The Synthesis and Characterization of Novel Bi-/Trimetallic Nanoparticles and Their Nanocomposite Membranes for Envisaged Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanoparticles and Nanocomposite Membranes
2.2.1. One-Pot Synthesis of HPEI-Stabilized Zerovalent Nanoparticles
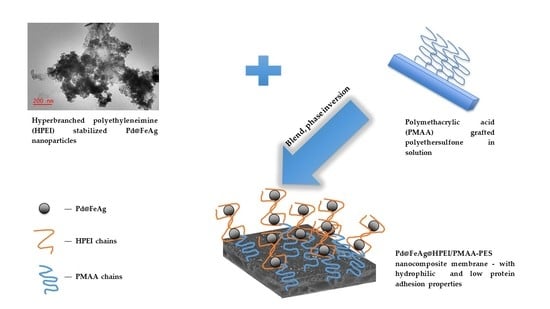
2.2.2. Nanocomposite Membrane Fabrication
2.3. Characterization of the Composite Membranes
2.3.1. ATR-FT-IR Analysis
2.3.2. XPS Analysis
2.3.3. XRD Analysis
2.3.4. ICP-OES Analysis
2.3.5. SEM Analysis
2.3.6. AFM Analysis
2.3.7. HRTEM Analysis
2.3.8. Membrane Hydrophilicity, Surface Tension, and Surface Free Energy Analyses
2.3.9. Water Uptake, Pore Size, Porosity, and Pure Water Permeation Fluxes
2.3.10. Protein Adhesion
3. Results and Discussion
3.1. Surface Chemistry
3.2. Membrane and Nanoparticle Morphology
3.3. Determination of Membrane-Embedded Metal Loading
3.4. Membrane Hydrophilicity and Water Interactions
3.5. Water Uptake and Porosity
3.6. Pore Size, Pure Water Permeation, and Protein Adhesion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, B.; Chen, X.; Li, K.; Zhang, C.; He, Y.; Du, R.; Wang, J.; Chen, L. Coupling Membrane and Fe–Pd Bimetallic Nanoparticles for Trichloroethene Removing from Water. J. Ind. Eng. Chem. 2019, 78, 198–209. [Google Scholar] [CrossRef]
- Dube, S.T.; Moutloali, R.M.; Malinga, S.P. Hyperbranched Polyethyleneimine/Multi-Walled Carbon Nanotubes Polyethersulfone Membrane Incorporated with Fe-Cu Bimetallic Nanoparticles for Water Treatment. J. Environ. Chem. Eng. 2020, 8, 103962. [Google Scholar] [CrossRef]
- Wan, H. Bimetallic Nanoparticles Integrated Membranes for Groundwater Remediation: Synthesis, Characterization and Applications. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2020. [Google Scholar]
- Malinga, S.P. β-Cyclodextrin Dendritic-Polymers and Nanostructured Materials for Water Treatment. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2013. [Google Scholar]
- Hou, X.; Chen, X.; Bi, S.; Li, K.; Zhang, C.; Wang, J.; Zhang, W. Catalytic Degradation of TCE by a PVDF Membrane with Pd-Coated Nanoscale Zero-Valent Iron Reductant. Sci. Total Environ. 2020, 702, 135030. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Lu, M.; Liang, L.; Zhang, H.; Wei, J. Biosynthesis Based Membrane Filtration Coupled with Iron Nanoparticles Reduction Process in Removal of Dyes. Chem. Eng. J. 2020, 387, 124202. [Google Scholar] [CrossRef]
- He, F.; Zhao, D.; Liu, J.; Roberts, C.B. Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichlorotheylene in soil and groundwater. Ind. Eng. Chem. 2007, 46, 29–34. [Google Scholar] [CrossRef]
- Tso, C.-P.; Shih, Y.-H. The influence of carboxymethylcellulose on the reactivity of Fe toward debrominated phenyl ether: The Ni doping, temperature, pH and anion effects. J. Hazard. Mater. 2017, 322, 145–151. [Google Scholar] [CrossRef]
- Ndlwana, L.; Sikhwivhilu, K.; Moutloali, R.M.; Ngila, J.C. Microwave assisted graft synthesis and characterization of polyethersulfone-g-poly(methacrylic acid). J. Phys. Chem. Earth A/B/C 2018, 106, 107–115. [Google Scholar] [CrossRef]
- Matabola, K.P.; Bambo, M.F.; Sikhwivhilu, K.; Vatsha, B.; Moutloali, R.M. Chemical Grafting of Polystyrene Sodium Sulfonate (PSS) onto Polyethersulfone (PES) Powder and Effect on the Characteristics of the Resultant Ultrafiltration Membranes. Mater. Today Proc. 2015, 2, 3957–3963. [Google Scholar] [CrossRef]
- Luo, L.; Chung, T.-S.; Weber, M.; Saudt, C.; Maletzko, C. Molecular interaction between acidic PPSU and basic HPEI polymers and its effects on membrane formation for ultrafiltration. J. Membr. Sci. 2017, 24, 33–42. [Google Scholar] [CrossRef]
- Vetriselvi, V.; Santhi Raj, R.J.J. Synthesis and characterization of poly acrylic acid modified dihydroxy benzene-redox polymer. Res. J. Chem. Sci. 2017, 4, 1–9. [Google Scholar]
- Shelyapina, M.G.; Gurgul, J.; Łątka, K.; Sánchez-López, P.; Bogdanov, D.; Kotolevich, Y.; Petranovskii, V.; Fuentes, S. Mechanism of Formation of Framework Fe3+ in Bimetallic Ag-Fe Mordenites—Effective Catalytic Centers for DeNOx Reaction. Microporous Mesoporous Mater. 2020, 299, 109841. [Google Scholar] [CrossRef]
- Mokete, R.; Eljamal, O.; Sugihara, Y. Exploration of the Reactivity of Nanoscale Zero-Valent Iron (NZVI) Associated Nanoparticles in Diverse Experimental Conditions. Chem. Eng. Process. Process. Intensif. 2020, 150, 107879. [Google Scholar] [CrossRef]
- Li, B.; Liu, W.; Zhao, X.G.; Ma, S.; Gong, W.J.; Feng, J.N.; Wang, F.; Zhang, Z.D. Ordering temperature of L10-FePd film reduced by Ag underlayer. Mater. Lett. 2013, 100, 58–61. [Google Scholar] [CrossRef]
- Jiang, G.; Li, X.; Lv, X.; Chen, L. Core/shell Fe/Pd/Pd catalyst with a superior activity to Pt in oxygen reduction reaction. Sci. Bull. 2016, 61, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Chen, G.-E.; Sun, W.-G.; Xu, Z.-L.; Kong, Y.-F.; Zheng, X.-P.; Xu, S.-J. Bio-inspired GO-Ag/PVDF/F127 membrane with improved anti-fouling for natural organic matter (NOM) resistance. Chem. Eng. J. 2017, 313, 450–460. [Google Scholar] [CrossRef]
- Xu, B.; Li, X.; Chen, Z.; Zhang, T.; Li, C. Pd@MIL-100(Fe) composite nanoparticles as efficient catalyst for the reduction of 2/3/4-nitrophenol: Synergistic effect between Pd and MIL-100(Fe). Microporous Mesoporous Mater. 2018, 255, 1–6. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, Y.-F.; Wang, J.; Chang, P.-J.; Tasi, C.-P.; Lu, C.-C.; Chiu, H.-T.; Yang, Y.-W. Correlation between N 1s XPS binding energy and bond distance inmetalamido, imido, and nitride complexes. Inorg. Chem. 2003, 42, 4516–4518. [Google Scholar] [CrossRef]
- Chauque, E.F.C.; Ngila, J.C.; Ray, S.C.; Ndlwana, L. Degradation of methyl orange on Fe/Ag nanoparticles immobilized on polyacrylonitrile nanofibers using EDTA chelating agents. J. Environ. Manag. 2019, 236, 481–489. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q.; Yang, H.; Alam, E.; Gao, B.; Gu, D. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: Characterization, kinetics and mechanisms. Colloids Surf. A Physiochemical Eng. Asp. 2017, 517, 63–71. [Google Scholar] [CrossRef]
- Yang, S.; Dong, J.; Yao, Z.; Shen, C.; Shi, S.; Tian, Y.; Lin, S.; Zhang, X. One-pot synthesis of graphene-supported monodisperse Pd nanoparticles as catalyst for formic acid electro-oxidation. Sci. Rep. 2014, 4, 4501–4506. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Yola, M.L.; Eren, T.; Kartal, F.; Caglayan, M.O.; Atar, N. Catalytic activity of Fe@Ag nanoparticle involved in calcium alginate beads for the reduction of nitrophenols. J. Mol. Liq. 2014, 190, 133–138. [Google Scholar] [CrossRef]
- Schrick, B.; Blough, J.L.; Jones, A.D.; Mallouk, T.E. Hydrodechlorination of Trichloroethylene to Hydrocarbons Using Bimetallic Nickel-Iron Nanoparticles. Chem. Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Sidkar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for the reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Choi, S.-I. Degradation of PCE, TCE and 1,1,1-TCA by nanosized FePd bimetallic nanoparticles under various experimental conditions. Chemosphere 2010, 81, 940–945. [Google Scholar] [CrossRef]
- Deng, B.; Yang, X.; Xie, L.; Li, J.; Hou, Z.; Yao, S.; Liang, G.; Sheng, K.; Huang, Q. Microfiltration membranes with pH-dependent property prepared from poly(methacrylic acid) grafted polyerthersulfone powder. J. Membr. Sci. 2009, 330, 363–368. [Google Scholar] [CrossRef]
- Masheane, M.L.; Nthunya, L.N.; Malinga, S.P.; Nxumalo, E.N.; Mamba, B.B.; Mhlanga, S.D. Synthesis of Fe-Ag/f-MWCNT/PES nanostructured-hybrid membranes for removal of Cr(VI) from water. Sep. Purif. Technol. 2017, 184, 79–87. [Google Scholar] [CrossRef]
- Prince, J.A.; Bhuvana, S.; Anbharasi, V.; Ayyanar, N.; Boodhoo, K.V.K.; Singh, G. Self-cleaning Metal Organic Framework (MOF) based ultra filtration membranes—A solution to bio-fouling in membrane separation processes. Sci. Rep. 2014, 4, 6555. [Google Scholar] [CrossRef] [Green Version]
- Dlamini, D.S.; Mamba, B.B.; Li, J. The role of nanoparticles in the performance of nano-enabled composite membranes—A critical scientific perspective. Sci. Total Environ. 2019, 656, 723–731. [Google Scholar] [CrossRef]
- Van Oss, C.J. Use of the Combined Lifshitz-van der Waals and Lewis acid-base Approaches in Determining the Apolar and Polar Contributions to Surface and Interfacial Tensions and Free Energies. J. Adhes. Sci. Technol. 2002, 16, 669–677. [Google Scholar] [CrossRef]
- Subhi, N.; Verliefde, A.R.D.; Chen, V.; Le-Clech, P. Assessment of Physicochemical Interactions in Hollow Fibre Ultrafiltration Membrane by Contact Angle Analysis. J. Membr. Sci. 2012, 403, 32–40. [Google Scholar] [CrossRef]
- Hwang, G.; Yang, J.-H.; Lee, C.-H.; Ahn, I.-S.; Mhin, B.J. New Selection Criterion for a Base Polar Liquid in the Lifshitz-van der Waals/Lewis Acid-Base Approach. J. Phys. Chem. C 2011, 115, 12458–12463. [Google Scholar] [CrossRef]
- Dumée, L.F.; Gray, S.; Duke, M.; Sears, K.; Schütz, J.; Finn, N. The Role of Membrane Surface Energy on Direct Contact Membrane Distillation Performance. Desalination 2013, 323, 22–30. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z.; Jagota, A.; Hui, C.-Y. Droplets on an Elastic Membrane: Configurational Energy Balance and Modified Young Equation. J. Mech. Phys. Solids 2020, 138, 103902. [Google Scholar] [CrossRef]
- Ndlwana, L.; Motsa, M.M.; Mamba, B.B. A unique method for dopamine-cross-linked graphene nanoplatelets within polyethersulfone membranes (GNP-pDA/PES) for enhanced mechanochemical resistance during NF and RO desalination. Eur. Polym. J. 2020, 135, 109889. [Google Scholar] [CrossRef]
- Arumugham, T.; Amimodu, R.G.; Kaleekkal, N.J.; Rana, D. Nano CuO/g-C3N4 sheets-based ultrafiltration membrane with enhanced interfacial affinity, antifouling and protein separation performances for water treatment application. J. Environ. Sci. 2019, 82, 57–69. [Google Scholar] [CrossRef]







| Membrane | |
|---|---|
| Short Name | Long Name |
| PES0 | Pristine PES |
| A0 (Ctrl) | PMAA-PES (Control) |
| A1 | Pd@Fe@HPEI/PMAA-PES |
| A2 | Pd@FeAg@HPEI/PMAA-PES |
| Membrane | Fe (mg) | Pd (mg) | Ag (mg) | Total (mg) |
|---|---|---|---|---|
| A1 | 18.5 ± 0.7 | 2.1 ± 0.2 | - | 20.6 ± 0.9 |
| A2 | 16.5 ± 0.4 | 1.7 ± 0.1 | 2.1 ± 0.2 | 21.7 ± 0.7 |
| Membrane | Contact Angle | Total ΔG (mJ/m2) | Apolar | Polar |
|---|---|---|---|---|
| PES0 | 72 ± 4 | 48.53 | 22.16 | 26.37 |
| A0 | 41 ± 2 | 43.18 | 21.64 | 21.54 |
| A1 | 38 ± 2 | 41.47 | 24.59 | 16.88 |
| A2 | 35 ± 2 | 40.79 | 25.21 | 15.58 |
| Membrane | Pore Size (µm) | Pure Water Flux (L/m2 h) | BSA Adsorption (µg cm−2) |
|---|---|---|---|
| PES0 | 0.280 | 89 ± 6 | 10.3 ± 0.7 |
| A0 | 0.377 | 277 ± 13 | 3.96 ± 0.5 |
| A1 | 0.227 | 136 ± 9 | 1.42 ± 0.2 |
| A2 | 0.022 | 107 ± 5 | 0.62 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndlwana, L.; Sikhwivhilu, K.; Moutloali, R.M.; Ngila, J.C. The Synthesis and Characterization of Novel Bi-/Trimetallic Nanoparticles and Their Nanocomposite Membranes for Envisaged Water Treatment. Membranes 2020, 10, 232. https://doi.org/10.3390/membranes10090232
Ndlwana L, Sikhwivhilu K, Moutloali RM, Ngila JC. The Synthesis and Characterization of Novel Bi-/Trimetallic Nanoparticles and Their Nanocomposite Membranes for Envisaged Water Treatment. Membranes. 2020; 10(9):232. https://doi.org/10.3390/membranes10090232
Chicago/Turabian StyleNdlwana, Lwazi, Keneiloe Sikhwivhilu, Richard Motlhaletsi Moutloali, and Jane Catherine Ngila. 2020. "The Synthesis and Characterization of Novel Bi-/Trimetallic Nanoparticles and Their Nanocomposite Membranes for Envisaged Water Treatment" Membranes 10, no. 9: 232. https://doi.org/10.3390/membranes10090232