The Water Flux Dynamic in a Hybrid Forward Osmosis-Membrane Distillation for Produced Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane and Materials
2.2. Properties of Feed and Draw Solution
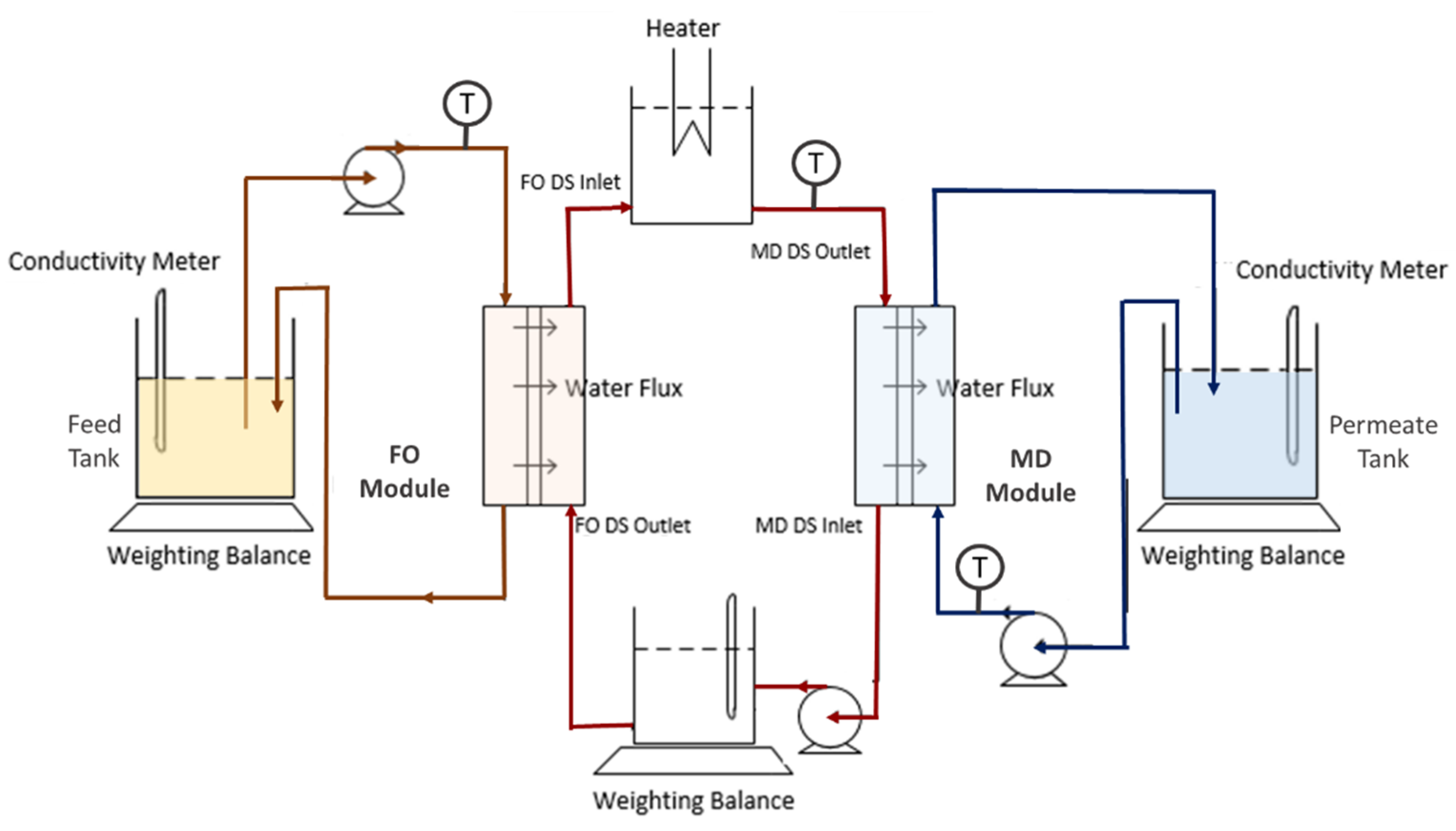
2.3. Filtration Setup
3. Results and Discussion
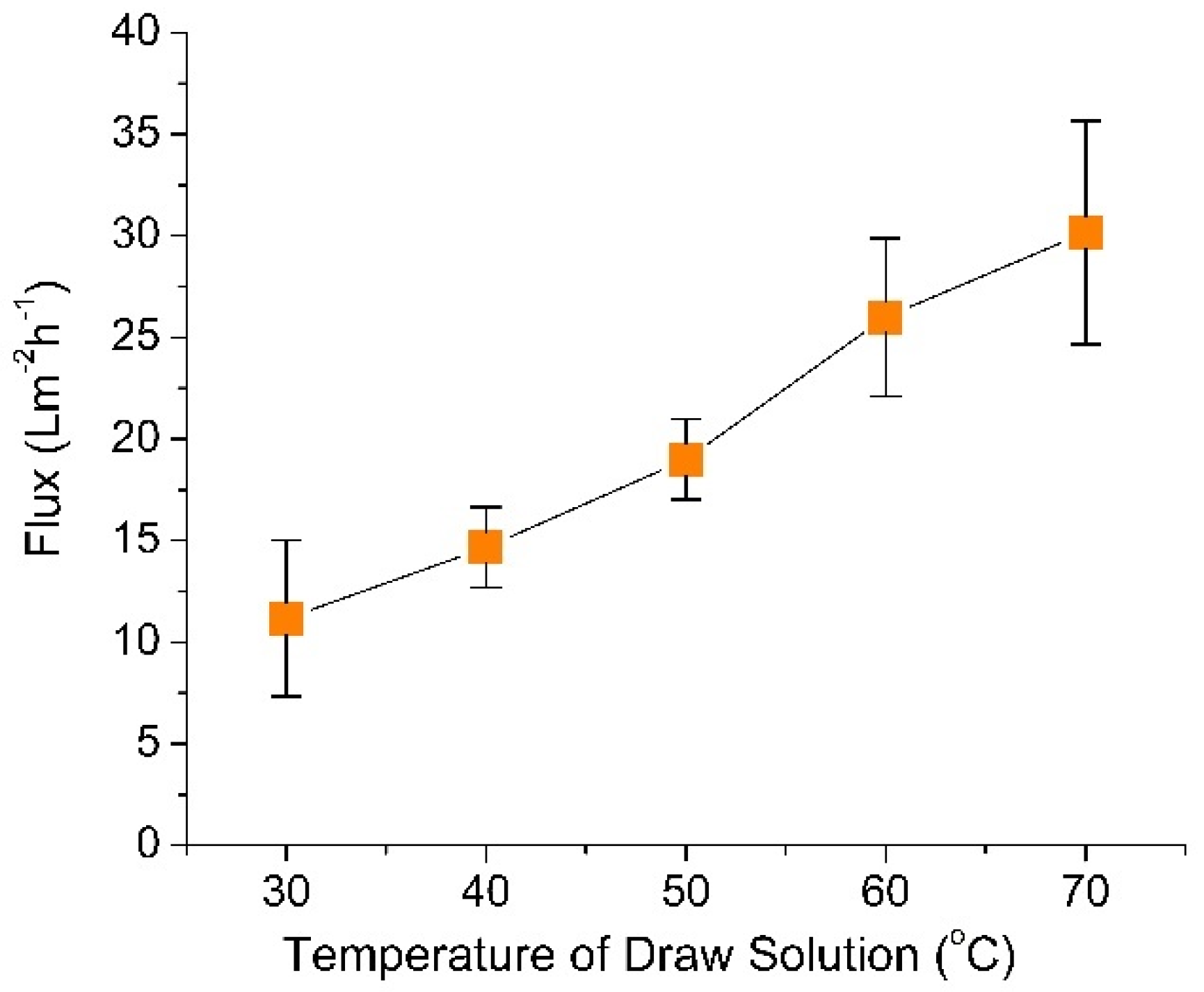
3.1. Effect of Draw Solution Temperature on the Standalone FO Water Flux
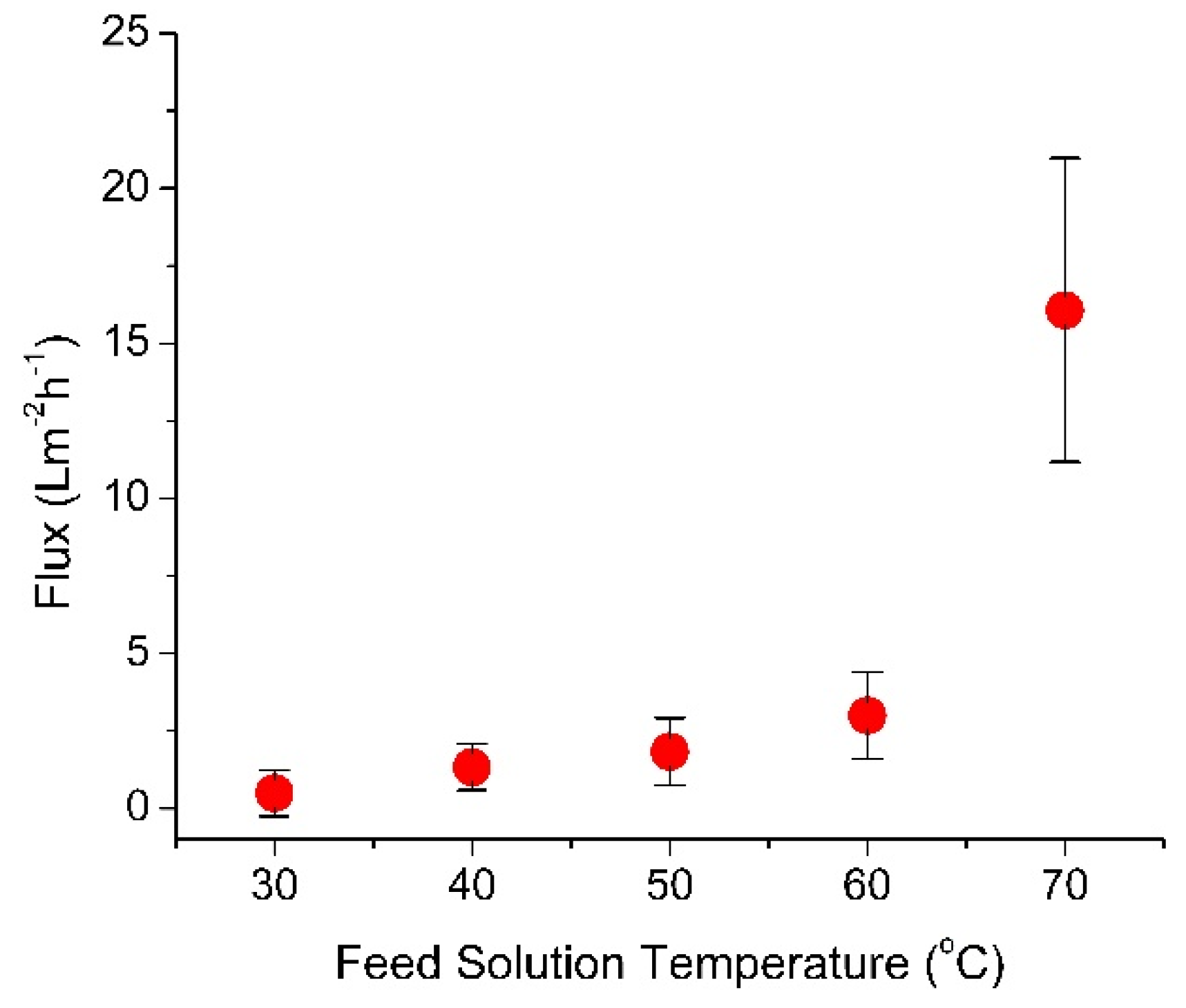
3.2. Effect of Feed Temperature on the Standalone MD Water Flux
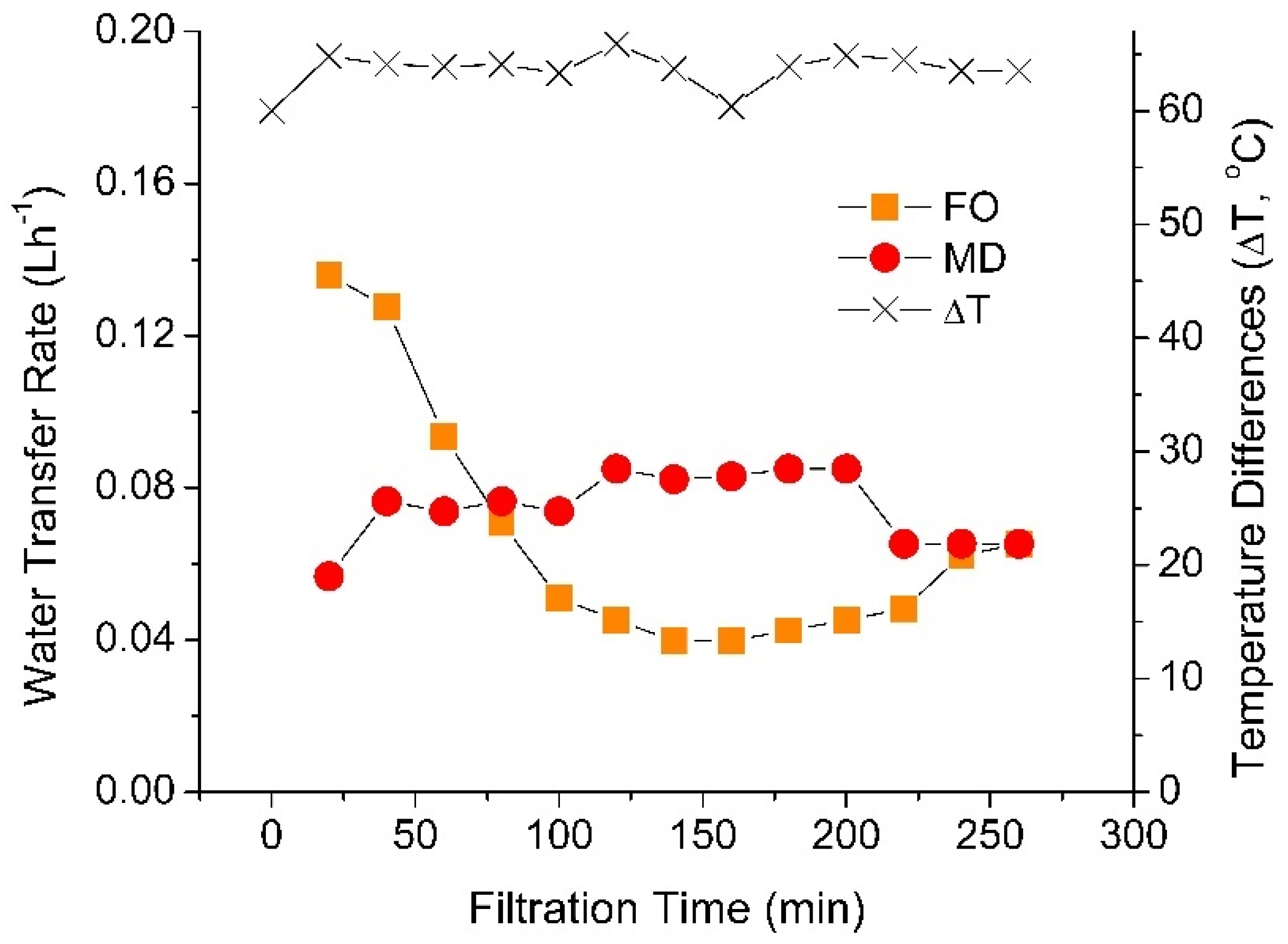
3.3. Effect of Temperature Difference on the Hybrid FO–MD Module Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mat Nawi, N.I.; Bilad, M.R.; Zolkhiflee, N.; Nordin, N.A.H.; Lau, W.J.; Narkkun, T.; Faungnawakij, K.; Arahman, N.; Mahlia, T.M.I. Development of A Novel Corrugated Polyvinylidene difluoride Membrane via Improved Imprinting Technique for Membrane Distillation. Polymers 2019, 11, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and wetting in the membrane distillation driven wastewater reclamation process—A review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.-S.; Ho, S.-T.; Nguyen, H.T.; Ray, S.S.; Nguyen, N.T.; Hsu, H.-T.; Le, N.C.; Tran, T.T. Optimising the recovery of EDTA-2Na draw solution in forward osmosis through direct contact membrane distillation. Sep. Purif. Technol. 2018, 198, 108–112. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Duong, H.C.; Nguyen, H.T.; Chen, S.-S.; Le, H.Q.; Ngo, H.H.; Guo, W.; Duong, C.C.; Le, N.C.; Bui, X.T. Forward osmosis–membrane distillation hybrid system for desalination using mixed trivalent draw solution. J. Membr. Sci. 2020, 603, 118029. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Gryta, M.; Grzechulska-Damszel, J.; Markowska, A.; Karakulski, K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J. Membr. Sci. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Wibisono, Y.; Bilad, M.R. Design of forward osmosis system. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Oxford, UK, 2020; pp. 57–83. [Google Scholar]
- Semiat, R. Energy issues in desalination processes. Environ. Sci. Technol. 2008, 42, 8193–8201. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward osmosis application in manufacturing industries: A short review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Qin, J.-J.; Lay, W.C.L.; Kekre, K.A. Recent developments and future challenges of forward osmosis for desalination: A review. Desalin. Water Treat. 2012, 39, 123–136. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, X.; Tang, C.Y.; Fu, Q.S.; Nie, S. Effect of draw solution concentration and operating conditions on forward osmosis and pressure retarded osmosis performance in a spiral wound module. J. Membr. Sci. 2010, 348, 298–309. [Google Scholar] [CrossRef]
- Qing, L.; Bilad, M.; Sun, G.; Jaafar, J.; Fane, A.G. Flow uneven-distribution and its impact on performances of forward osmosis module. J. Water Process Eng. 2020, 33, 101014. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Arifin, S.N.H.M.; Hizam, S.M.; Rampun, E.L.A.; Bilad, M.R.; Elma, M.; Khan, A.L.; Wibisono, Y.; Jaafar, J. Chlorella vulgaris broth harvesting via standalone forward osmosis using seawater draw solution. Bioresour. Technol. Rep. 2020, 9, 100394. [Google Scholar] [CrossRef]
- Wibisono, Y.; Agung Nugroho, W.; Akbar Devianto, L.; Adi Sulianto, A.; Roil Bilad, M. Microalgae in food-energy-water nexus: A review on progress of forward osmosis applications. Membranes 2019, 9, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, D.; Gupta, L.; Dhingra, R. Forward osmosis in India: Status and comparison with other desalination technologies. Int. Sch. Res. Not. 2014, 2014, 175464. [Google Scholar] [CrossRef] [PubMed]
- Chekli, L.; Phuntsho, S.; Kim, J.E.; Kim, J.; Choi, J.Y.; Choi, J.-S.; Kim, S.; Kim, J.H.; Hong, S.; Sohn, J. A comprehensive review of hybrid forward osmosis systems: Performance, applications and future prospects. J. Membr. Sci. 2016, 497, 430–449. [Google Scholar] [CrossRef]
- Aydiner, C.; Topcu, S.; Tortop, C.; Kuvvet, F.; Ekinci, D.; Dizge, N.; Keskinler, B. A novel implementation of water recovery from whey:“Forward–reverse osmosis” integrated membrane system. Desalin. Water Treat. 2013, 51, 786–799. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. A novel forward osmosis-nano filtration integrated system for coke-oven wastewater reclamation. Chem. Eng. Res. Des. 2015, 100, 542–553. [Google Scholar] [CrossRef]
- Kwon, Y.-N.; Kim, M.-J.; Lee, Y.T. Application of a FO/MD-combined system for the desalination of saline solution. Desalin. Water Treat. 2016, 57, 14347–14354. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhao, B.; Wang, Z.; Xie, M.; Song, J.; Nghiem, L.D.; He, T.; Yang, C.; Li, C.; Chen, G. Water reclamation from shale gas drilling flow-back fluid using a novel forward osmosis–vacuum membrane distillation hybrid system. Water Sci. Technol. 2014, 69, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Ong, R.C.; Wang, P.; Chung, T.S.; Helmer, B.J.; de Wit, J.S. Advanced FO membranes from newly synthesized CAP polymer for wastewater reclamation through an integrated FO-MD hybrid system. AIChE J. 2013, 59, 1245–1254. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. A forward osmosis–membrane distillation hybrid process for direct sewer mining: System performance and limitations. Environ. Sci. Technol. 2013, 47, 13486–13493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husnain, T.; Liu, Y.; Riffat, R.; Mi, B. Integration of forward osmosis and membrane distillation for sustainable wastewater reuse. Sep. Purif. Technol. 2015, 156, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hou, D.; Li, K.; Zhang, Y.; Wang, J.; Zhang, X. Domestic wastewater treatment by forward osmosis-membrane distillation (FO-MD) integrated system. Water Sci. Technol. 2018, 77, 1514–1523. [Google Scholar] [CrossRef]
- Yusoff, M.; Hizami, M.; Nyunt, E.K.; Bilad, M.R.; Arahman, N.; Mulyati, S.; Rizal, S.; Nordin, N.A.H.; Leam, J.J.; Khan, A.L. Hybrid membrane distillation and wet scrubber for simultaneous recovery of heat and water from flue gas. Entropy 2020, 22, 178. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.Y.; Teoh, M.M.; Nugroho, A.; Chung, T.-S. Integrated forward osmosis–membrane distillation (FO–MD) hybrid system for the concentration of protein solutions. Chem. Eng. Sci. 2011, 66, 2421–2430. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, P.; Wan, C.; Chung, T.-S. Polyelectrolyte-promoted forward osmosis–membrane distillation (FO–MD) hybrid process for dye wastewater treatment. Environ. Sci. Technol. 2012, 46, 6236–6243. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Zhao, L.; Ma, W.; Liu, H.; Ma, J. Integrated forward osmosis-membrane distillation process for human urine treatment. Water Res. 2016, 91, 45–54. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Hong, S. Treatment of industrial wastewater produced by desulfurization process in a coal-fired power plant via FO-MD hybrid process. Chemosphere 2018, 210, 44–51. [Google Scholar] [CrossRef]
- Song, H.; Xie, F.; Chen, W.; Liu, J. FO/MD hybrid system for real dairy wastewater recycling. Environ. Technol. 2018, 39, 2411–2421. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, M.; Deng, Q.; Cai, T. Combination and performance of forward osmosis and membrane distillation (FO-MD) for treatment of high salinity landfill leachate. Desalination 2017, 420, 99–105. [Google Scholar] [CrossRef]
- Al-Furaiji, M.; Benes, N.; Nijmeijer, A.; McCutcheon, J.R. Use of a forward osmosis–membrane distillation integrated process in the treatment of high-salinity oily wastewater. Ind. Eng. Chem. Res. 2018, 58, 956–962. [Google Scholar] [CrossRef]
- Kim, Y.; Li, S.; Francis, L.; Li, Z.; Linares, R.V.; Alsaadi, A.S.; Abu-Ghdaib, M.; Son, H.S.; Amy, G.; Ghaffour, N. Osmotically and thermally isolated forward osmosis–membrane distillation (FO–MD) integrated module. Environ. Sci. Technol. 2019, 53, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Hizam, S.; Nordin, N.; Bilad, M.; Wirzal, M.; Putra, Z.; Sambudi, N.; Nasef, M. The Effect of Concentration Staging on Performance of Produced Water Treatment Using Forward Osmosis. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 12021. [Google Scholar]
- Nguyen, T.P.N.; Jun, B.-M.; Lee, J.H.; Kwon, Y.-N. Comparison of integrally asymmetric and thin film composite structures for a desirable fashion of forward osmosis membranes. J. Membr. Sci. 2015, 495, 457–470. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Shon, H.K.; Hong, S. Organic fouling mechanisms in forward osmosis membrane process under elevated feed and draw solution temperatures. Desalination 2015, 355, 169–177. [Google Scholar] [CrossRef]
- Phuntsho, S.; Vigneswaran, S.; Kandasamy, J.; Hong, S.; Lee, S.; Shon, H.K. Influence of temperature and temperature difference in the performance of forward osmosis desalination process. J. Membr. Sci. 2012, 415, 734–744. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; McCutcheon, J.R. Elucidating the impact of temperature gradients across membranes during forward osmosis: Coupling heat and mass transfer models for better prediction of real osmotic systems. J. Membr. Sci. 2018, 553, 189–199. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L. Effects of working temperature on separation performance, membrane scaling and cleaning in forward osmosis desalination. Desalination 2011, 278, 157–164. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zhang, S.; Wang, P.; Fu, X.; Chung, T.-S. Sustainable water recovery from oily wastewater via forward osmosis-membrane distillation (FO-MD). Water Res. 2014, 52, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Manawi, Y.M.; Khraisheh, M.A.M.M.; Fard, A.K.; Benyahia, F.; Adham, S. A predictive model for the assessment of the temperature polarization effect in direct contact membrane distillation desalination of high salinity feed. Desalination 2014, 341, 38–49. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M. Heat transport in the membrane distillation process. J. Membr. Sci. 1998, 144, 211–222. [Google Scholar] [CrossRef]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane fouling and wetting in a DCMD process for RO brine concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Martínez-Díez, L.; Vázquez-González, M.I. Temperature and concentration polarization in membrane distillation of aqueous salt solutions. J. Membr. Sci. 1999, 156, 265–273. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Membr. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R. Direct contact membrane distillation: Effect of mass transfer on heat transfer. J. Membr. Sci. 2001, 188, 137–143. [Google Scholar] [CrossRef]
- Zohrabian, L.; Hankins, N.P.; Field, R.W. Hybrid forward osmosis-membrane distillation system: Demonstration of technical feasibility. J. Water Process Eng. 2020, 33, 101042. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. 3-Membrane distillation—Principles, applications, configurations, design, and implementation. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 55–106. [Google Scholar] [CrossRef]

| Feed | Draw Solution | Membranes | Performances | Remarks | Ref. |
|---|---|---|---|---|---|
| Protein solution | NaCl (0.5–2.0 M) | FO: PBI MD: PVDF–PTFE | Feed solution can be concentrated from 1-g/L to roughly 2.1-g/L in 4 h | Water transfer rate of FO–MD balance at 50 to 60 °C | [29] |
| Dyed wastewater | Poly (acrylic acid) sodium (0.2–0.5 M) | FO: CA MD: PVDF | Highest FO and MD fluxes is 32.0 and 25.0 LMH, respectively, achieved at 80 °C | Water transfer rate of FO–MD most stable at 66 °C | [30] |
| Synthetic wastewater | NaCl (0.5–2.0 M) | FO: CA–propionate MD: PVDF | Highest FO and MD fluxes is 19.9 and 16.2 LMH, respectively, achieved at 70 °C | Most similar flux at 0.5-g/L feed concentration | [24] |
| Landfill leachate | NaCl (optimal: 4.8 M) | FO: TFC MD: PTFE–PVDF | Highest FO and MD fluxes is 4.3 and 6.2 LMH, respectively | Water transfer rate of FO and MD stable at 62.5 °C (100,000-mg/L NaCl in feed solution) | [34] |
| Domestic wastewater | NaCl (0.6 M) | FO: CTA MD: PVDF | Water flux maintained at 17.6 LHM for 120 h operation | After 120 h operation, fouling on FO membrane reduces the flux | [27] |
| Produced water | NaCl (5 M) KCl (4 M) MgCl2 (4 and 4.8 M) LiCl (4.8 and 10 M) | FO: HTI–TFC MD: 3 M–PP | Stable fluxes (3.0–4.0 LMH) for 20 h operation achieved at 4.8-M MgCl2 of draw solution | NaCl, KCl, LiCl show high MD flux and low or negative FO flux | [35] |
| DI water | MgCl2 (0.37, 0.62 and 1.44 M) | FO: CTA MD: PTFE | FO flux 13.0 LMH at 50 °C and MD flux 102 LMH at temperature difference of 60 °C | Initial flowrate and concentration are the most important factors for stable integrated module | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Nawi, N.I.; Bilad, M.R.; Anath, G.; Nordin, N.A.H.; Kurnia, J.C.; Wibisono, Y.; Arahman, N. The Water Flux Dynamic in a Hybrid Forward Osmosis-Membrane Distillation for Produced Water Treatment. Membranes 2020, 10, 225. https://doi.org/10.3390/membranes10090225
Mat Nawi NI, Bilad MR, Anath G, Nordin NAH, Kurnia JC, Wibisono Y, Arahman N. The Water Flux Dynamic in a Hybrid Forward Osmosis-Membrane Distillation for Produced Water Treatment. Membranes. 2020; 10(9):225. https://doi.org/10.3390/membranes10090225
Chicago/Turabian StyleMat Nawi, Normi Izati, Muhammad Roil Bilad, Ganeswaran Anath, Nik Abdul Hadi Nordin, Jundika Candra Kurnia, Yusuf Wibisono, and Nasrul Arahman. 2020. "The Water Flux Dynamic in a Hybrid Forward Osmosis-Membrane Distillation for Produced Water Treatment" Membranes 10, no. 9: 225. https://doi.org/10.3390/membranes10090225










