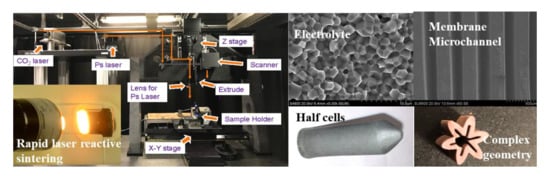
A Novel Laser 3D Printing Method for the Advanced Manufacturing of Protonic Ceramics
Abstract
:1. Introduction
2. Experimental Section
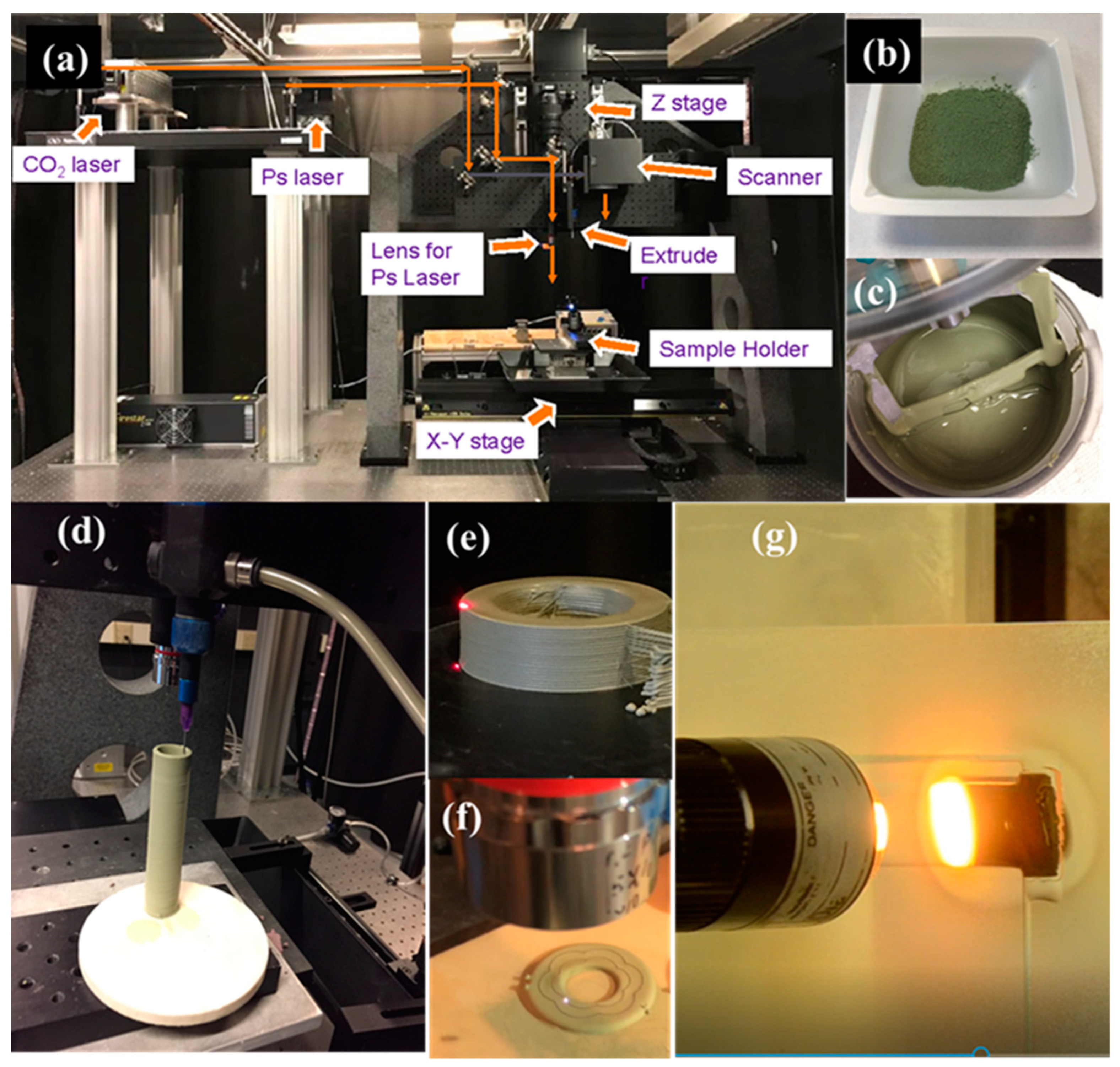
2.1. L3DP System
2.1.1. Preparation of Printable Pastes
2.1.2. Microextrusion-Based 3D Printing
2.1.3. Rapid Laser Drying
2.1.4. Precise Laser Machining
2.1.5. Rapid Laser Reactive Sintering
2.2. Post Treatment
2.3. Characterization
3. Results and Discussion
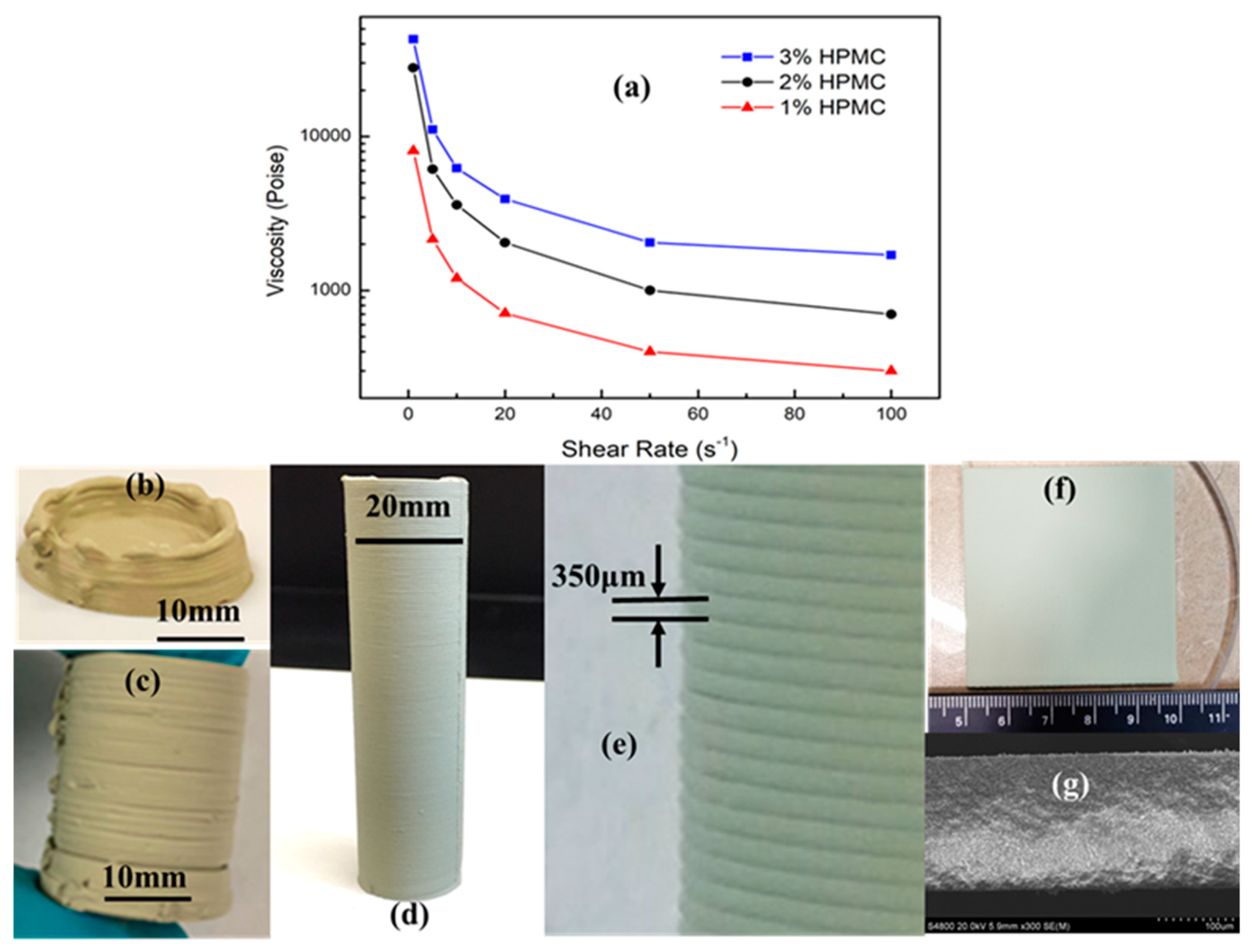
3.1. Printable Pastes
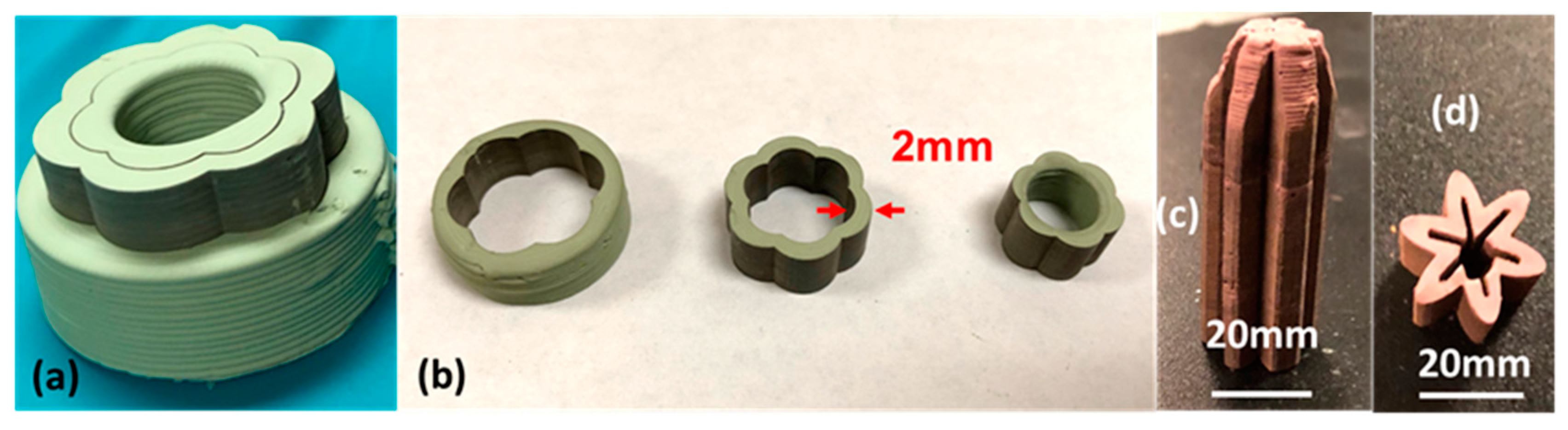
3.2. 3D Printing of Complex PCED Green Anode Parts
3.3. Laser Machining-Assisted 3D Printing of High-Quality PC Green Parts
3.4. Manufacturing of PC Parts by L3DP Followed by Post-Treatment
3.4.1. Electrochemical Half Cells
3.4.2. Microchannel Membranes
3.5. Manufacturing of PC Parts by Direct L3DP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Sengodan, S.; Kim, S.; Kwon, O.; Bu, Y.; Kim, G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2019, 109, 606–618. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Hayre, R.O.; Crumlin, E.J.; Hook, D.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H., Il; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Lee, H.W.; Kim, B.K.; Son, J.W.; Yoon, K.J.; Kim, H.; Shin, D.; Ji, H., Il; Lee, J.H. A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm−2 at 600 °C. Nat. Energy 2018, 3, 870–875. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; OHayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z.; Liu, M. Enhanced Sulfur and Coking tolerance of a MIEC for SOFC: BCZYYb. Science 2009, 326, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Vøllestad, E.; Strandbakke, R.; Tarach, M.; Catalán-Martínez, D.; Fontaine, M.L.; Beeaff, D.; Clark, D.R.; Serra, J.M.; Norby, T. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 2019, 18, 752–759. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Choi, S.; Davenport, T.C.; Haile, S.M. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: Exceptional reversibility, stability, and demonstrated faradaic efficiency. Energy Environ. Sci. 2019, 12, 206–215. [Google Scholar] [CrossRef]
- Huang, H.; Cheng, S.; Gao, J.; Chen, C.; Yi, J. Phase-inversion tape-casting preparation and significant performance enhancement of Ce0. 9Gd0. 1O1. 95–La0. 6Sr0. 4Co0. 2Fe0. 8O3−δ dual-phase asymmetric membrane for oxygen separation. Mater. Lett. 2014, 137, 245–248. [Google Scholar] [CrossRef]
- Huang, H.; Lin, J.; Wang, Y.; Wang, S.; Xia, C.; Chen, C. Facile one-step forming of NiO and yttrium-stabilized zirconia composite anodes with straight open pores for planar solid oxide fuel cell using phase-inversion tape casting method. J. Power Sources 2015, 274, 1114–1117. [Google Scholar] [CrossRef]
- Non-oxidative, H.E. Fluorescent Aliphatic Hyperbranched Polyether: Chromophores-free and without any N and P Atoms. Phys. Chem. Chem. Phys. 2016. [Google Scholar] [CrossRef]
- Malerød-Fjeld, H.; Clark, D.; Yuste-Tirados, I.; Zanón, R.; Catalán-Martinez, D.; Beeaff, D.; Morejudo, S.H.; Vestre, P.K.; Norby, T.; Haugsrud, R.; et al. Thermo-electrochemical production of compressed hydrogen from methane with near-zero energy loss. Nat. Energy 2017, 2, 923–931. [Google Scholar] [CrossRef]
- Morejudo, S.H.; Zanón, R.; Escolástico, S.; Yuste-Tirados, I.; Malerød-Fjeld, H.; Vestre, P.K.; Coors, W.G.; Martínez, A.; Norby, T.; Serra, J.M.; et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 2016, 353, 563–566. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Kjølseth, C.; Serra, J.M. Outstanding hydrogen permeation through CO2-stable dual-phase ceramic membranes. Energy Environ. Sci. 2014, 7, 3736–3746. [Google Scholar] [CrossRef]
- Rebollo, E.; Mortalò, C.; Escolástico, S.; Boldrini, S.; Barison, S.; Serra, J.M.; Fabrizio, M. Exceptional hydrogen permeation of all-ceramic composite robust membranes based on BaCe0.65Zr0.20Y0.15O3-δ and Y- or Gd-doped ceria. Energy Environ. Sci. 2015, 8, 3675–3686. [Google Scholar] [CrossRef]
- Yin, J.; Wang, X.; Xu, J.; Wang, H.; Zhang, F.; Ma, G. Ionic conduction in BaCe0.85-XZrxEr 0.15O3-α and its application to ammonia synthesis at atmospheric pressure. Solid State Ion. 2011, 185, 6–10. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Ma, G. Proton conduction in BaxCe0.8Y0.2O 3-α + 0.04ZnO at intermediate temperatures and its application in ammonia synthesis at atmospheric pressure. J. Mater. Sci. 2011, 46, 4690–4694. [Google Scholar] [CrossRef]
- Robinson, S.; Manerbino, A.; Grover Coors, W. Galvanic hydrogen pumping in the protonic ceramic perovskite BaCe0.2Zr0.7Y0.1O3-δ. J. Membr. Sci. 2013, 446, 99–105. [Google Scholar] [CrossRef]
- Vasileiou, E.; Kyriakou, V.; Garagounis, I.; Vourros, A.; Stoukides, M. Ammonia synthesis at atmospheric pressure in a BaCe0.2Zr0.7Y0.1O2.9 solid electrolyte cell. Solid State Ion. 2015, 275, 110–116. [Google Scholar] [CrossRef]
- Klinsrisuk, S.; Irvine, J.T.S. Electrocatalytic ammonia synthesis via a proton conducting oxide cell with BaCe0.5Zr0.3Y0.16Zn0.04O3-Δ electrolyte membrane. Catal. Today 2017, 286, 41–50. [Google Scholar] [CrossRef]
- Sofie, S.W. Fabrication of functionally graded and aligned porosity in thin ceramic substrates with the novel freeze-tape-casting process. J. Am. Ceram. Soc. 2007, 90, 2024–2031. [Google Scholar] [CrossRef]
- Jang, W.S.; Hyun, S.H.; Kim, S.G. Preparation of YSZ/YDC and YSZ/GDC composite electrolytes by the tape casting and sol-gel dip-drawing coating method for low-temperature SOFC. J. Mater. Sci. 2002, 37, 2535–2541. [Google Scholar] [CrossRef]
- Hotza, D.; Greil, P. Aqueous Tape Casting of Ceramic Powders. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 1995, 202, 206–217. [Google Scholar] [CrossRef]
- Hammel, E.C.; Ighodaro, O.L.R.; Okoli, O.I. Processing and properties of advanced porous ceramics: An application based review. Ceram. Int. 2014, 40, 15351–15370. [Google Scholar] [CrossRef]
- Colombo, P. Conventional and novel processing methods for cellular ceramics. Philos. Trans. R. Soc. A 2006, 364, 109–124. [Google Scholar] [CrossRef]
- Huang, Y.; Leu, M.C.; Mazumder, J.; Donmez, A. Additive Manufacturing: Current State, Future Potential, Gaps and Needs, and Recommendations. J. Manuf. Sci. Eng. 2015, 137, 014001. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. CAD Comput. Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Santos, E.C.; Shiomi, M.; Osakada, K.; Laoui, T. Rapid manufacturing of metal components by laser forming. Int. J. Mach. Tools Manuf. 2006, 46, 1459–1468. [Google Scholar] [CrossRef]
- Duley, W.W.; Mueller, R.E. CO2 laser welding of polymers. Polym. Eng. Sci. 1992, 32, 582–585. [Google Scholar] [CrossRef]
- Mu, S.; Zhao, Z.; Lei, J.; Hong, Y.; Hong, T.; Jiang, D.; Song, Y.; Jackson, W.; Brinkman, K.S.; Peng, F.; et al. Engineering of microstructures of protonic ceramics by a novel rapid laser reactive sintering for ceramic energy conversion devices. Solid State Ion. 2018, 320, 369–377. [Google Scholar] [CrossRef]
- Hong, Y.; Lei, J.; Heim, M.; Song, Y.; Yuan, L.; Mu, S.; Bordia, R.K.; Xiao, H.; Tong, J.; Peng, F. Fabricating Ceramics with Embedded Microchannels using an Integrated Additive Manufacturing and Laser Machining Method. J. Am. Ceram. Soc. 2018, 1–12. [Google Scholar] [CrossRef]
- Blakeley, B.; Sullivan, N. Fuel processing in a ceramic microchannel reactor: Expanding operating windows. Int. J. Hydrogen Energy 2016, 41, 3794–3802. [Google Scholar] [CrossRef]
- Cohen, J.L.; Volpe, D.J.; Westly, D.A.; Pechenik, A.; Abruña, H.D. A dual electrolyte H2/O2 planar membraneless microchannel fuel cell system with open circuit potentials in excess of 1.4 V. Langmuir 2005, 21, 3544–3550. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Sanders, M.; O’Hayre, R. Solid-state reactive sintering mechanism for large-grained yttrium-doped barium zirconate proton conducting ceramics. J. Mater. Chem. 2010, 20, 6333. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Hoban, M.; O’Hayre, R. Cost-effective solid-state reactive sintering method for high conductivity proton conducting yttrium-doped barium zirconium ceramics. Solid State Ion. 2010, 181, 496–503. [Google Scholar] [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive manufacturing of ceramic-based materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Huang, F.-M.; Chang, Y.-C. Fine ceramic lattices prepared by extrusion freeforming. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 340–344. [Google Scholar] [CrossRef]
- Ren, X.B.; Zhang, Z.L.; Nyhus, B. Effect of residual stresses on the crack-tip constraint in a modified boundary layer model. Int. J. Solids Struct. 2009, 46, 2629–2641. [Google Scholar] [CrossRef] [Green Version]
- Moreno, A.M.; Wilhite, B.A. Autothermal hydrogen generation from methanol in a ceramic microchannel network. J. Power Sources 2010, 195, 1964–1970. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, S.; Hong, Y.; Huang, H.; Ishii, A.; Lei, J.; Song, Y.; Li, Y.; Brinkman, K.S.; Peng, F.; Xiao, H.; et al. A Novel Laser 3D Printing Method for the Advanced Manufacturing of Protonic Ceramics. Membranes 2020, 10, 98. https://doi.org/10.3390/membranes10050098
Mu S, Hong Y, Huang H, Ishii A, Lei J, Song Y, Li Y, Brinkman KS, Peng F, Xiao H, et al. A Novel Laser 3D Printing Method for the Advanced Manufacturing of Protonic Ceramics. Membranes. 2020; 10(5):98. https://doi.org/10.3390/membranes10050098
Chicago/Turabian StyleMu, Shenglong, Yuzhe Hong, Hua Huang, Akihiro Ishii, Jincheng Lei, Yang Song, Yanjun Li, Kyle S. Brinkman, Fei Peng, Hai Xiao, and et al. 2020. "A Novel Laser 3D Printing Method for the Advanced Manufacturing of Protonic Ceramics" Membranes 10, no. 5: 98. https://doi.org/10.3390/membranes10050098