |
| 42 | N115 | 80 | 96.64 | 86 | 83 | [32] | 81 | N117 | 10 | 72.5 | 97.5 | 71 | [87] |
| 43 | N115 | 80 | 94 | 87 | 81.8 | [88] | 81 | N117 | 80 | 92.5 | 84 | 78 | [87] |
| 44 | N115 | 50 | 97 | 89 | 86.3 | [89] | 82 | N117 | 40 | 89 | 91 | 81 | [90] |
| 44 | N115 | 150 | 98 | 70 | 68.6 | [89] | 82 | N117 | 200 | 94 | 67 | 63 | [90] |
| 45 | N115 | 50 | 96 | 89 | 86 | [91] | 83 | N117 | 40 | 90 | 92 | 83 | [92] |
| 46 | N115 | 40 | 93 | 93 | 86.5 | [93] | 83 | N117 | 200 | 95 | 67 | 63.7 | [92] |
| 46 | N115 | 120 | 96 | 80 | 76.8 | [93] | 84 | N117 | 40 | 95.6 | 91 | 86.9 | [94] |
| 47 | N115 | 40 | 96.26 | 94.3 | 90.77 | [95] | 85 | N117 | 40 | 94 | 91 | 86 | [96] |
| 47 | N115 | 160 | 98.11 | 79.98 | 78.47 | [95] | 85 | N117 | 100 | 96 | 80 | 76.8 | [96] |
| 48 | N115 | 80 | 93 | 88 | 82 | [97] | 86 | N117 | 40 | 95 | 90 | 85.5 | [98] |
| 49 | N115 | 40 | 91 | 93 | 85 | [99] | 86 | N117 | 140 | 96 | 77 | 74 | [98] |
| 49 | N115 | 160 | 94 | 78 | 73 | [99] | 87 | N117 | 20 | 94 | 95 | 89.3 | [100] |
| 50 | N115 | 50 | 91.3 | 91.9 | 84 | [101] | 87 | N117 | 80 | 96 | 83 | 79.7 | [100] |
| 51 | N115 | 80 | 92 | 88 | 82 | [102] | 88 | N117 | 40 | 95.9 | 89.7 | 86 | [103] |
| 13 | N115 | 80 | 95 | 90 | 86 | [27] | 89 | N117 | 20 | 81 | 87 | 72 | [104] |
| 13 | N115 | 320 | 97 | 67 | 65 | [27] | 89 | N117 | 80 | 94 | 67 | 65 | [104] |
| 90 | N117 | 20 | 86.2 | 90.3 | 77.8 | [105] | |||||||
| Polymer | Group | Structure Examples |
|---|---|---|
| PFSA, PTFE, PVDF, ETFE | fluoro-carbons | -C-F |
| Poly (phenylene) | hydro-carbon |  |
| Poly (ether ketone) | hydro-carbon | 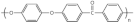 |
| Poly (ether sulfone) | hydro-carbon | 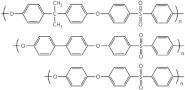 |
| Poly (fluorenyl ether) | hydro-carbon | 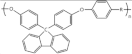 |
| Poly (phenylene ether) | hydro-carbon | 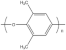 |
| other | hydro-carbon | - |
| Poly (benzimidazole) | N-heterocycles |  |
| Poly (phthalazinone ether ketone) | N-heterocycles | 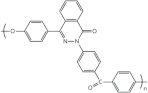 |
| Poly (imide) | N-heterocycles | 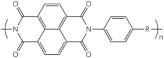 |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
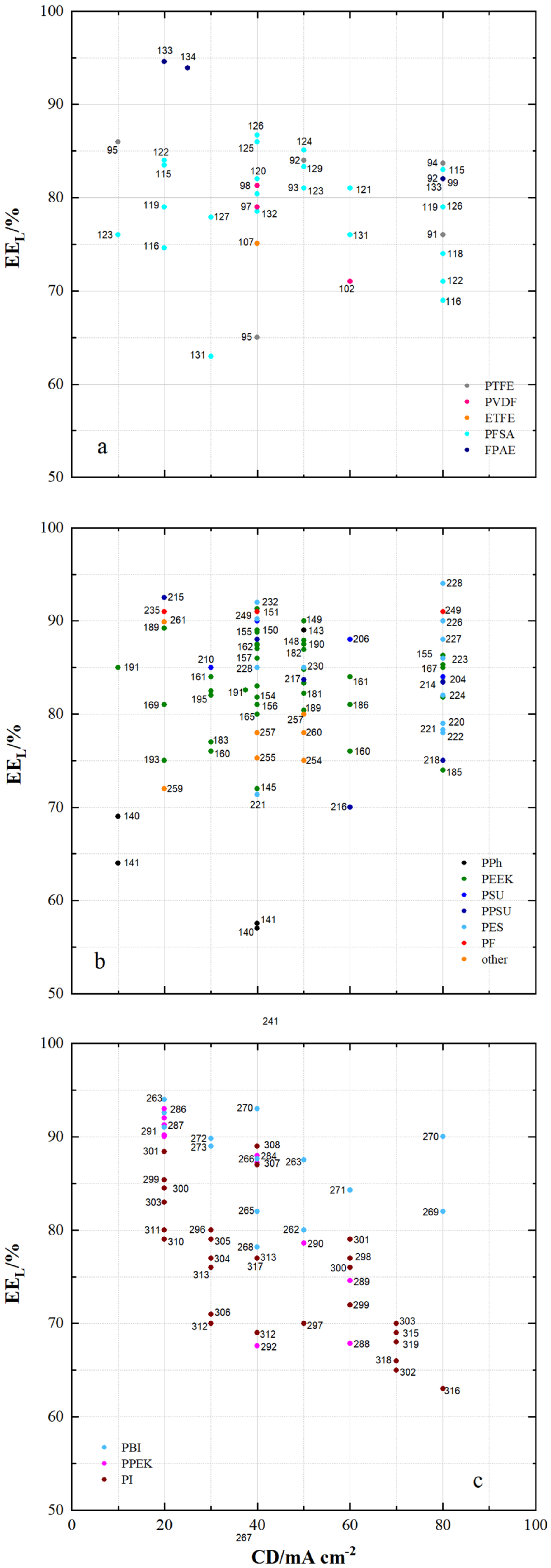
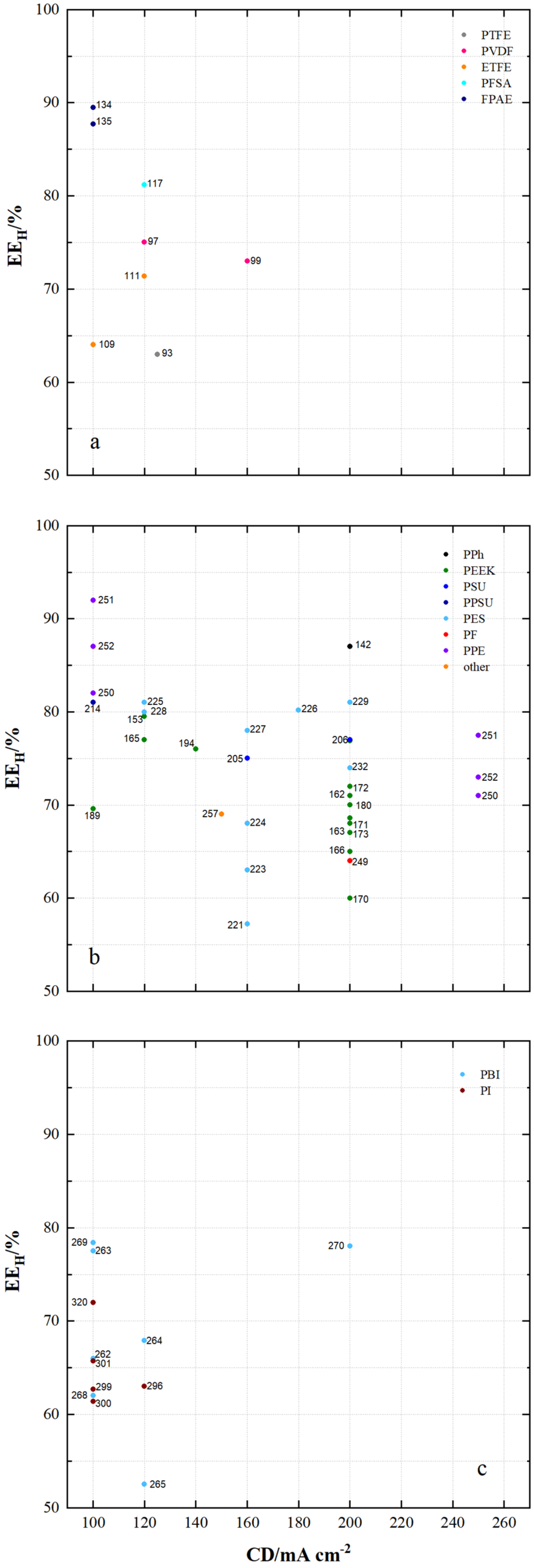
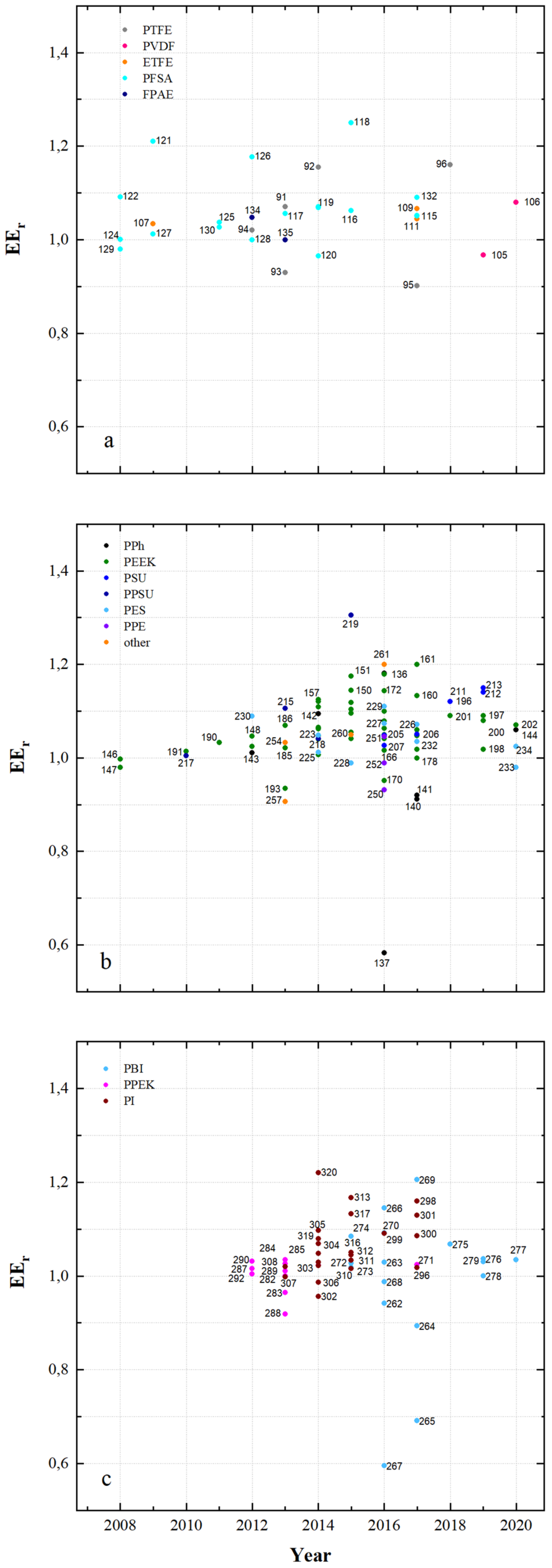
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
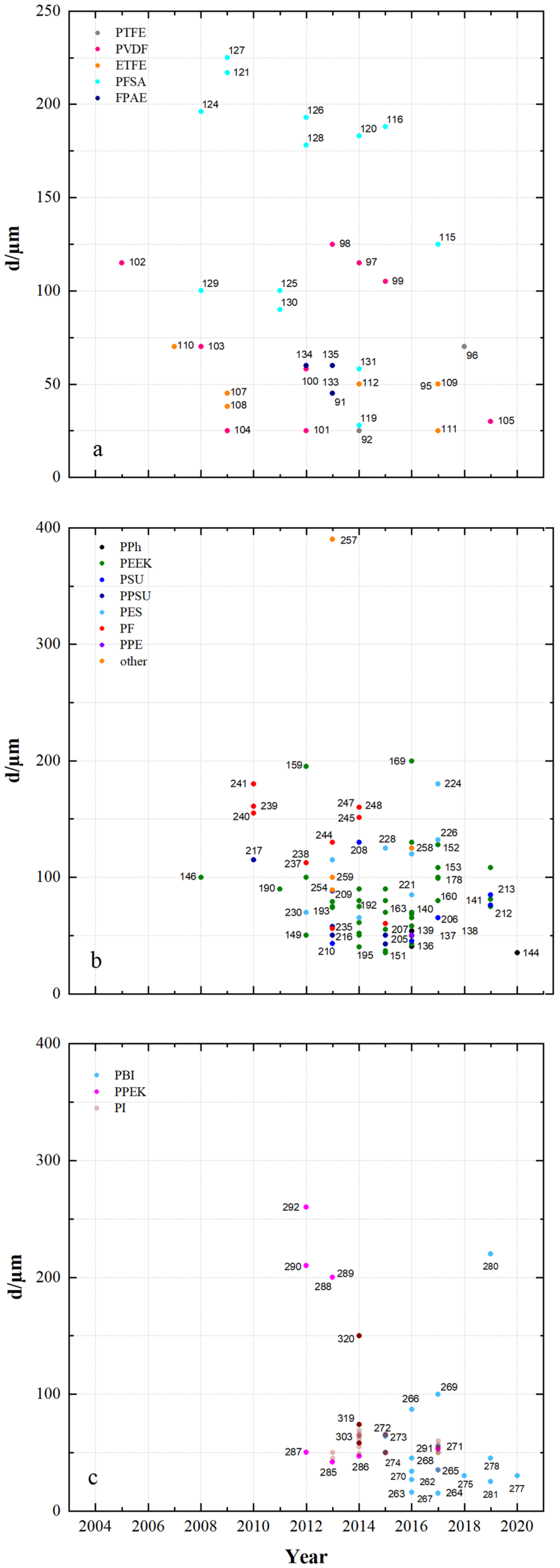
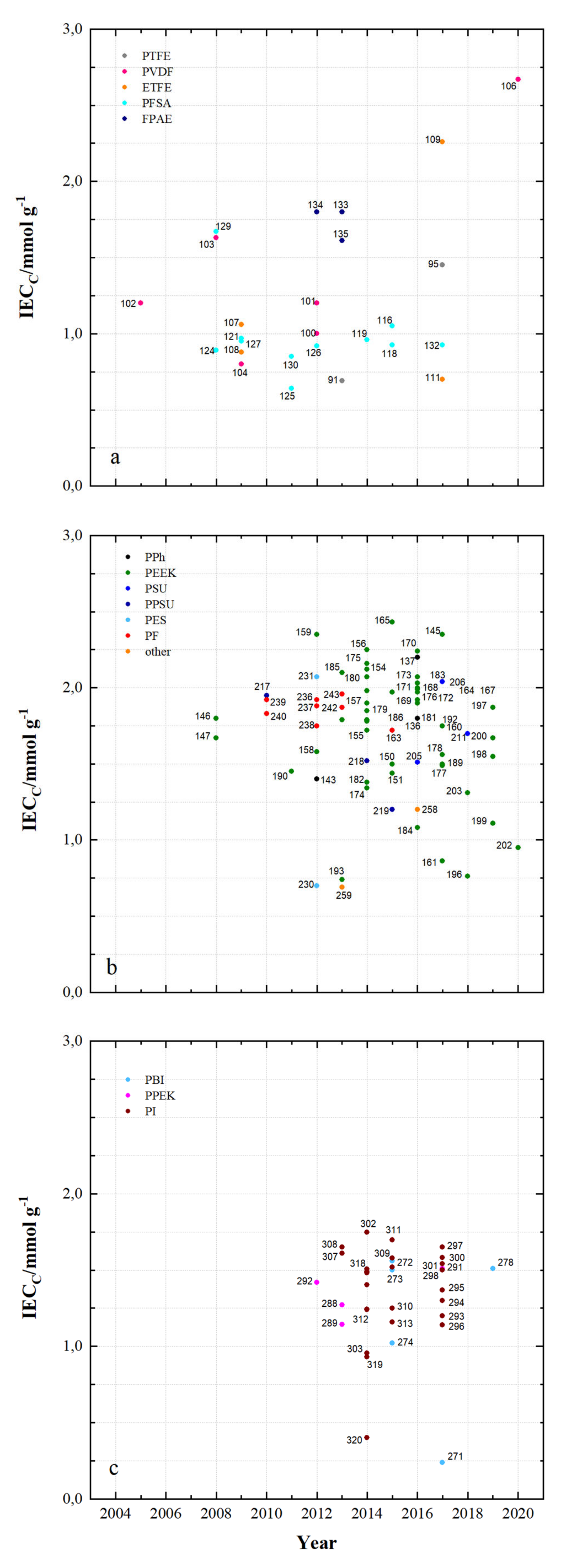
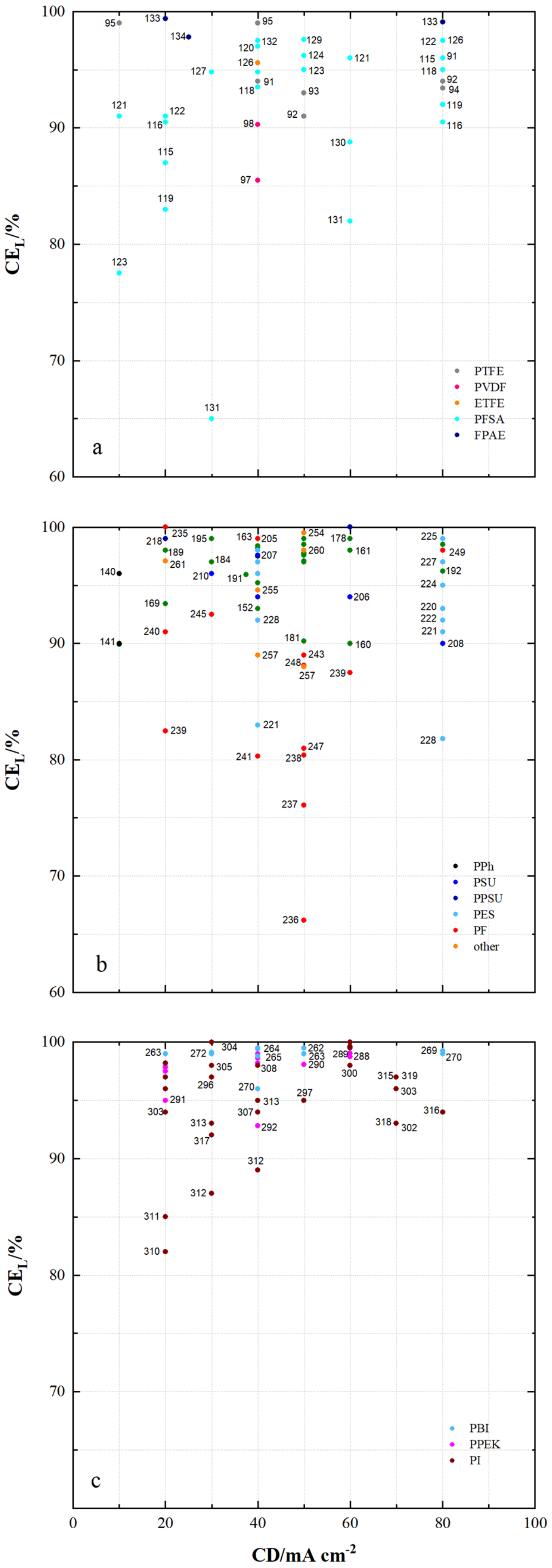
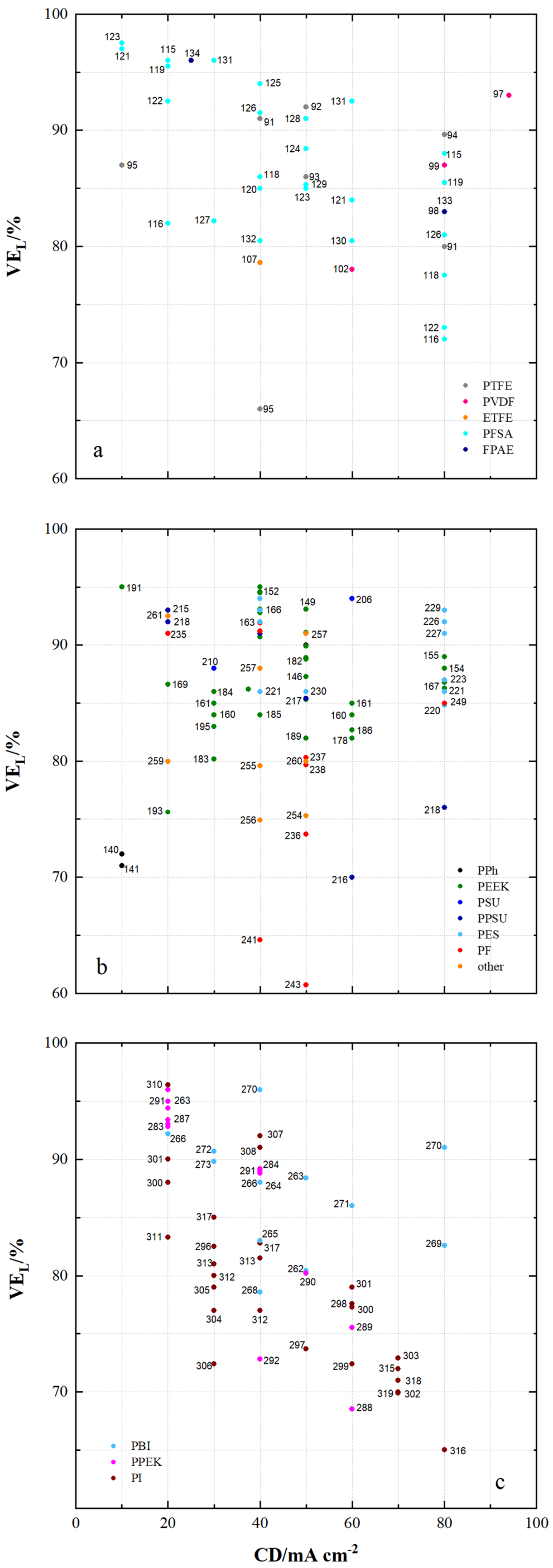
| 91 | PTFE/Nafion/P/N | dhe | CEM | 45 | 0.69 | - | 24.9 | 4.62 × 10−8 | 0.7 | 80 | 96 | 80 | 76 | 1.070 | N212 | [53] |
| 92 | PTFE/P/N/S-7 | dhe | CEM | 25 | - | - | 65.5 | 9 × 10−8 | 0.45 | 80 | 94 | 87 | 82 | 1.155 | P/N | [115] |
| 93 | PTFE/SiO2 | sym | - | - | - | - | 48 | - | - | 50 | 93 | 86 | 80 | 0.930 | N115 | [91] |
| 94 | PTFE/SPEEK/SP60 | dhe | CEM | - | - | - | 36 | - | - | 80 | 93 | 90 | 84 | 1.021 | N115 | [88] |
| 95 | PTFE/ZrP | dhe | CEM | 50 | 1.45 | - | - | 3.66 × 10−7 | 0.275 | 40 | 99 | 65 | 64 | 0.901 | N115 | [86] |
| 96 | PTFE/SE3/P | dhe | CEM | 70 | - | - | 29.8 | 7.1 × 10−7 | 0.178 | 100 | 99 | 79 | 78 | 1.147 | N117 | [116] |
| 97 | PVDF/M7 | sym | - | 115 | - | - | - | - | - | 120 | 94 | 79 | 75 | - | - | [117] |
| 98 | PVDF/M-23-125 | asym | - | 125 | - | - | - | 7.9 × 10−7 | 0.664 | 80 | 95 | 83 | 79 | - | - | [118] |
| 99 | PVDF/M2 | asym | - | 105 | - | - | - | - | - | 80 | 94 | 87 | 82 | 1.012 | N115 | [119] |
| 100 | PVDF-g-St-co-/AIEM | dho | AIEM | 58 | 1 | - | - | 1.18 × 10−7 | 0.153 | - | - | - | - | - | N117 | [120] |
| 101 | PVDF g/AIEM | dho | AIEM | 25 | 1.2 | - | 48 | 6.9 × 10−8 | 0.087 | - | - | - | - | - | N117 | [121] |
| 102 | PVDF-g-PSSA/22 | dho | CEM | 115 | 1.2 | - | 26.4 | 2.53 × 10−7 | 0.084 | 60 | 91 | 78 | 73 | 1.082 | N117 | [68] |
| 103 | PVDF-g-PSSA-co-PMAc | dho | AIEM | 70 | 1.63 | - | - | 7.3 × 10−, | 0.089 | N117 | [122] | |||||
| 104 | PVDF-g-St-co-./AIEM | dho | AIEM | 25 | 0.8 | 0.7 | 36 | 6.7 × 10−8 | 0.084 | - | - | - | - | - | N117 | [123] |
| 105 | PVDF/SiO2-SO3H_42 | dhe | CEM | 30 | - | - | 52.1 | 1.12 × 10−7 | 0.108 | 60 | 90.3 | 83.5 | 75.6 | 0.967 | N115 | [124] |
| 106 | PVDF/HA-45 | dho | CEM | - | 2.67 | - | 46 | 2.5 × 10−7 | - | 100 | 95 | 84 | 80 | 1.08 | N117 | [125] |
| 107 | ETFE-g-PSSA-c- AIEM-II | dho | AIEM | 45 | 1.06 | 1.24 | 36.1 | 2.9 × 10−9 | 0.004 | 40 | 96 | 79 | 75 | 1.034 | N117 | [126] |
| 108 | ETFE-g-PSSA | dho | CEM | 38 | 0.88 | - | 14.7 | 3.9 × 10−8 | 0.057 | - | - | - | - | - | N117 | [126] |
| 109 | ETFE-g-GMA- DG225 | dho | CEM | 50 | 2.4 | - | 181 | - | - | 100 | 87 | 73 | 64 | 1.067 | N117 | [56] |
| 110 | ETFE-g-PDMAEMA/40% | dho | AEM | 70 | - | 1.7 | 20 | 3.6 × 10−8 | 0.042 | - | - | - | - | - | N117 | [127] |
| 111 | ETFE-g-poly(VP) | dho | AIEM | 25 | 0.7 | - | - | - | - | 120 | 98 | 73 | 71 | 1.044 | N117 | [128] |
| 112 | ETFE-VB-DABACO | dho | AEM | 50 | - | 1.55 | 38 | - | - | - | - | - | - | - | N115 | [129] |
| 113 | ETFE-VB-DMA | dho | AEM | 50 | - | 1.33 | 8.8 | - | - | - | - | - | - | - | N115 | [129] |
| 114 | ETFE-VB-TMA | dho | AEM | 50 | - | 1.64 | 38 | - | - | - | - | - | - | - | N115 | [129] |
| 115 | PFSA AATMS | dhe | AIEM* | 125 | - | - | 8.5 × 10−7 | 0.218 | 80 | 96 | 88 | 83 | 1.051 | N115 | [71] | |
| 116 | PFSA AATMS (a-SiO2) | dhe | AIEM* | 188 | 1.05 | - | 35.1 | 2.32 × 10−7 | 0.268 | 80 | 91 | 72 | 69 | 1.062 | N117 | [64] |
| 117 | PFSA CC (CCM) | dhe | CEM | - | - | - | - | - | - | 120 | 95 | 86 | 81 | 1.056 | N115 | [130] |
| 118 | PFSA FC (N/FC-5) | dhe | CEM | - | 0.925 | - | 31 | 4.1 × 10−8 | 0.5 | 80 | 95 | 78 | 74 | 1.250 | N117 | [112] |
| 119 | PFSA GO (GO-0.01) | dhe | CEM | 27.75 | 0.96 | - | 22.41 | - | - | 80 | 92 | 86 | 79 | 1.068 | N117 | [51] |
| 120 | PFSA ND (AMH-3) | dhe | CEM | 183 | - | - | - | 8.64 × 10−7 | 0.281 | 40 | 97 | 85 | 82 | 0.965 | N117 | [47] |
| 121 | PFSA Ormosil (N/O) | dhe | CEM | 217 | 0.97 | - | 23.6 | 1.85 × 10−7 | 0.050 | 80 | 96 | 84 | 81 | 1.210 | N117 | [131] |
| 122 | PFSA [PDDA PSS]5 | dhe | AIEM | - | - | - | - | 2.85 × 10−7 | 0.095 | 80 | 98 | 73 | 72 | 1.091 | N117 | [85] |
| 123 | PFSA [PDDA ZrP]3 | dhe | AIEM | - | - | - | 8 | - | - | 50 | 95 | 85 | 81 | 1.052 | N115 | [132] |
| 124 | PFSA PEI (N/P2.5) | dhe | AIEM* | 196 | 0.89 | 5.23 × 10−7 | 50 | 96.2 | 88.4 | 85.1 | 1.001 | N117 | [67] | |||
| 125 | PFSA PVDF (N/P0.2) | dhe | CEM | 100 | 0.64 | - | 16.2 | - | - | 80 | 94 | 88 | 84 | 1.037 | D-520 | [133] |
| 126 | PFSA (N-sDDS) | dhe | CEM | 193 | 0.92 | - | 14.3 | - | - | 70 | 96 | 85 | 81 | 1.177 | N117 | [134] |
| 127 | PFSA (N/Si/Ti) | dhe | CEM | 225 | 0.95 | - | 22.5 | 4.3 × 10−7 | - | 30 | 95 | 82 | 78 | 1.012 | N117 | [76] |
| 128 | PFSA (N-SiO2) | dhe | CEM | 178 | - | - | - | 8.64 × 10−7 | - | 50 | 93 | 91 | 84 | 1.000 | N117 | [135] |
| 129 | PFSA SPEEK (N/S) | dhe | CEM | 100 | 1.67 | - | - | 1.93 × 10−7 | 0.053 | 50 | 98 | 85 | 83 | 0.980 | N117 | [136] |
| 130 | PFSA (N-TiO2) | dhe | CEM | 90 | 0.85 | - | 19.13 | 6.72 × 10−6 | 0.297 | 60 | 89 | 81 | 72 | 1.027 | N117 | [77] |
| 131 | PFSA (CS/PWA) | dhe | AIEM* | 58 | - | - | - | - | - | 60 | 82 | 93 | 76 | 1.070 | N212 | [44] |
| 132 | PFSA (ZrNT) | dhe | CEM | 155 | 0.927 | - | - | 3.6 × 10−9 | 0.010 | 40 | 98 | 81 | 79 | 1.090 | N117 | [137] |
| 133 | SFPAE 1.8_45 | dho | CEM | 45 | 1.8 | - | - | - | - | 80 | 99 | 83 | 82 | - | - | [113] |
| 134 | SFPAE 1.8 | dho | CEM | 60 | 1.8 | - | 48 | 1.16 × 10−8 | 0.219 | 100 | 98 | 92 | 90 | 1.047 | N212 | [39] |
| 135 | SFPAE (PVDF-co-/10%) | dhe | CEM | 60 | 1.6 | - | 35 | - | - | 100 | 99 | 88 | 88 | 0.999 | SFPAE | [138] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 136 | PP/SDAPP1.8 | dho | CEM | 41 | 1.8 | - | - | - | - | 200 | 96 | 88 | 85 | 1.181 | N117 | [139] |
| 137 | PP/SDAPP2.2 | dho | CEM | 50 | 2.2 | - | - | - | - | 200 | 48 | 88 | 42 | 0.583 | N117 | [139] |
| 138 | PP/QDAPP0.8 | dho | AEM | 54 | - | 0.8 | - | - | - | 200 | 96 | 88 | 85 | 1.181 | N117 | [139] |
| 139 | PP/QDAPP1.2 | dho | AEM | 54 | - | 1.2 | - | - | - | 200 | 96 | 88 | 85 | 1.181 | N117 | [139] |
| 140 | PP/AMPP11 | dho | AEM | 80 | - | 1.1 | 31.6 | 3.3 × 10−9 | 0.034 | 40 | 99 | 57 | 57 | 0.912 | N117 | [140] |
| 141 | PP/AMPP15 | dho | AEM | 80 | - | 1.5 | 45.2 | 3.2 × 10−8 | 0.330 | 40 | 98 | 59 | 58 | 0.920 | N117 | [140] |
| 142 | PP/QDAPP2 | dho | AEM | - | - | 0.8 | 77 | 1.4 × 10−6 | 0.666 | 200 | 97 | 90 | 87 | 1.094 | N212 | [52] |
| 143 | PP/SDAPP (Sample1) | dho | CEM | - | 1.4 | - | 36 | 4.4 × 10−7 | 0.094 | 50 | 99 | 90 | 89 | 1.011 | N117 | [43] |
| 144 | PP/p-TPN1 | dho | AEM | 35 | - | 2.15 | 18 | 7.4 × 10−8 | 0.018 | 80 | 100 | 85 | 85 | 1.06 | N212 | [141] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 145 | SPEEK/DS92 | dho | CEM | 65 | 2.35 | - | 88 | 3.06 × 10−6 | 0.243 | 200 | 93 | 82 | 77 | 1.048 | N212 | [55] |
| 146 | SPEEK | dho | CEM | 100 | 1.8 | - | - | 2.43 × 10−7 | 0.067 | 50 | 97 | 87 | 85 | 0.998 | N117 | [136] |
| 147 | SPEEK (N/S) | dhe | CEM | 100 | 1.67 | - | - | 1.93 × 10−7 | 0.053 | 50 | 98 | 85 | 83 | 0.980 | N117 | [136] |
| 148 | SDPEEK (SD4-6-100) | dho | CEM | 100 | 1.2 | - | 42.5 | 0.38 × 10−7 | 0.027 | 50 | 98 | 90 | 88 | 1.046 | N115 | [101] |
| 149 | SDPEEK (C-SD5-5-50) | dho | CEM | 50 | 1.65 | - | 29.8 | 0.48 × 10−7 | 0.034 | 50 | 97 | 93 | 90 | 1.024 | N115 | [101] |
| 150 | SPEEK | dho | CEM | 35 | 1.5 | - | 27 | 6.88 × 10−7 | 0.433 | 40 | 98 | 91 | 89 | 1.145 | N112 | [33] |
| 151 | SPEEK (PANI 80/20) | dhe | CEM | 37 | 1.44 | - | 21 | 2.67 × 10−7 | 0.168 | 40 | 98 | 93 | 91 | 1.175 | N112 | [33] |
| 152 | SPEEK | dho | CEM | 128 | - | - | 60.6 | 1.61 × 10−6 | 0.797 | 40 | 93 | 95 | 88 | 1.060 | N115 | [142] |
| 153 | SPEEK (SPEEK/RP) | dhe | CEM | 108 | - | - | 51.8 | 6.9 × 10−7 | 0.342 | 120 | 98 | 81 | 80 | 1.060 | N115 | [142] |
| 154 | SPEEK-co-PEEK | dho | CEM | 65 | 2.12 | - | 55 | 1.84 × 10−6 | 0.571 | 80 | 93 | 88 | 82 | 1.062 | N117 | [58] |
| 155 | SPEEK (S/SBA-20) | dhe | CEM | 61 | 1.72 | - | 31.8 | 6.2 × 10−7 | 0.193 | 80 | 97 | 89 | 86 | 1.121 | N117 | [58] |
| 156 | SPEEK | dho | CEM | 75 | 2.25 | - | 86 | 3.06 × 10−6 | 0.812 | 200 | 96 | 72 | 70 | 1.094 | N117 | [60] |
| 157 | SPEEK (SPEEK-15%) | dho | CEM | 75 | 1.9 | - | 62 | 1.9 × 10−6 | 0.496 | 200 | 98 | 72 | 72 | 1.125 | N117 | [60] |
| 158 | SPEEK/DS 57.99 | dho | CEM | 195 | 1.58 | - | 30.57 | 2.42 × 10−8 | 0.008 | - | - | - | - | - | N117 | [143] |
| 159 | SPEEK/DS 86.49 | dho | CEM | 195 | 2.35 | - | 83.02 | 2.28 × 10−6 | 0.740 | - | - | - | - | - | N117 | [143] |
| 160 | SPEEK | dho | CEM | 80 | 1.75 | - | 32.6 | 4.2 × 10−6 | 0.636 | 60 | 90 | 84 | 76 | 1.133 | N117 | [72] |
| 161 | SPEEK (g-C3N4-1.5) | dhe | CEM | 80 | 0.86 | - | 20.7 | 4 × 10−7 | 0.061 | 60 | 98 | 85 | 84 | 1.200 | N117 | [72] |
| 162 | SPEEK | dho | CEM | 70 | - | - | - | 1.04 × 10−7 | 0.299 | 200 | 97 | 73 | 71 | 1.118 | N117 | [82] |
| 163 | SPEEK (PDA-0.5h) | dho | CEM | 70 | - | - | - | 1.67 × 10−7 | 0.048 | 200 | 99 | 68 | 67 | 1.055 | N117 | [82] |
| 164 | SPEEK/S67-DMF | dho | CEM | 55 | 1.97 | - | 38 | 2 × 10−7 | 0.053 | 120 | 97 | 83 | 81 | 1.095 | N117 | [84] |
| 165 | SPEEK/S87-DMF | dho | CEM | 55 | 2.43 | - | 81 | 3.5 × 10−6 | 0.921 | 120 | 94 | 83 | 77 | 1.041 | N117 | [84] |
| 166 | SPEEK | dho | CEM | 70 | - | - | - | - | - | 200 | 100 | 65 | 65 | 1.016 | N115 | [144] |
| 167 | PTFE/SPEEK/PTFE | dhe | CEM | 130 | - | - | - | - | - | 80 | 99 | 86 | 85 | - | N115 | [144] |
| 168 | SPEEK | dho | CEM | 200 | 2 | - | 50 | 1.14 × 10−6 | 0.877 | - | - | - | - | - | N117 | [105] |
| 169 | SPEEK/PVDF/TPA | dhe | CEM | 200 | 1.9 | - | 35.3 | 5.17 × 10−7 | 0.398 | 20 | 93 | 87 | 81 | 1.041 | N117 | [105] |
| 170 | SPEEK | dho | CEM | 65 | 2.24 | - | 62.6 | 9 × 10−7 | 0.281 | 200 | 98 | 61 | 60 | 0.952 | N117 | [92] |
| 171 | SPEEK (S/A 5%) | dhe | CEM | 65 | 1.99 | - | 53.5 | 2 × 10−7 | 0.063 | 200 | 97 | 70 | 68 | 1.079 | N117 | [92] |
| 172 | SPEEK (S/S 5%) | dhe | CEM | 58 | 1.92 | - | 52.7 | 2 × 10−7 | 0.063 | 200 | 98 | 73 | 72 | 1.143 | N117 | [92] |
| 173 | SPEEK (S/T 5%) | dhe | CEM | 68 | 2.07 | - | 60.6 | 3.5 × 10−7 | 0.109 | 200 | 99 | 68 | 67 | 1.063 | N117 | [92] |
| 174 | C-SPEEK-50 | dhe | CEM | 90 | 1.34 | - | 50 | - | - | 80 | 98 | 87 | 85 | 1.040 | N115 | [59] |
| 175 | SPEEK | dho | CEM | 80 | 2.16 | - | 30.9 | 1.56 × 10−6 | 0.467 | - | - | - | -- | - | N117 | [145] |
| 176 | SPEEK (S/G) | dhe | CEM | 90 | 1.98 | - | 49.4 | 8.7 × 10−7 | 0.261 | 80 | 98 | 86 | 84 | 1.063 | N117 | [145] |
| 177 | SPEEK | dho | CEM | 99 | 1.49 | - | 26.7 | 1.56 × 10−6 | 0.427 | - | - | - | - | - | N117 | [146] |
| 178 | SPEEK (S/OCN-1) | dhe | CEM | 100 | 1.56 | - | 48.2 | 9.09 × 10−7 | 0.249 | 60 | 98 | 86 | 84 | 1.000 | N117 | [146] |
| 179 | SPEEK | dho | CEM | 52 | 1.85 | - | 37.1 | 1.15 × 10−6 | 0.330 | 200 | 98 | 68 | 67 | 1.047 | N117 | [38] |
| 180 | SPEEK (S/GO 3) | dhe | CEM | 50 | 2.07 | - | 44.9 | 5.9 × 10−7 | 0.171 | 200 | 98 | 72 | 71 | 1.109 | N117 | [38] |
| 181 | SPEEK (S/P-0) | dho | CEM | - | 1.79 | - | 38.4 | 1.12 × 10−7 | 1.172 | 50 | 90 | 91 | 82 | 1.007 | N117 | [66] |
| 182 | SPEEK (S/P-3/PEI) | dhe | CEM | - | 1.38 | - | 32.9 | 4.78 × 10−8 | 0.073 | 50 | 97 | 89 | 87 | 1.065 | N117 | [50] |
| 183 | SPEEK | dho | CEM | - | 2.03 | - | 43 | 4.2 × 10−6 | 0.627 | 30 | 96 | 80 | 77 | 1.100 | N117 | [147] |
| 184 | SPEEK (PPD-GO-1) | dhe | CEM | - | 1.08 | - | 22 | 1.2 × 10−6 | 0.179 | 30 | 97 | 86 | 83 | 1.179 | N117 | [147] |
| 185 | SPEEK | dho | CEM | 79 | 2.1 | - | 47.3 | 2.5 × 10−7 | 0.104 | 80 | 97 | 76 | 74 | 1.021 | N117 | [40] |
| 186 | SPEEK (S/P 15) | dhe | CEM | 74 | 1.79 | - | 39.6 | 1 × 10−7 | 0.042 | 60 | 98 | 83 | 81 | 1.069 | N117 | [40] |
| 187 | SPEEK | dho | CEM | 80 | - | - | - | 4.03 × 10−7 | 0.490 | - | - | - | - | - | N212 | [148] |
| 188 | SPEEK/SCCT | dhe | CEM | 90 | - | - | - | 3.22 × 10−7 | 0.391 | 50 | 99 | 86 | 85 | 1.104 | N212 | [148] |
| 189 | SPEEK (TiO2 5%) | dhe | CEM | - | 1.5 | - | 23 | 2.45 × 10−7 | 0.076 | 50 | 98 | 82 | 80.4 | 1.084 | N117 | [149] |
| 190 | SPEEK (SPEEK-40) | dho | CEM | 90 | 1.45 | - | - | 0.36 × 10−7 | 0.045 | 50 | 99 | 89 | 88 | 1.033 | N115 | [83] |
| 191 | SPEEK (TPA/PP) | dhe | CEM | 240 | - | - | - | 4.78 × 10−7 | 0.581 | 35.7 | 96 | 86 | 83 | 1.014 | N212 | [150] |
| 192 | SPEEK (S/PAN 20) | dho | CEM | 75 | 1.78 | - | 58 | 11.3 × 10−7 | 0.300 | 80 | 96 | 87 | 84 | 1.065 | N117 | [151] |
| 193 | SPEEK (PSP) | dho | CEM | 75 | 0.74 | - | 7.8 | 1.37 × 10−8 | 0.050 | 20 | 99 | 76 | 75 | 0.935 | N117 | [152] |
| 194 | PEEK-QADMPEK 3 | dho | AEM | 43 | - | 1.75 | 18.8 | 7.64 × 10−7 | 0.244 | 80 | 99 | 85 | 84 | 1.050 | N117 | [98] |
| 195 | QPEK-C-TMA | dho | AEM | 40 | - | 1.4 | 36 | 8.2 × 10−9 | 0.028 | 30 | 99 | 83 | 82 | - | N212 | [153] |
| 196 | SPEKS/sGO 0.5 | dhe | CEM | - | 0.76 | - | 31 | 5 × 10−8 | 0.161 | 40 | 99 | 83.3 | 82.5 | 1.12 | N212 | [154] |
| 197 | SPEEK/ZC-GO-2 | dhe | AIEM | 75 | 1.87 | - | 36.5 | 12.7 × 10−7 | 0.189 | 50 | 98.5 | 92.3 | 91.4 | 1.09 | N117 | [155] |
| 198 | S/TPAM-1% | dho | AIEM | 108 | 1.55 | - | 39.3 | 3.04 × 10−7 | 0.074 | 60 | 97.5 | 86.6 | 83.8 | 1,018 | N115 | [156] |
| 199 | SPEEK/L15 | dho | CEM | 81 | 1.11 | - | 29.62 | 1.7 × 10−8 | 0.086 | 120 | 99.5 | 83.9 | 83.5 | - | N212 | [157] |
| 200 | CrSPK45-S | dho | CEM | - | 1.67 | - | 22.16 | 6.1 × 10−9 | 0.12 | 80 | 98 | 86.7 | 85 | 1.08 | N117 | [158] |
| 201 | Q2-ADMPEK-4 | dho | AEM | - | - | 2.07 | 24,05 | - | - | 80 | 99 | 88.5 | 87.6 | 1.09 | N212 | [159] |
| 202 | CQSPK-6 | dho | AEIM | 0.95 | - | 21.6 | 1.05 × 10−9 | 0.064 | 60 | 98.4 | 82.7 | 81.4 | 1.07 | N117 | [160] | |
| 203 | SPAEK/Ce2Zr2O7 2% | dhe | CEM | - | 1.31 | - | 52 | 1.29 × 10−9 | 0.037 | 40 | 99.9 | 82.6 | 82.1 | 1.087 | N212 | [161] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 204 | PSU/PVDF/imi sIPN | dhe | AEM | - | - | - | 21 | - | - | 80 | 99 | 85 | 84 | - | - | [162] |
| 205 | PSU/CMPSF 72 | sym | AEM | 45 | - | 1.51 | - | - | - | 80 | 99 | 87 | 86 | 1.048 | N115 | [102] |
| 206 | PSU/ImPSf/SPEEK | dhe | AIEM | 65 | 2.04 | - | 56 | 1.5 × 10−8 | 0.071 | 200 | 98 | 79 | 77 | 1.069 | N212 | [55] |
| 207 | PSU/PVP 50 | dhe | AIEM | 50 | - | - | - | - | - | 100 | 99 | 79 | 78 | 1.026 | N212 | [163] |
| 208 | PSU/PVP/PS M90 | asym | AIEM | 130 | - | - | - | - | - | 80 | 90 | 87 | 78 | - | - | [164] |
| 209 | PSU/CPSF-Py | sym | AEM | 88 | - | - | - | - | - | 100 | 97 | 89 | 86 | 1.051 | N115 | [165] |
| 210 | PSU/TMA | dho | AEM | 43 | - | - | - | 2.6 × 10−8 | - | 30 | 96 | 88 | 85 | 1.012 | N212 | [166] |
| 211 | PSU/PSf-c-PTA-1.4 | dho | AEM | - | - | 1.7 | 36.7 | 2.57 × 10−7 | 0.198 | 120 | 98.4 | 85.7 | 84.3 | 1.12 | N115 | [167] |
| 212 | PSU/SPSF-62 | dho | CEM | 76 | 1.26 | - | 24.5 | 2.94 × 10−6 | 0.438 | 100 | 98.8 | 87.2 | 86.2 | 1.14 | N117 | [168] |
| 213 | PSU/SPSF/g-C3N4-1 | dhe | AIEM | 85 | 1.11 | - | 19.9 | 7 × 10−7 | - | 100 | 98 | 89.1 | 87.3 | 1.15 | N117 | [169] |
| 214 | PPSU/CMP-2 | dho | AEM | 42.5 | - | 1.95 | 35.3 | 1.5 × 10−8 | 0.005 | 80 | 97 | 86 | 83 | 1.049 | N117 | [96] |
| 215 | PPSU/QA-1.7 | dho | AEM | 57.5 | - | 1.7 | 16 | - | - | 80 | 100 | 75 | 70 | 1.106 | N212 | [114] |
| 216 | PPSU/AEM | dho | AEM | 50 | - | - | - | - | - | 60 | 100 | 70 | 70 | - | N212 | [170] |
| 217 | PPSU/S-needle | dho | CEM | 115 | 1.95 | - | - | 2.07 × 10−7 | 0.161 | 50 | 98 | 85 | 84 | 1.005 | N117 | [48] |
| 218 | PPSU/BPSH35 | dho | CEM | - | 1.52 | - | 40 | 1.6 × 10−9 | 0.123 | 80 | 99 | 76 | 75 | 1.042 | N212 | [49] |
| 219 | PPSU/S2B2 | dho | AIEM* | 50 | 1.2 | - | 40.2 | 100 | 99 | 79 | 77 | 1.305 | N117 | [34] | ||
| 220 | PES/PVP M3 | asym | AEM | 115 | - | - | - | 4 × 10−6 | - | 80 | 93 | 85 | 79 | - | - | [171] |
| 221 | PES/SPEEK M-35-13 | sym | CEM | 85 | - | - | - | - | - | 80 | 91 | 86 | 78 | - | - | [172] |
| 222 | PES/SPEEK M-35-6 | asym | CEM | 160 | - | - | - | - | - | 80 | 92 | 85 | 78 | - | - | [173] |
| 223 | PES/SPEEK M3 | dhe | CEM | 65 | - | - | - | - | - | 80 | 99 | 87 | 86 | 1.049 | N115 | [99] |
| 224 | PES/SPEEK/FT 10% | asym | CEM | 180 | - | - | - | 3.98 × 10−6 | - | 80 | 95 | 86 | 82 | - | N115 | [174] |
| 225 | PES/SPEEK/N 2.56 | asym | CEM | - | - | - | - | - | - | 80 | 99 | 87 | 86 | 1.012 | N115 | [81] |
| 226 | PES/SPEEK/PDDA 7.5 | sym | AIEM | 132 | - | - | - | - | - | 80 | 98 | 92 | 90 | 1.071 | N115 | [175] |
| 227 | PES/SPEEK/PPy | asym | AIEM* | 120 | - | - | - | - | - | 80 | 97 | 91 | 88 | 1.073 | N115 | [95] |
| 228 | PES/SPEEK/SiO2 M2 | dhe | CEM | 125 | - | - | 25 | - | - | 80 | 82 | 87 | 94 | 0.989 | N115 | [93] |
| 229 | PES/SPEEK/ZSM-35 | asym | CEM | - | - | - | - | - | - | 80 | 98 | 93 | 91 | 1.109 | N115 | [97] |
| 230 | SPES/SPEEK | dhe | CEM | 70 | 0.7 | - | 21.6 | - | - | 50 | 98 | 86 | 85 | 1.090 | N212 | [176] |
| 231 | SPES | dho | CEM | - | 2.07 | - | 121.93 | 2.5 × 10−6 | 0.809 | - | - | - | - | - | - | [143] |
| 232 | SPAES S/N | dhe | CEM | - | - | - | - | - | - | 200 | 99 | 75 | 74 | 1.035 | N115 | [69] |
| 233 | SPES (IL-30) | asym | CEM | - | - | - | - | 1.41 × 10−8 | - | 140 | 99 | 80.13 | 79.3 | 0.98 | N212 | [177] |
| 234 | MD2.0-10 | dhe | AEIM | - | - | - | - | 1.7 × 10−7 | 0.134 | 80 | 99.3 | 82.6 | 82 | 1.025 | N115 | [178] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 235 | QA-PFE | dho | AEM | 56 | - | 2.0 | - | - | - | 60 | 100 | 70 | 70 | 1.0 | N212 | [37] |
| 236 | SPECIAL | dho | CEM | 112.5 | 1.92 | - | 29 | - | 0.25 | 50 | 66.2 | 73.7 | 48.8 | - | N117 | [179] |
| 237 | F-SPFEK | dho | CEM | 112.5 | 1.88 | - | 33 | - | 0.75 | 50 | 76.1 | 80.3 | 61.1 | - | N117 | [179] |
| 238 | F-SPFEK-APTES | dho | CEM | 112.5 | 1.75 | - | 26 | - | 0.50 | 50 | 80.4 | 79.7 | 64.1 | - | N117 | [179] |
| 239 | SPECIAL | dho | CEM | 161 | 1.92 | - | 39 | - | 0.21 | 60 | 87.5 | - | - | - | N117 | [180] |
| 240 | SPFEK/3%SIO2 | dhe | CEM | 155 | 1.83 | - | 36.6 | - | 0.29 | 60 | 87.5 | - | - | - | N117 | [180] |
| 241 | SPECIAL | dho | CEM | 180 | 1.92 | - | 27.8 | 9.85 × 10−7 | 0.40 | 40 | 80.3 | 64.6 | 51.9 | - | N117 | [180] |
| 242 | SPECIAL | dho | CEM | - | 1.87 | - | - | - | - | - | - | - | - | - | N117 | [181] |
| 243 | SPFEK/5ZrPSPP | dhe | CEM | - | 1.96 | - | - | - | - | 50 | 89 | 60.7 | 54 | - | N117 | [181] |
| 244 | SPFEK-[PDDA/PSS]n2 | dhe | AIEM | 130 | - | - | - | - | - | - | - | - | - | - | N117 | [182] |
| 245 | SPFEK 20.7 [PDA/PSS]2 | sym | AIEM | 151 | - | - | - | 5.92 × 10−7 | 0.36 | - | - | - | - | - | N115 | [183] |
| 246 | SPECIAL | dho | CEM | 160 | 1.57 | - | 36.5 | 2.67 × 10−7 | 0.13 | - | - | - | - | - | N115 | [184] |
| 247 | SPFEKA 10%. | dho | AIEM | 160 | 1.52 | - | 30.6 | 1.56 × 10−7 | 0.08 | - | - | - | - | - | N115 | [184] |
| 248 | SPFEKA-20% | dho | AIEM | 160 | 1.47 | - | 30.9 | 0.88 × 10−7 | 0.04 | - | - | - | - | - | N115 | [184] |
| 249 | HSPAEK | dho | CEM | 60 | 1.72 | - | 38.5 | 5.5 × 10−7 | 0.16 | 80 | 98 | 85 | 83 | 1.05 | N117 | [90] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 250 | BrPPO/Py-42 | dho | AEM | 50 | - | - | 13 | 0.12 × 10−7 | 0.02 | 100 | 98.1 | 84 | 82 | 0.932 | N212 | [35] |
| 251 | BrPPO/Py-56 | dho | AEM | 50 | - | - | 18 | 0.2 × 10−7 | 0.03 | 100 | 97.7 | 94 | 92 | 1.045 | N212 | [35] |
| 252 | BrPPO/Py-70 | dho | AEM | 50 | - | - | 21 | 0.36 × 10−7 | 0.05 | 100 | 96.7 | 90 | 87 | 0.989 | N212 | [35] |
| 253 | SPPO-GO | dhe | CEM | - | 1.17 | - | 16.3 | 1.1 × 10−8 | 0.05 | 40 | 98 | 71 | 69.6 | - | N212 | [185] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 254 | QPTM | dho | AEM | 89 | - | 2.08 | 8.8 | 1.19 × 10−7 | 0.034 | 50 | 100 | 75 | 75 | 1.033 | N117 | [41] |
| 255 | QVTD 2-3 | dho | AEM | - | - | - | - | - | - | 40 | 95 | 80 | 75 | - | - | [186] |
| 256 | VBC AVSH-3 | dho | AEM | - | - | - | - | - | - | 40 | 95 | 75 | 75 | - | - | [187] |
| 257 | PVC/silica | sym | - | 390 | - | - | - | - | - | 40 | 89 | 88 | 78 | 0.907 | N115 | [188] |
| 258 | Si-PWA/PVA | dhe | CEM | 125 | 1.2 | - | - | 6.9 × 10−8 | 0.119 | - | - | - | - | - | N115 | [189] |
| 259 | DHIM-375 | dho | CEM | 100 | 0.69 | - | 31 | 1.56 × 10−7 | 0.052 | 20 | 91 | 80 | 72 | - | N117 | [190] |
| 260 | ZPPT-6 | dho | AIEM | 80 | - | 1.22 | 30 | - | - | 50 | 98 | 80 | 78 | 1.05 | N117 | [57] |
| 261 | PIM-1 | asym | - | “0.75” | - | - | - | - | - | 20 | 97.1 | 92.5 | 89.9 | 1.2 | N112- | [191] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 262 | mPBI | dho | AEM | 27 | - | - | - | 2.86 × 10−9 | 0.008 | 50 | 99.5 | 80.4 | 80 | 0.941 | N115 | [65] |
| 263 | BlpPBI | dho | AEM | 27 | - | - | - | 3.45 × 10−8 | 0.099 | 50 | 99 | 88.4 | 87.5 | 1.029 | N115 | [65] |
| 264 | mPBI-15 | dho | AEM | 15 | - | - | - | 0 | - | 120 | 99.8 | 68 | 67.9 | 0.893 | N212 | [192] |
| 265 | mPBI-35 | dho | AEM | 35 | - | - | - | 0 | - | 120 | 99 | 53 | 52.5 | 0.691 | N212 | [192] |
| 266 | p-PBI | sym | AEM | 87 | - | - | - | 4.5 × 10−8 | 0.028 | 40 | 99 | 88 | 87 | 1.145 | N112 | [193] |
| 267 | PBI-0% | dho | AEM | 16 | - | - | - | - | - | 40 | 95 | 50 | 47 | 0.595 | N117 | [70] |
| 268 | PBI 10%. | sym | AEM | 45 | - | - | - | 1.17 × 10−7 | - | 40 | 99 | 79 | 78 | 0.987 | N117 | [70] |
| 269 | PBI-200 | asym | AEM | 100 | - | - | - | 3 × 10−9 | - | 80 | 99 | 83 | 82 | 1.206 | N211 | [194] |
| 270 | PBI-O/PBI-34 | sym | AEM | 34 | - | - | - | - | - | 80 | 99 | 91 | 90 | 1.092 | N115 | [195] |
| 271 | CSOPBI-NH2 (9/1) | dho | AIEM* | 55 | 0.24 | - | 47.4 | 6 × 10−9 | 0.001 | 60 | 98 | 86 | 84 | 1.024 | N117 | [87] |
| 272 | -6F-co-10%BI | dho | AIEM* | 64 | 1.56 | - | - | 2.24 × 10−11 | - | 30 | 99 | 91 | 90 | 1.027 | N117 | [63] |
| 273 | -6F-co-10%BI-cld | dho | AIEM* | 65 | 1.50 | - | - | 1.28 × 10−11 | - | 30 | 99 | 90 | 89 | 1.018 | N117 | [63] |
| 274 | FPAE-6F-PBI S1B1 | dhe | AIEM* | 50 | 1.02 | - | 23.8 | - | - | 100 | 100 | 64 | 64 | 1.085 | N117 | [34] |
| 275 | B20N10 | dho | AEIM | 30 | - | - | 23.6 | 1.95 × 10−9 | 0.006 | 80 | 100 | 82.2 | 82.2 | 1.068 | N115 | [196] |
| 276 | CPBI-70-NMG | dho | AEM | - | - | - | - | - | - | 120 | 99 | 86 | 85.3 | 1.036 | N212 | [197] |
| 277 | 0,7µm PBI | asym | AEM | 30 | - | - | - | - | - | 120 | 98.5 | 85 | 83 | 1.034 | N212 | [198] |
| 278 | PWN/F6PBI(9/1) | dho | AIEM | 45 | 1.51 | - | - | - | - | 40 | 99 | 81 | 81 | 1 | N212 | [199] |
| 279 | PVDF-PBI | asym | AEM | - | - | - | - | - | - | 60 | 98.4 | 83.3 | 82 | 1.03 | N117 | [200] |
| 280 | sPBI | dho | AEIM | 220.2 | - | - | 58.1 | 5.74 × 10−7 | - | 242 | 93 | 86 | 81 | - | N212 | [201] |
| 281 | PE/PBI | dhe | AEM | 25 | - | - | 20.9 | 5.04 × 10−7 | 0.346 | 200 | 99 | 81 | 80.11 | 1.03 | N212 | [202] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 282 | PyPPEK-2 | dho | AEM | - | - | 1.4 | 17.4 | - | - | 60 | 99 | 85 | 84 | 1.000 | N117 | [203] |
| 283 | QAPPEK-2 | dho | AEM | - | - | 1.5 | 21 | - | - | 60 | 99 | 83 | 82 | 0.964 | N117 | [203] |
| 284 | QAPPEKK | dho | AEM | - | - | 1.56 | - | - | - | 40 | 99 | 89 | 88 | 1.026 | N117 | [103] |
| 285 | PyPPEKK-4 | dho | AEM | 42 | - | 1.55 | 16.5 | - | - | 40 | 98 | 90 | 89 | 1.034 | N117 | [103] |
| 286 | QBPPEK 80 | dho | AEM | 47 | - | 1.53 | 23.8 | - | - | 40 | 99 | 89 | 88 | 1.023 | N117 | [204] |
| 287 | QAPPEKK-4 | dho | AEM | 50 | - | 1.56 | 20.8 | - | - | 20 | 98 | 93 | 91.3 | 1.016 | N117 | [100] |
| 288 | SPEC | dho | CEM | 200 | 1.272 | - | 32.34 | 2.77 × 10−7 | 0.024 | 60 | 99 | 69 | 68 | 0.919 | N117 | [80] |
| 289 | SPPEK TPA-17 | dhe | CEM | 200 | 1.142 | - | 33.28 | 5.75 × 10−7 | 0.049 | 60 | 99 | 76 | 75 | 1.010 | N117 | [80] |
| 290 | SPPEK/WO3 | dhe | CEM | 210 | - | - | 48.15 | 3.97 × 10−7 | 0.034 | 50 | 98 | 80 | 79 | 1.032 | N117 | [79] |
| 291 | SPPEK P-90 | dho | CEM | 53 | 1.51 | - | 23.2 | - | - | 40 | 98 | 89 | 87 | 1.023 | N115 | [61] |
| 292 | SPPES/SP-02 | dho | CEM | 260 | 1.42 | - | 17.42 | 1.24 × 10−7 | 0.055 | 40 | 93 | 73 | 68 | 1.004 | N117 | [205] |
| No | Membrane Sample | Membrane | Membrane Properties | VRFB Properties | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Polymer/Sample Name | Struc | Chem | d | IECC | IECA | WU | DC | Dr | CD | CE | VE | EE | EEr | Mem | Pub |
| µm | mmol g−1 | mmol g−1 | wt.% | cm2 min−1 | % | mA cm−2 | % | % | % | |||||||
| 293 | SPI (ODA) | dho | CEM | 60 | 1.2 | - | 21.93 | 2.17 × 10−7 | 0.127 | - | - | - | - | - | N117 | [74] |
| 294 | bSPI (APABI) | dho | AIEM* | 54 | 1.3 | - | 28.80 | 1.75 × 10−7 | 0.102 | - | - | - | - | - | N117 | [74] |
| 295 | bSPI(MDA) | dho | CEM | 55 | 1.37 | - | 34.88 | 4.43 × 10−7 | 0.259 | - | - | - | - | - | N117 | [74] |
| 296 | bSPI(BAPP) | dho | CEM | 57 | 1.14 | - | 20.03 | 2.89 × 10−7 | 0.169 | 120 | 99 | 64 | 63 | 1.018 | N117 | [74] |
| 297 | SPI(H) | dho | CEM | 50 | 1.65 | - | - | - | - | 50 | 95 | 74 | 70 | - | - | [206] |
| 298 | s-FSPI | dho | CEM | - | 1.50 | - | 17.78 | 7.4 × 10−8 | 0.055 | 60 | 100 | 77 | 77 | 1.160 | N115 | [207] |
| 299 | 6F-SPI-50 | dho | CEM | - | - | - | - | 2.27 × 10−7 | 0.172 | 60 | 99.5 | 72.4 | 72 | 1.091 | N115 | [75] |
| 300 | SPI | dho | CEM | 50 | 1.58 | - | 25.7 | 2.25 × 10−7 | 0.165 | 60 | 98 | 78 | 76 | 1.086 | N115 | [78] |
| 301 | 6F-s-bSPI | dho | CEM | 35 | 1.54 | - | 16.5 | 1.18 × 10−7 | 0.087 | 60 | 100 | 79 | 79 | 1.129 | N115 | [78] |
| 302 | SPI | dho | CEM | 69 | 1.75 | - | 39.92 | 1.89 × 10−7 | 0.111 | 70 | 93 | 70 | 65 | 0.956 | N117 | [104] |
| 303 | SPI/AlOOH-10 | dhe | CEM | 58 | 0.95 | - | 48.59 | 1.14 × 10−7 | 0.067 | 70 | 96 | 73 | 70 | 1.029 | N117 | [104] |
| 304 | SPI(APABI) | dho | AIEM* | 65 | 1.24 | - | 22.79 | 7.2 × 10−8 | 0.042 | 30 | 100 | 77 | 77 | 1.069 | N117 | [208] |
| 305 | SPI(BAPP) | dho | CEM | 62 | 1.49 | - | 27.08 | 4.8 × 10−8 | 0.028 | 30 | 100 | 79 | 79 | 1.097 | N117 | [208] |
| 306 | SPI(MDA) | dho | CEM | 64 | 1.48 | - | 26.94 | 2.36 × 10−7 | 0.138 | 30 | 98 | 72 | 71 | 0.986 | N117 | [208] |
| 307 | SPI | dho | CEM | 45 | 1.61 | - | 41.40 | 1.89 × 10−7 | 0.123 | 40 | 94 | 92 | 87 | 0.998 | N117 | [94] |
| 308 | SPI/CS | dhe | AIEM* | 50 | 1.65 | - | 28.66 | 1.12 × 10−7 | 0.073 | 40 | 98 | 91 | 89 | 1.020 | N117 | [94] |
| 309 | SPI | dho | CEM | 65 | 1.58 | - | 37.14 | 2.37 × 10−7 | 0.139 | - | - | - | - | - | N117 | [209] |
| 310 | SPI/MoS2 | dhe | CEM | 65 | 1.25 | - | 29.36 | 2.02 × 10−7 | 0.119 | 80 | 95 | 65 | 62 | 1.016 | N117 | [209] |
| 311 | SPI/s-MoS2 | dhe | CEM | 66 | 1.70 | - | 32.20 | 1.23 × 10−7 | 0.072 | 80 | 96 | 66 | 63 | 1.033 | N117 | [209] |
| 312 | SPI | dho | CEM | 50 | 1.25 | - | 54.7 | 2.6 × 10−6 | 0.388 | 40 | 89 | 77 | 69 | 1.045 | N117 | [210] |
| 313 | SPI/PEI-GO-2 | dhe | AIEM* | 50 | 1.16 | - | 44.2 | 7 × 10−7 | 0.104 | 40 | 95 | 82 | 77 | 1.167 | N117 | [210] |
| 314 | SPI | dho | CEM | 55 | 1.40 | - | 38.46 | 1.9 × 10−7 | 0.111 | - | - | - | - | - | N117 | [54] |
| 315 | SPI/TiO2 | dhe | CEM | 49 | 1.24 | - | 32.94 | 2.02 × 10−7 | 0.118 | 70 | 97 | 72 | 69 | 1.022 | N117 | [54] |
| 316 | SPI | dho | CEM | 50 | 1.25 | - | 54.7 | 2.6 × 10−6 | 0.388 | 80 | 94 | 65 | 63 | 1.050 | N117 | [211] |
| 317 | SPI/ZGO-4 | dhe | AIEM* | 50 | 1.52 | - | 63.1 | 1.2 × 10−6 | 0.179 | 40 | 93 | 83 | 77 | 1.132 | N117 | [211] |
| 318 | SPI | dho | CEM | 66 | 1.51 | - | 37.52 | 2.37 × 10−7 | 0.139 | 70 | 93 | 71 | 66 | 1.048 | N117 | [212] |
| 319 | SPI/ZrO2-15 | dhe | CEM | 74 | 0.93 | - | 53.19 | 2.18 × 10−7 | 0.127 | 70 | 97 | 70 | 68 | 1.079 | N117 | [212] |
| 320 | SPI | dho | CEM | 150 | 0.40 | - | - | - | - | 100 | 98 | 73 | 72 | 1.220 | N117 | [213] |
| MS | Polymer Used | EEr | CD | Dr | d | WU | IEC | Ref. |
|---|---|---|---|---|---|---|---|---|
| - | mA cm−2 | - | µm | wt.% | mmol g−1 | |||
| 219 | PPSU | 1.305 | 100 | - | 50 | - | 1.2 | [34] |
| 118 | PFSA | 1.250 | 80 | 0.5 | - | 31 | 0.925 | [112] |
| 320 | PI | 1.220 | 100 | - | 150 | - | 0.4 | [213] |
| 121 | PFSA | 1.210 | 80 | 0.05 | 217 | 23.6 | 0.97 | [131] |
| 269 | PBI | 1.206 | 80 | - | - | - | - | [194] |
| 261 | other | 1.200 | 20 | - | - | - | - | [191] |
| 161 | SPEEK | 1.200 | 60 | 0.061 | 80 | 20.7 | 0.86 | [72] |
| 136 | sDAPP | 1.181 | 200 | - | 41 | - | 1.8 | [139] |
| 139 | qDAPP | 1.181 | 200 | - | 54 | - | 1.2 | [139] |
| 184 | SPEEK | 1.179 | 30 | 0.179 | - | 22 | 1.08 | [147] |
| 126 | PFSA | 1.177 | 70 | - | 193 | 14.3 | 0.92 | [134] |
| 151 | SPEEK | 1.175 | 40 | 0.168 | 37 | 21 | 1.44 | [33] |
| 313 | PI | 1.167 | 40 | 0.104 | 50 | 44.2 | 1.16 | [210] |
| 298 | PI | 1.160 | 60 | 0.055 | - | 17.8 | 1.5 | [207] |
| 92 | PTFE | 1.155 | 80 | 0.45 | 25 | 65.5 | - | [115] |
| Polymer | MS | Cycles | mL | mA cm−2 | Ref. | Polymer | MS | Cycles | mL | mA cm−2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PTFE | 94 | 700 | - | 80 | [88] | PES | 224 | 70 | 40 | 80 | [174] |
| PVDF | 97 | 50 | 30 | 80 | [117] | 230 | 100 | 80 | 50 | [176] | |
| 98 | 1000 | 30 | 80 | [118] | PPE | 251 | 500 | 25 | 200 | [35] | |
| 99 | 300 | 30 | 80 | [119] | other | 254 | 4000 | - | 50 | [41] | |
| 102 | 230 | 40 | 60 | [68] | 255 | 150 | 3 | 40 | [186] | ||
| PFSA | 116 | 150 | 60 | 80 | [64] | 256 | 500 | 3 | 40 | [187] | |
| 117 | 300 | - | 120 | [130] | 259 | 120 | 3 | 20 | [190] | ||
| DAPP | 143 | 400 | 100 | 50 | [43] | PBI | 262 | 200 | 100 | 50 | [65] |
| PEEK | 155 | 120 | 50 | 60 | [58] | 270 | 13000 | 60 | 80–120 | [195] | |
| 157 | 100 | 50 | 80 | [60] | 271 | 300 | 10 | 60 | [87] | ||
| 161 | 300 | 10 | 30 | [72] | 272 | 220 | 20| 30 | [63] | ||
| 167 | 1000 | - | 80 | [144] | PPEK | 290 | 100 | 120 | 50 | [79] | |
| 174 | 180 | 30 | 80 | [59] | 291 | 100 | 30 | 60 | [61] | ||
| 176 | 500 | 50 | 80 | [145] | PI | 296 | 500 | 30 | 30–120 | [74] | |
| 180 | 50 | 50 | 60 | [38] | 297 | 750 | 30 | 50 | [206] | ||
| 194 | 100 | 30 | 80 | [98] | 298 | 100 | - | 60 | [207] | ||
| PSU | 204 | 900 | - | 80 | [162] | 301 | 100 | 60 | 60 | [78] | |
| 205 | 6000 | 100 | 120 | [102] | 311 | 500 | 30 | 25–70 | [209] | ||
| 207 | 500 | 50 | 80 | [163] | 313 | 100 | 8 | 40 | [210] | ||
| 208 | 300 | 30 | 80 | [164] | 317 | 100 | 8 | 40 | [211] | ||
| PES | 220 | 150 | 30 | 80 | [171] | Nafion | 32 | 200 | 50 | 160 | [45] |
| 221 | 250 | 60 | 80 | [172] | 56 | 200 | 50 | 160 | [45] |
| MS | Membrane Polymer | Ref. | MS | Membrane Polymer | Ref. |
|---|---|---|---|---|---|
| 224 | PES | [174] | 129 | PFSA | [136] |
| 151 | PEEK | [33] | 167 | PEEK/PTFE | [144] |
| 263 | PBI | [65] | 93 | PTFE | [91] |
| 191 | PEEK | [150] | 98 | PVDF | [118] |
| 217 | PPSU | [48] | 251 | PPE | [35] |
| 297 | PI | [206] | 207 | PSU | [163] |
| 266 | PBI | [193] | 286 | PPEK | [204] |
| 243 | PF | [181] | 209 | PSU | [165] |
| 258 | Other | [189] | 10 | PFSA | [27] |
| Membrane Chemistry and Structure | Efficiency | Membrane Material and Structure | Cost | ||
|---|---|---|---|---|---|
| CE | VE | EE | |||
| CEM | + | +++ | ++ | fluoro-carbon | + |
| AEM | +++ | + | ++ | hydro-carbon | +++ |
| AIEM | ++ | ++ | +++ | N-heterocycle | ++ |
| dense | +++ | ++ | +++ | dense | +++ |
| sym | + | +++ | ++ | sym | ++ |
| asym | ++ | ++ | ++ | asym | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Düerkop, D.; Widdecke, H.; Schilde, C.; Kunz, U.; Schmiemann, A. Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review. Membranes 2021, 11, 214. https://doi.org/10.3390/membranes11030214
Düerkop D, Widdecke H, Schilde C, Kunz U, Schmiemann A. Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review. Membranes. 2021; 11(3):214. https://doi.org/10.3390/membranes11030214
Chicago/Turabian StyleDüerkop, Dennis, Hartmut Widdecke, Carsten Schilde, Ulrich Kunz, and Achim Schmiemann. 2021. "Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review" Membranes 11, no. 3: 214. https://doi.org/10.3390/membranes11030214






