Interfacial Modulation of Graphene by Polythiophene with Controlled Molecular Weight to Enhance Thermal Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of P3HT with Controllable Molecular Weight
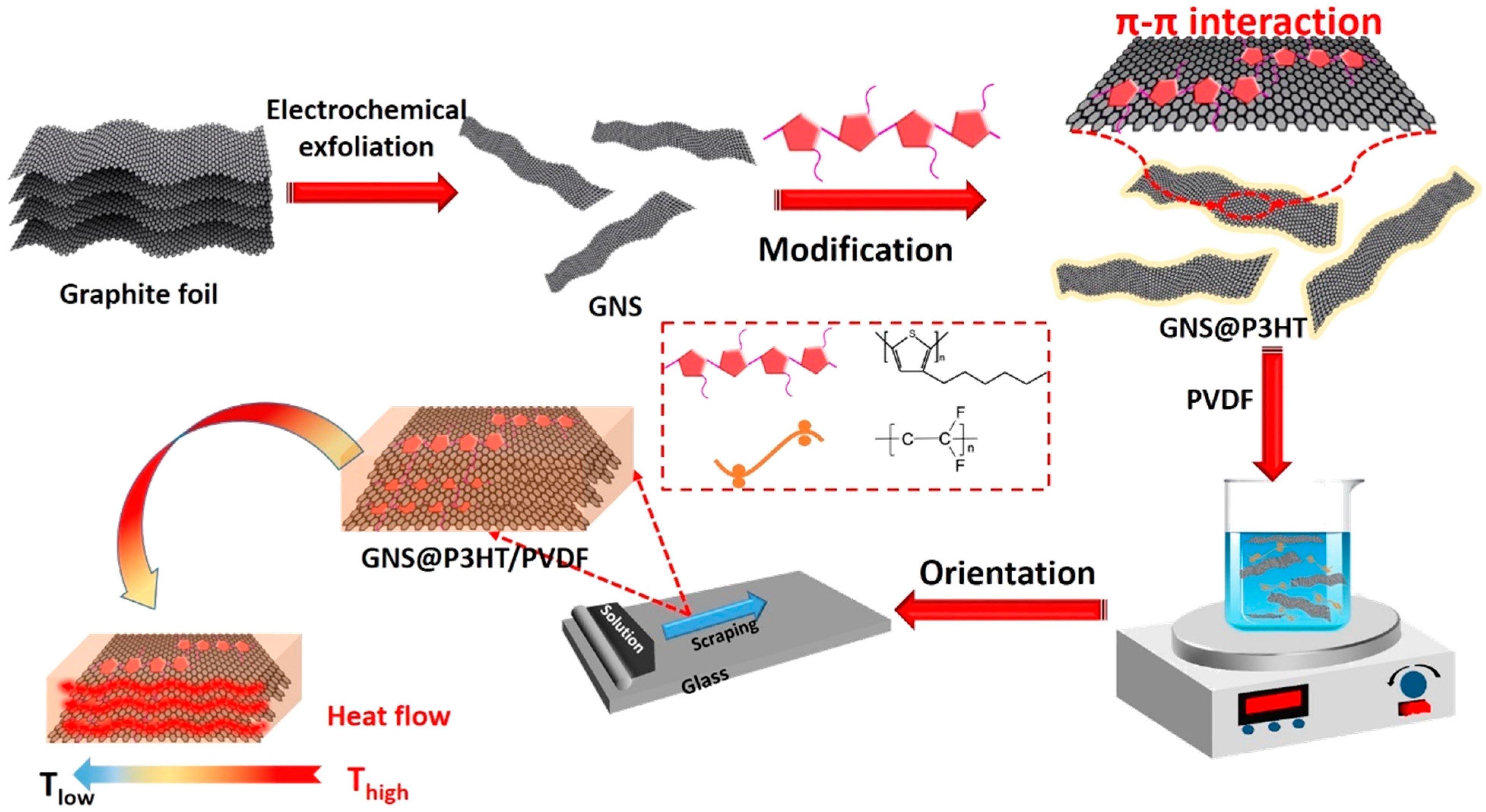
2.3. Preparation of GNS
2.4. Preparation of GNS@P3HT Filler by π–π Stacking
2.5. Fabrication of GNS@P3HT(X)/PVDF Membranes
2.6. Characterization
3. Results and Discussion
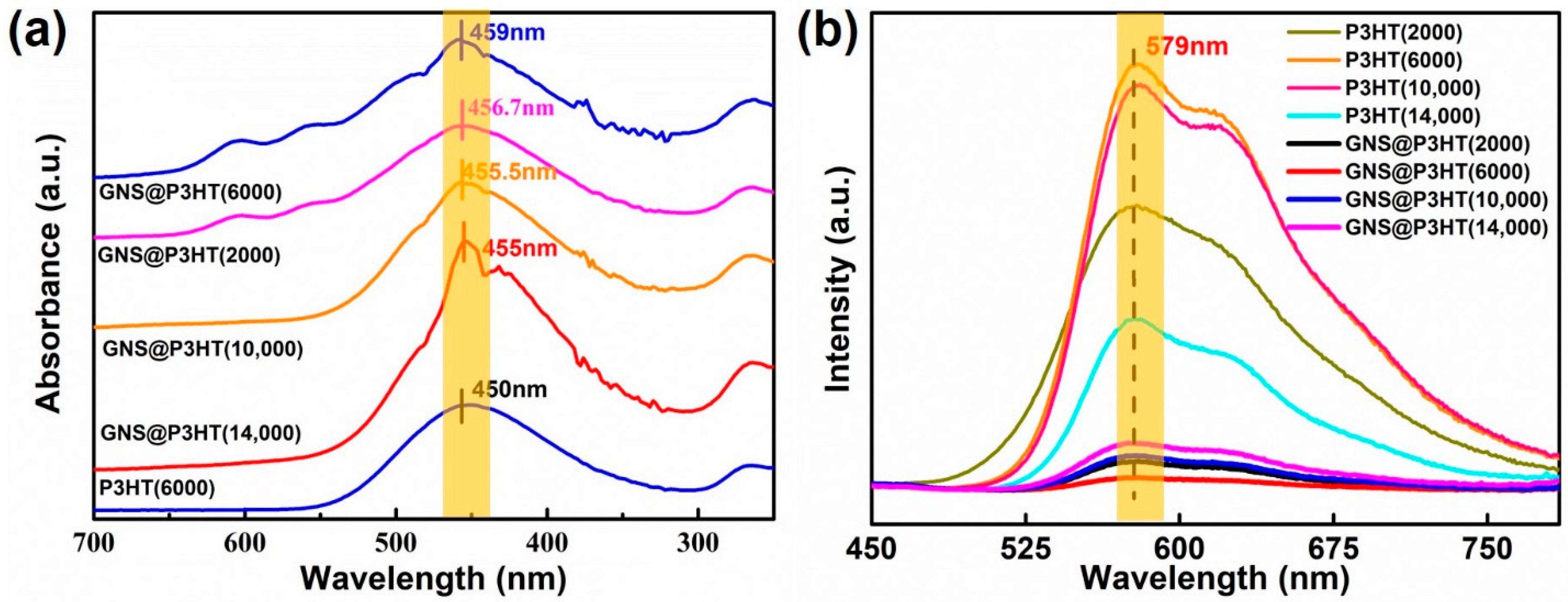
3.1. Structural Characterization of P3HT
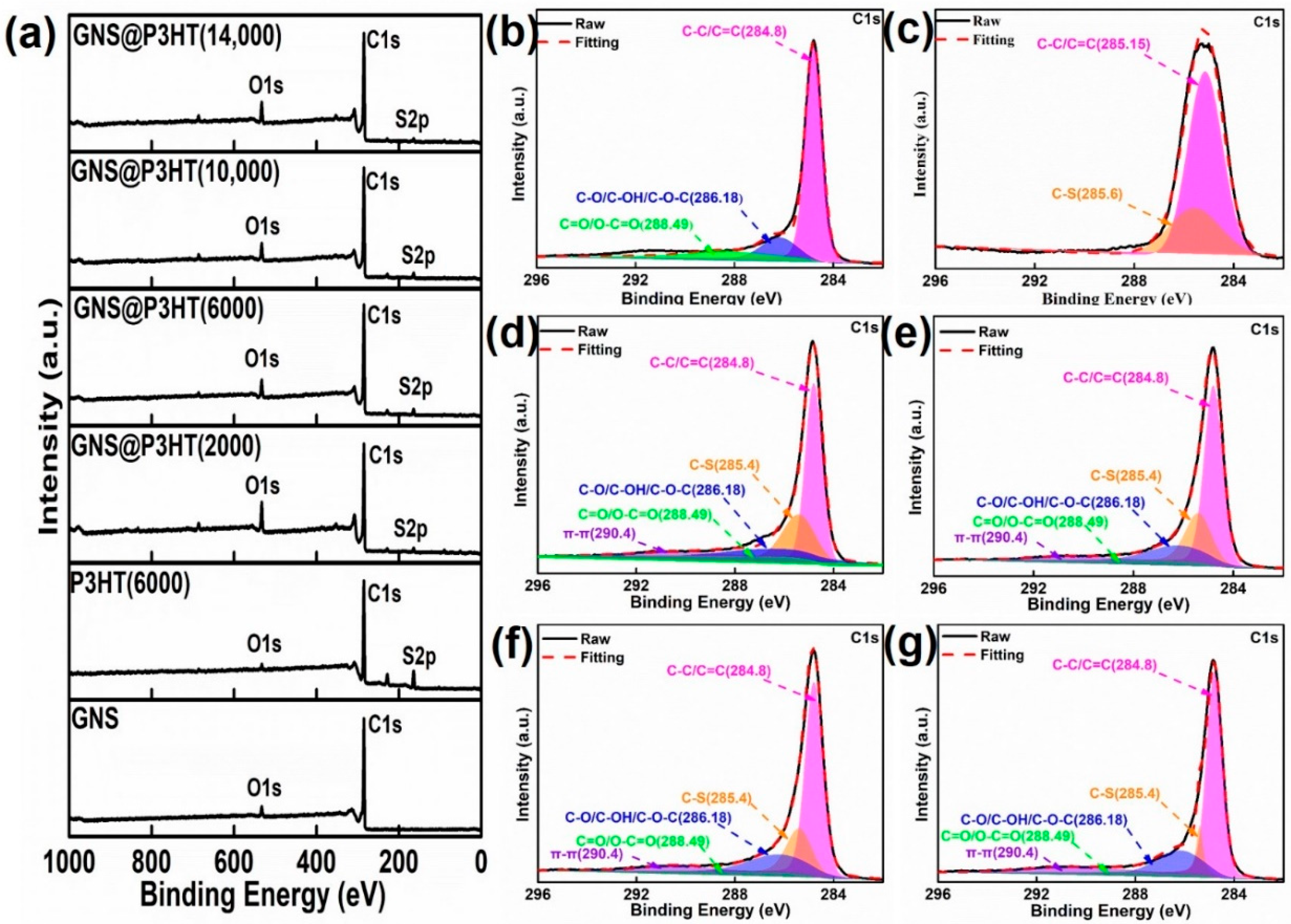
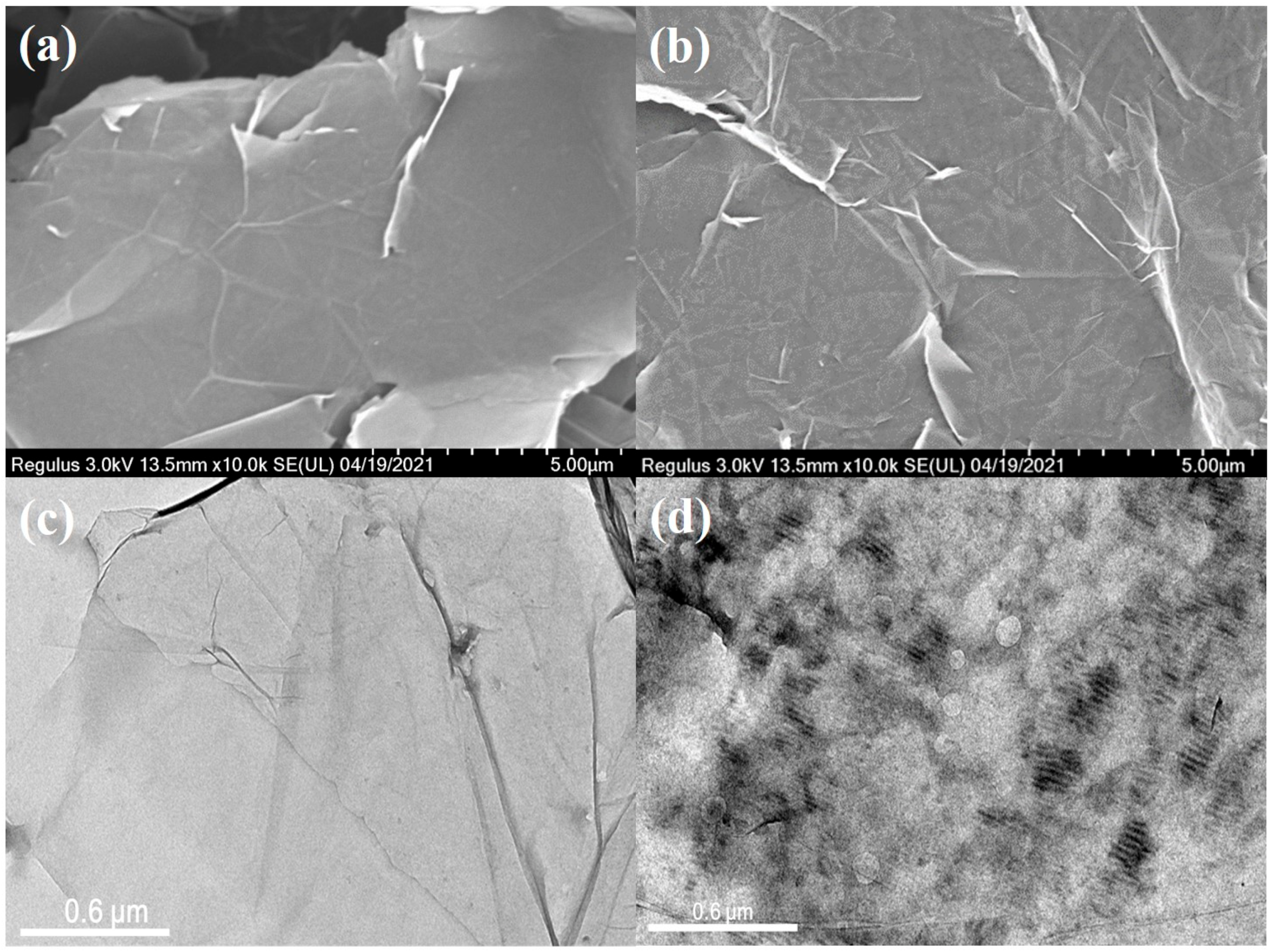
3.2. Fabrication of GNS@P3HT(X) and GNS@P3HT(X)/PVDF Membranes
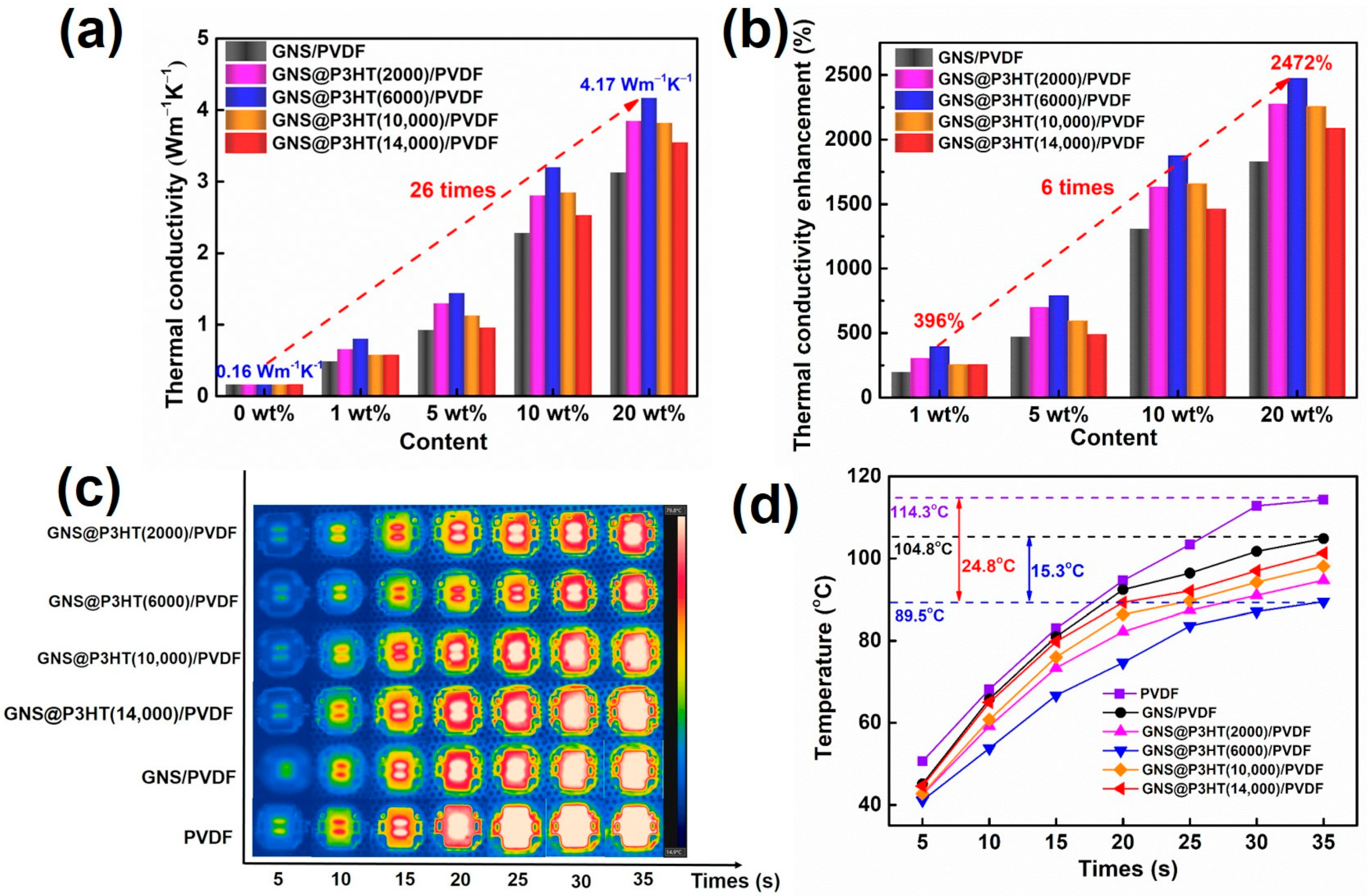
3.3. Thermal Properties of GNS@P3HT/PVDF Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ying, J.; Tan, X.; Lv, L.; Wang, X.; Gao, J.; Yan, Q.; Ma, H.; Nishimura, K.; Li, H.; Yu, J.; et al. Tailoring Highly Ordered Graphene Framework in Epoxy for High-Performance Polymer-Based Heat Dissipation Plates. ACS Nano 2021, 15, 12922–12934. [Google Scholar] [CrossRef]
- Yao, Y.; Ye, Z.; Huang, F.; Zeng, X.; Zhang, T.; Shang, T.; Han, M.; Zhang, W.; Ren, L.; Sun, R.; et al. Achieving Significant Thermal Conductivity Enhancement Via an Ice-Templated and Sintered Bn-Sic Skeleton. ACS Appl. Mater. Interfaces 2020, 12, 2892–2902. [Google Scholar] [CrossRef]
- Pan, X.L.; Debije, M.G.; Schenning, A.P.H.J. High Thermal Conductivity in Anisotropic Aligned Polymeric Materials. ACS Appl. Polym. Mater. 2021, 3, 578–587. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, H.; Fu, Q. Recent Progress on Thermal Conductive and Electrical Insulating Polymer Composites. Compos. Commun. 2018, 8, 74–82. [Google Scholar] [CrossRef]
- Song, H.; Liu, J.; Liu, B.; Wu, J.; Cheng, H.-M.; Kang, F. Two-Dimensional Materials for Thermal Management Applications. Joule 2018, 2, 442–463. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Qiu, H.; Han, Y.; Gu, H.; Song, P.; Wang, L.; Kong, J.; Cao, D.; Gu, J. Superior Electromagnetic Interference Shielding 3d Graphene Nanoplatelets/Reduced Graphene Oxide Foam/Epoxy Nanocomposites with High Thermal Conductivity. J. Mater. Chem. C 2019, 7, 2725–2733. [Google Scholar] [CrossRef]
- Kim, K.; Ju, H.; Kim, J. Vertical Particle Alignment of Boron Nitride and Silicon Carbide Binary Filler System for Thermal Conductivity Enhancement. Compos. Sci. Technol. 2016, 123, 99–105. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar]
- Su, Z.; Wang, H.; Tian, K.; Huang, W.; Guo, Y.; He, J.; Tian, X. Multifunctional Anisotropic Flexible Cycloaliphatic Epoxy Resin Nanocomposites Reinforced by Aligned Graphite Flake with Non-Covalent Biomimetic Functionalization. Compos. Part A Appl. Sci. Manuf. 2018, 109, 472–480. [Google Scholar] [CrossRef]
- Lin, S.; Buehler, M.J. The Effect of Non-Covalent Functionalization on the Thermal Conductance of Graphene/Organic Interfaces. Nanotechnology 2013, 24, 165702–165708. [Google Scholar] [CrossRef]
- Namasivayam, M.; Andersson, M.R.; Shapter, J. Role of Molecular Weight in Polymer Wrapping and Dispersion of Mwnt in a Pvdf Matrix. Polymers 2019, 11, 162. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Luo, Y.; Yang, W.; Ye, X.; Zhang, H.; Zhu, J.; Wu, S. Study on the Thermal and Dielectric Properties of Covalently Modified Go/Xnbr Composites. Mater. Des. 2021, 198, 109335. [Google Scholar] [CrossRef]
- Singh, V.; Bougher, T.L.; Weathers, A.; Cai, Y.; Bi, K.; Pettes, M.T.; McMenamin, S.A.; Lv, W.; Resler, D.P.; Gattuso, T.R.; et al. High Thermal Conductivity of Chain-Oriented Amorphous Polythiophene. Nat. Nanotechnol. 2014, 9, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Taphouse, J.H.; Smith, O.L.; Marder, S.R.; Cola, B.A. A Pyrenylpropyl Phosphonic Acid Surface Modifier for Mitigating the Thermal Resistance of Carbon Nanotube Contacts. Adv. Funct. Mater. 2014, 24, 465–471. [Google Scholar] [CrossRef]
- Osaka, I.; McCullough, R.D. Advances in Molecular Design and Synthesis of Regioregular Polythiophenes. Acc. Chem. Res. 2008, 41, 1202–1214. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhou, J.; Song, B.; Jiang, Z.; Lee, E.M.Y.; Huberman, S.; Gleason, K.K.; Chen, G. Molecular Engineered Conjugated Polymer with High Thermal Conductivity. Sci. Adv. 2018, 4, 3031–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvez, K.; Wu, Z.-S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Mullen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [Green Version]
- Hiorns, R.C.; Khoukh, A.; Gourdet, B.; Dagron-Lartigau, C. Extremely Regio-Regular Poly(3-Alkylthiophene)S from Simplified Chain-Growth Grignard Metathesis Polymerisations and the Modification of Their Chain-Ends. Polym. Int. 2006, 55, 608–620. [Google Scholar] [CrossRef]
- Miyakoshi, R.; Yokoyama, A.; Yokozawa, T. Catalyst-Transfer Polycondensation. Mechanism of Ni-Catalyzed Chain-Growth Polymerization Leading to Well-Defined Poly(3-Hexylthiophene). J. Am. Chem. Soc. 2005, 127, 17542–17547. [Google Scholar] [CrossRef]
- Jang, K.S.; Eom, Y.S.; Lee, T.W.; Kim, D.O.; Oh, Y.S.; Jung, H.C.; Nam, J.D. Fabrication of Poly(3-Hexylthiophene) Thin Films by Vapor-Phase Polymerization for Optoelectronic Device Applications. ACS Appl. Mater. Interfaces 2009, 1, 1567–1571. [Google Scholar] [CrossRef]
- Nicho, M.E.; García-Escobar, C.H.; Arenas, M.C.; Altuzar-Coello, P.; Cruz-Silva, R.; Güizado-Rodríguez, M. Influence of P3ht Concentration on Morphological, Optical and Electrical Properties of P3ht/Ps and P3ht/Pmma Binary Blends. Mater. Sci. Eng. B 2011, 176, 1393–1400. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, J.; Singh, R.; Kant, R.; Chand, S.; Kumar, V. Micromorphology, Photophysical and Electrical Properties of Pristine and Ferric Chloride Doped Poly(3-Hexylthiophene) Films. Mater. Chem. Phys. 2007, 104, 390–396. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, J.; Singh, R.; Kant, R.; Rastogi, R.C.; Chand, S.; Kumar, V. Structure–Conductivity Correlation in Ferric Chloride-Doped Poly(3-Hexylthiophene). New J. Phys. 2006, 8, 112. [Google Scholar] [CrossRef]
- Stefan, M.C.; Bhatt, M.P.; Sista, P.; Magurudeniya, H.D. Grignard Metathesis (Grim) Polymerization for the Synthesis of Conjugated Block Copolymers Containing Regioregular Poly(3-Hexylthiophene). Polym. Chem. 2012, 3, 1693–1701. [Google Scholar] [CrossRef]
- Novak, T.G.; Kim, J.; Song, S.H.; Jun, G.H.; Kim, H.; Jeong, M.S.; Jeon, S. Fast P3ht Exciton Dissociation and Absorption Enhancement of Organic Solar Cells by Peg-Functionalized Graphene Quantum Dots. Small 2016, 12, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cai, K.F.; Shen, S.Z.; Casey, P.S. Preparation and Characterization of Graphene Nanosheets/Poly(3-Hexylthiophene) Thermoelectric Composite Materials. Synth. Met. 2012, 162, 2102–2106. [Google Scholar] [CrossRef]
- Presto, D.; Song, V.; Boucher, D. P3ht/Graphene Composites Synthesized Using in Situ Grim Methods. J. Polym. Sci. Part B Polym. Phys. 2016, 55, 60–76. [Google Scholar] [CrossRef]
- Resmi, R.; Amrutha, S.R.; Jayakannan, M. Control of Molecular Aggregation in Symmetrically Substituted Π-Conjugated Bulky Poly(P-Phenylenevinylene)S and Their Copolymers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2631–2646. [Google Scholar] [CrossRef]
- Xiao, K.; Shen, Y.; Sun, J.; Liang, S.; Fan, H.; Tan, J.; Wang, X.; Huang, X.; Waite, T.D. Correlating Fluorescence Spectral Properties with Dom Molecular Weight and Size Distribution in Wastewater Treatment Systems. Environ. Sci. Water Res. Technol. 2018, 4, 1933–1943. [Google Scholar] [CrossRef]
- Zheng, J.; Zong, Y.; Zhao, G.; Yu, Z.; Wang, M.; Zhu, C.; Li, C.; Liu, J.; Gui, D. Nematic Liquid Crystal 4-Cyano-4′-Pentylbiphenyl Functionalization of Mwnts for Improved Thermal and Mechanical Properties of Silicone Pressure Sensitive Adhesives. Int. J. Adhes. Adhes. 2019, 98, 102457–102461. [Google Scholar] [CrossRef]
- Wei, C.; Li, J.; Xiao, X.; Yue, T.; Zhao, D. The One-Step Preparation of Green-Emission Carbon Dots Based on the Deactivator-Reducing Reagent Synergistic Effect and the Study on Their Luminescence Mechanism. RSC Adv. 2018, 8, 20016–20024. [Google Scholar] [CrossRef] [Green Version]
- Xiao, K.; Sun, J.-Y.; Shen, Y.-X.; Liang, S.; Liang, P.; Wang, X.-M.; Huang, X. Fluorescence Properties of Dissolved Organic Matter as a Function of Hydrophobicity and Molecular Weight: Case Studies from Two Membrane Bioreactors and an Oxidation Ditch. RSC Adv. 2016, 6, 24050–24059. [Google Scholar] [CrossRef]
- Shang, C.; Wei, N.; Zhuo, H.; Shao, Y.; Zhang, Q.; Zhang, Z.; Wang, H. Highly Emissive Poly(Maleic Anhydride-Alt-Vinyl Pyrrolidone) with Molecular Weight-Dependent and Excitation-Dependent Fluorescence. J. Mater. Chem. C 2017, 5, 8082–8090. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, J.; Tang, P.; Qiu, F. Preparation and Characterization of Highly Stable and Aqueous Dispersion of Conjugated Polyelectrolyte/Single-Walled Carbon Nanotube Nanocomposites. Acta Chim. Sin. 2018, 76, 453–459. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Shi, J.; Shi, G.; Cao, S. Nonvolatile Rewritable Memory Device Based on Solution-Processable Graphene/Poly(3 Hexylthiophene) Nanocomposite. Mater. Chem. Phys. 2013, 142, 626–632. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films Via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. JACS 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Mahakul, P.C.; Sa, K.; Subramanyam, B.V.R.S.; Patra, K.C.; Mahanandia, P. Investigation of Optical and Electrical Properties of Mwcnt/Rgo/Poly(3-Hexylthiophene) Ternary Composites. J. Mater. Sci. 2018, 53, 8151–8160. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.; Durstock, M.; Baek, J.-B.; Dai, L. Soluble P3ht-Grafted Graphene for Efficient Bilayer-Heterojunction Photovoltaic Devices. ACS Nano 2010, 4, 5633–5640. [Google Scholar] [CrossRef] [Green Version]
- Hilal, M.; Han, J.I. Significant Improvement in the Photovoltaic Stability of Bulk Heterojunction Organic Solar Cells by the Molecular Level Interaction of Graphene Oxide with a Pedot: Pss Composite Hole Transport Layer. Sol. Energy 2018, 167, 24–34. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal Conductivity and Structure of Non-Covalent Functionalized Graphene/Epoxy Composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Hontoria-Lucas, C.; López-Peinado, A.J.; de López-González, J.D.; Rojas-Cervantes, M.L.; Martín-Aranda, R.M. Study of Oxygen-Containing Groups in a Series of Graphite Oxides: Physical and Chemical Characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Hao, L.; Kang, J.; Shi, J.; Xu, J.; Cao, J.; Wang, L.; Liu, Y.; Pan, C. Enhanced Thermoelectric Performance of Poly(3-Substituted Thiophene)/Single-Walled Carbon Nanotube Composites Via Polar Side Chain Modification. Compos. Sci. Technol. 2020, 199, 108359. [Google Scholar] [CrossRef]
- Xia, H.; Ye, Z.; Liu, X.; Peng, J.; Qiu, F. Synthesis, Characterization, and Solution Structure of All-Conjugated Polyelectrolyte Diblock Copoly(3-Hexylthiophene)S. RSC Adv. 2014, 4, 19646–19653. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X. Improved Dispersibility of Graphene Oxide in O-Dichlorobenzene by Adding a Poly(3-Alkylthiophene). Carbon 2012, 50, 4566–4572. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Grey, J.K. Resonance Chemical Imaging of Polythiophene/Fullerene Photovoltaic Thin Films: Mapping Morphology-Dependent Aggregated and Unaggregated Cdc Species. J. Am. Chem. Soc. 2009, 131, 9654–9662. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Martin, T.P.; Thomas, A.K.; Grey, J.K. Resonance Raman Spectroscopic- and Photocurrent Imaging of Polythiophene/Fullerene Solar Cells. J. Phys. Chem. Lett. 2009, 1, 178–182. [Google Scholar] [CrossRef]
- Wang, T.; Jing, L.C.; Zhu, Q.; Ethiraj, A.S.; Fan, X.; Liu, H.; Tian, Y.; Zhu, Z.; Meng, Z.; Geng, H.Z. Tannic Acid Modified Graphene/Cnt Three-Dimensional Conductive Network for Preparing High-Performance Transparent Flexible Heaters. J. Colloid Interface Sci. 2020, 577, 300–310. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Wen, Y.; Liu, J.; Li, X.; Zeng, H.; Xue, Z.; Zhou, X.; Xie, X. Noncovalent Engineering of Carbon Nanotube Surface by Imidazolium Ionic Liquids: A Promising Strategy for Enhancing Thermal Conductivity of Epoxy Composites. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105517–105526. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Y.; Lin, Y.; Yan, H.; Zhang, W.; Bao, Y.; Ma, J. Enhanced Mechanical and Tribological Properties of Graphene/Bismaleimide Composites by Using Reduced Graphene Oxide with Non-Covalent Functionalization. Compos. Part B Eng. 2019, 165, 491–499. [Google Scholar] [CrossRef]
- Ryu, S.H.; Cho, H.-B.; Kwon, Y.-T.; Song, Y.; Lee, J.; Lee, S.-B.; Choa, Y.-H. Quasi-Isotropic Thermal Conduction in Percolation Networks: Using the Pore-Filling Effect to Enhance Thermal Conductivity in Polymer Nanocomposites. ACS Appl. Polym. Mater. 2020, 3, 1293–1305. [Google Scholar] [CrossRef]
- Michael, S.; Nadiv, R.; Buzaglo, M.; Kahil, K.; Regev, O. Thermally Conductive Graphene-Polymer Composites: Size, Percolation, and Synergy Effects. Chem. Mater. 2015, 27, 2100–2106. [Google Scholar]
- Kargar, F.; Barani, Z.; Salgado, R.; Debnath, B.; Lewis, J.S.; Aytan, E.; Lake, R.K.; Balandin, A.A. Thermal Percolation Threshold and Thermal Properties of Composites with High Loading of Graphene and Boron Nitride Fillers. ACS Appl. Mater. Interfaces 2018, 10, 37555–37565. [Google Scholar] [CrossRef]
- Qi, X.; Pu, K.Y.; Li, H.; Zhou, X.; Wu, S.; Fan, Q.L.; Liu, B.; Boey, F.; Huang, W.; Zhang, H. Amphiphilic Graphene Composites. Angew. Chem. Int. Ed. Engl. 2010, 49, 9426–9429. [Google Scholar] [CrossRef] [PubMed]



| Mn (g/mol) | Molar Ratio of CH3BrMg/C10H14Br2S | Molar Ratio of C10H14Br2S/Ni(dppp)Cl2 | T/°C | t/h |
|---|---|---|---|---|
| 2000 | 1.1:1 | 100:1 | 25 | 2 |
| 6000 | 1.1:1 | 100:1 | 30 | 2 |
| 10,000 | 1.1:1 | 100:1 | 40 | 2 |
| 14,000 | 1.1:1 | 125:1 | 40 | 2 |
| Sample | C (%) | S (%) | S/C (%) |
|---|---|---|---|
| GNS | 94.66 | - | - |
| P3HT (6000) | 89.1 | 8.48 | 9.52 |
| GNS@P3HT (2000) | 79.64 | 2.62 | 3.29 |
| GNS@P3HT (6000) | 89.44 | 3.1 | 3.47 |
| GNS@P3HT (10,000) | 84.49 | 2.13 | 2.52 |
| GNS@P3HT (14,000) | 88.73 | 1.78 | 2.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Y.; Chen, P.; Xia, R.; Wu, B.; Qian, J. Interfacial Modulation of Graphene by Polythiophene with Controlled Molecular Weight to Enhance Thermal Conductivity. Membranes 2021, 11, 895. https://doi.org/10.3390/membranes11110895
Li Y, Wang Y, Chen P, Xia R, Wu B, Qian J. Interfacial Modulation of Graphene by Polythiophene with Controlled Molecular Weight to Enhance Thermal Conductivity. Membranes. 2021; 11(11):895. https://doi.org/10.3390/membranes11110895
Chicago/Turabian StyleLi, Ya, Yu Wang, Peng Chen, Ru Xia, Bin Wu, and Jiasheng Qian. 2021. "Interfacial Modulation of Graphene by Polythiophene with Controlled Molecular Weight to Enhance Thermal Conductivity" Membranes 11, no. 11: 895. https://doi.org/10.3390/membranes11110895







