Synthesis and Characterization of Novel Nanoporous Gl-POSS-Branched Polymeric Gas Separation Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
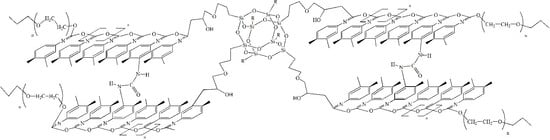
2.2. Synthesis of Polymers Based on TDI, PPEG, and Gl-POSS (POI-Gl-POSS)
 ] groups (E/K) was taken into account, when calculating the content of Gl-POSS. Table 1 shows the ratio of these groups depending on the content of Gl-POSS. The calculation was performed on 100 g of PPEG.
] groups (E/K) was taken into account, when calculating the content of Gl-POSS. Table 1 shows the ratio of these groups depending on the content of Gl-POSS. The calculation was performed on 100 g of PPEG. 2.3. Polymer Characterization
2.3.1. Tensile Stress–Strain Measurements
2.3.2. Infrared Spectroscopy Analysis
2.3.3. Thermomechanical Analysis
2.3.4. Mechanical Loss Tangent Measurements (MLT)
2.3.5. Thermal Gravimetric Analysis
2.3.6. Dielectric Loss Tangents Measurements
2.3.7. Atomic Force Microscopy Topology Analysis
2.3.8. Contact Angle Measurements
2.3.9. Porometry
2.3.10. Permeability Measurements
2.3.11. X-ray Analysis
3. Results and Discussion
3.1. POI-Gl-POSS Synthesis and Characterization
3.2. Morphology Characterization of POI-Gl-POSS
3.3. Water Contact Angle
3.4. Thermal–Mechanical and Dynamic Mechanical Behavior of POI-Gl-POSS
3.5. The Temperature Dependence of the Dielectric Loss Tangents of POI-Gl-POSS
3.6. Mechanical Properties of POI-Gl-POSS
3.7. X-ray Analysis
3.8. Gas Separation Performance of POI-Gl-POSS Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PPEG | block copolymer of propylene oxide with ethylene oxide |
| TDI | 2,4-tolylene diisocyanate |
| POSS | polyhedral oligomeric silsesquioxane |
| Gl-POSS | octaglycidyl polyhedral oligomeric silsesquioxane |
| POI-0 | the polymer based on TDI and PPEG |
| POI-Gl-POSS | the polymer based on TDI, PPEG and Gl-POSS |
| ICN | polyisocyanurate |
| E/K | molar ratio of epoxy groups to potassium alcoholate groups |
| TMA | thermomechanical analysis |
| TGA | thermogravimetric analysis |
| AFM | atomic force microscopy |
| DLT | dielectric loss tangent |
| MLT | mechanical loss tangent |
| MI | macroinitiator |
| PS | pressure sensor |
| VG | vacuum gauge |
| RMM | radial membrane module |
References
- Yampolskii, Y. Polymeric gas separation membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.J.; Macedonio, F. Membrane engineering in process intensification-An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K. Membrane-based gas separation. J. Membr. Sci. 1993, 83, 1–80. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers, Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Baker, R. Membrane Technology and Application, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2004. [Google Scholar]
- Vorotyntsev, I.V.; Drozdov, P.N.; Mochalov, G.M.; Smirnova, N.N.; Suvorov, S.S. Sorption of ammonia and nitrogen on cellulose acetate. Russ. J. Phys. Chem. A. 2006, 80, 2020–2023. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M. High purification of gases by diffusion through polymer membranes. Pet. Chem. 2015, 55, 259–275. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V. High purification of substances by a gas separation method. Desalination 2009, 240, 301–305. [Google Scholar] [CrossRef]
- Vorotyntsev, I.V.; Drozdov, P.N.; Shablikin, D.N.; Gamajunova, T.V. Ammonia separation and purification by absorbing pervaporation. Desalination 2006, 200, 379–380. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Belyaev, E.S. Deep gas cleaning of highly permeating impurities using a membrane module with a feed tank. Pet. Chem. 2011, 51, 595–600. [Google Scholar] [CrossRef]
- Drozdov, A. Viscoelastoplasticity of amorphous glassy polymers. Eur. Pol. Jour. 2000, 36, 2063–2074. [Google Scholar] [CrossRef]
- Volkov, V.V. Free volume structure and transport properties of glassy polymers-materials for separating membranes. Polym. J. 1991, 23, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Buonemenna, M.; Yave, W.; Goleme, G. Some approaches for high performance polymer based membranes for gas separation: Block copolymers, carbon molecular sieves and mixed matrix membranes. RCS Adv. 2012, 2, 10745–10773. [Google Scholar] [CrossRef]
- Park, S.; Yavuzcetin, O.; Kim, B.; Tuominen, M.; Russell, T. A Simple Top-Down/Bottom-Up Approach to Sectored, Ordered Arrays of Nanoscopic Elements Using Block Copolymers. Small 2009, 5, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Shin, C.; Kim, E.; Ryu, D.; Jeong, U.; Russell, T.; Hawker, C. Microdomain Orientation of PS-b-PMMA by Controlled Interfacial Interactions. Macromolecules 2008, 41, 6431–6437. [Google Scholar] [CrossRef]
- Webster, O. Living Polymerization Methods. Science 1991, 251, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Matsen, M.W.; Bates, F.S. Origins of Complex Self-Assembly in Block Copolymers. Macromolecules 1996, 29, 7641–7644. [Google Scholar] [CrossRef]
- Darlin, S. Directing the self-assembly of block copolymers. Prog. Polym. Sci. 2007, 32, 1152–1204. [Google Scholar] [CrossRef]
- Powell, C.; Qiao, G. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Membr. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Shishatskiy, S.; Pauls, J.; Nunes, S.; Peineman, K. Quaternary ammonium membrane materials for CO2 separation. J. Membr. Sci. 2010, 359, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, G.; Houde, A.; Stern, S. Poly(ether urethane) and poly(ether urethane urea) membranes with high H2S/CH4 selectivity. J. Membr. Sci. 1997, 135, 99–106. [Google Scholar] [CrossRef]
- Scholes, C.; Stevens, G.; Kentish, S. The effect of hydrogen sulfide, carbon monoxide and water on the performance of a PDMS membrane in carbon dioxide/nitrogen separation. J. Membr. Sci. 2010, 350, 189–199. [Google Scholar] [CrossRef]
- Tricoli, V.; Cussler, E. Ammonia selective hollow fibers. J. Membr. Sci. 1995, 104, 19–26. [Google Scholar] [CrossRef]
- Makhloufi, C.; Belaissaoui, B.; Roizard, D.; Favre, E. Interest of Poly[bis(trifluoroethoxy)phosphazene] Membranes for Ammonia Recovery–Potential Application in Haber Process. Procedia Eng. 2012, 44, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Makhloufi, C.; Roizard, D.; Favre, E. Reverse selective NH3/CO2 permeation in fluorinated polymers using membrane gas separation. J. Membr. Sci. 2013, 441, 63–72. [Google Scholar] [CrossRef]
- Semenova, S.; Smirnov, S.; Ohya, H. Performances of glassy polymer membranes plasticized by interacting penetrants. J. Membr. Sci. 2000, 172, 75–89. [Google Scholar] [CrossRef]
- Vorotyntsev, I.V.; Drozdov, P.N.; Karyakin, N.V. Ammonia permeability of a cellulose acetate membrane. Inorg. Mater. 2006, 42, 231–235. [Google Scholar] [CrossRef]
- Kulprathipanja, S.; Kulkarni, S.S. UOP Inc., Des Plaines, IL (United States). Separation of Polar Gases from Nonpolar Gases. US Patent US 4,608,060 A, 26 August 1986. [Google Scholar]
- Appl, M. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Pez, G.; Laciak, D. Air Products, Chemicals Inc., assignee. Ammonia Separation Using Semipermeable Membranes. U.S. Patent US 4,762,535, 9, 9 August 1988. Patent CA 1304697, -CA 567740. [Google Scholar]
- Davletbaeva, I.M.; Zaripov, I.I.; Mazilnikov, A.I.; Davletbaev, R.S.; Sharifullin, R.R.; Atlaskin, A.A.; Sazanova, T.S.; Vorotyntsev, I.V. Synthesis and Study of Gas Transport Properties of Polymers Based on Macroinitiators and 2,4-Toluene Diisocyanate. Membranes 2019, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Siavashi, F.; Saidi, M.; Rahimpour, M. Purge gas recovery of ammonia synthesis plant by integrated configuration of catalytic hydrogen-permselective membrane reactor and solid oxide fuel cell as a novel technology. J. Power Sources 2014, 267, 104–116. [Google Scholar] [CrossRef]
- Karami, M.R.; Keshavarz, P.; Khorram, M.; Mehdipour, M. Analysis of ammonia separation from purge gases in microporous hollow fiber membrane contactors. J. Hazard. Mat. 2013, 260, 576–584. [Google Scholar] [CrossRef]
- Klinsrisuk, S.; Tao, S.; Irvine, J. Membrane Reactors for Ammonia Production. In Membrane Reactors for Energy Applications and Basic Chemical Production; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 543–563. [Google Scholar]
- Khakpay, A.; Scovazzo, P. Reverse-selective behavior of room temperature ionic liquid based membranes for natural gas processing. J. Membr. Sci. 2018, 545, 204–212. [Google Scholar] [CrossRef]
- Hernández, F.; Ríos, A.; Tomás-Alonso, F.; Gomez, D.; Villora, G. Improvement in the separation efficiency of transesterification reaction compounds by the use of supported ionic liquid membranes based on the dicyanamide anion. J. Membr. Sci. 2009, 244, 122–129. [Google Scholar] [CrossRef]
- De los Rios, A.P.; Hernandez-Fernandez, F.J.; Tomas-Alonso, F.; Palacios, J.M.; Gómez, D.; Rubio, M.; Víllora, G. A SEM–EDX study of highly stable supported liquid membranes based on ionic liquids. J. Membr. Sci. 2007, 300, 88–94. [Google Scholar] [CrossRef]
- Bara, J.E.; Hatakeyama, E.S.; Gin, D.L.; Noble, R.D. Improving CO2 permeability in polymerized room-temperature ionic liquid gas separation membranes through the formation of a solid composite with a room-temperature ionic liquid. Polym. Adv. Technol. 2008, 19, 1415–1420. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Wiesenauer, E.F.; Nicodemus, G.D.; Gin, D.L.; Noble, R.D. Ideal CO2/Light Gas Separation Performance of Poly(vinylimidazolium) Membranes and Poly(vinylimidazolium)-Ionic Liquid Composite Films. Ind. Eng. Chem. Res. 2013, 52, 1023–1032. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Emelina, O.Yu.; Vorotyntsev, I.V.; Davletbaev, R.S.; Grebennikova, E.S.; Petukhov, A.N.; Ahkmetshina, A.I.; Sazanova, T.S.; Loskutov, V.V. Synthesis and properties of novel polyurethanes based on amino ethers of boron acid for gas separation membranes. RSC Adv. 2015, 5, 65674–65683. [Google Scholar] [CrossRef]
- Ríos-Dominguez, H.; Ruiz-Treviño, F.A.; Contreras-Reyes, R.; González-Montiel, A. Syntheses and evaluation of gas transport properties in polystyrene–POSS membranes. J. Membr. Sci. 2006, 271, 94–100. [Google Scholar] [CrossRef]
- Joly, C.; Samaihi, M.; Porcar, L.; Noble, R.D. Polyimide−Silica Composite Materials: How Does Silica Influence Their Microstructure and Gas Permeation Properties. Chem. Mater. 1999, 11, 2331–2338. [Google Scholar] [CrossRef]
- Peng, F.; Sun, L.; Lu, H.; Wang, Y.; Liu, J.; Jiang, Z. Hybrid Organic−Inorganic Membrane: Solving the Tradeoff between Permeability and Selectivity. Chem. Mater. 2005, 17, 6790–6796. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Chung, T.S.; Kawi, S. Facilitated transport by hybrid POSS®–Matrimid®–Zn2+ nanocomposite membranes for the separation of natural gas. J. Membr. Sci. 2010, 365, 14–21. [Google Scholar] [CrossRef]
- Chua, M.L.; Shao, L.; Low, B.T.; Xiao, Y.; Chung, T.S. Polyetheramine–polyhedral oligomeric silsesquioxane organic–inorganic hybrid membranes for CO2/H2 and CO2/N2 separation. J. Membr. Sci. 2011, 385, 40–48. [Google Scholar] [CrossRef]
- Iyer, P.; Iyer, G.; Coleman, M. Gas transport properties of polyimide–POSS nanocomposites. J. Membr. Sci. 2010, 358, 26–32. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Khan, M.M.; Gacal, B.N.; Abetz, V. Functionalization of POSS nanoparticles and fabrication of block copolymer nanocomposite membranes for CO2 separation. Reac. & Funct. Polym. 2015, 86, 125–133. [Google Scholar]
- Duan, J.; Litwiller, E.; Pinnau, I. Solution-diffusion with defects model for pressure-assisted forward osmosis. J. Membr. Sci. 2014, 470, 323–333. [Google Scholar] [CrossRef]
- Kopesky, E.T.; Haddad, T.S.; Cohen, R.E.; McKinley, G.H. Thermomechanical Properties of Poly(methyl methacrylate)s Containing Tethered and Untethered Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2004, 37, 8992–9004. [Google Scholar] [CrossRef]
- Zhao, Y.; Schiraldi, D.A. Thermal and mechanical properties of polyhedral oligomeric silsesquioxane (POSS)/polycarbonate composites. Polymer 2005, 46, 11640–11647. [Google Scholar] [CrossRef]
- Xu, H.; Kuo, S.W.; Lee, J.S.; Chang, F.C. Preparations, Thermal Properties, and Tg Increase Mechanism of Inorganic/Organic Hybrid Polymers Based on Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2002, 35, 8788–8793. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Mazilnikov, A.I.; Zaripov, I.I.; Davletbaev, R.S.; Gumerov, A.M.; Parfenov, V.V. Synthesis of Block Copolymers Based on a Macroinitiator and 2,4-Toluene Diisocyanate. Polym. Sci. Ser. B 2018, 60, 51–57. [Google Scholar] [CrossRef]
- Davletbaev, R.S.; Akhmetshina, A.I.; Gumerov, A.M.; Davletbaeva, I.M.; Parfenov, V.V. Supramolecular Architecture of Polymers as the Basis of Obtaining Mesoporous Polymers. Comp. Int. 2014, 21, 611–621. [Google Scholar] [CrossRef]
- Bokare, U.M.; Gandhi, K.S. Effect of simultaneous polyaddition reaction on the curing of epoxides. J. Polym. Sci. 1980, 18, 857–870. [Google Scholar] [CrossRef]
- Hou, G.; Gao, J.; Tian, C.T.; Wu, X. Preparation and characterization of nanocomposite films by hybrid cationic ring opening polymerization of glycidyl-POSS. Mater. Chem. Phys. 2014, 148, 236–244. [Google Scholar] [CrossRef]
- Ghosal, K.; Freeman, B.D. Gas separation using polymer membranes: An overview. Polym. Adv. Technol. 1994, 5, 673–697. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Davletbaev, R.S.; Davletbaeva, I.M.; Mikhailova, A.V.; Gumerov, A.M.; Deberdeev, R. Ya. Immobilization of Organic Reagents on Optically Transparent Mesoporous Polymers and Its Analytical Use. Rus. J. App. Chem. 2015, 3, 495–501. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Davletbaeva, I.M.; Grebenschikova, E.S.; Sazanova, T.S.; Petukhov, A.N.; Atlaskin, A.A.; Razov, E.N.; Zaripov, I.I.; Martins, C.F.; Neves, L.A.; et al. The Effect of Microporous Polymeric Support Modification on Surface and Gas Transport Properties of Supported Ionic Liquid Membranes. Membranes 2016, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Smirnov, K.Y. Nitrous oxide high purification by membrane gas separation. Inorg. Mater. 2009, 45, 1263–1266. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Smirnov, K.Y. Germane high purification by membrane gas separation. Desalination 2006, 200, 232–233. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Murav’ev, D.V. Fine gas purification to remove slightly penetrating impurities using a membrane module with a feed reservoir. Dokl. Chem. 2006, 411, 243–245. [Google Scholar] [CrossRef]
- Drozdov, P.N.; Kirillov, Y.P.; Kollotilov, E.Y.; Vorotynsev, I.V. High purification of gas in radial membrane element. Desalination 2002, 146, 249–254. [Google Scholar] [CrossRef]
- Trubyanov, M.M.; Drozdov, P.N.; Atlaskin, A.A.; Battalov, S.V.; Puzanov, E.S.; Vorotyntsev, A.V.; Petukhov, A.N.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Unsteady-state membrane gas separation by novel pulsed retentate mode for improved membrane module performance: Modelling and experimental verification. J. Membr. Sci. 2017, 530, 53–64. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, NY, USA, 1975. [Google Scholar]
- Small Angle. X-ray Scattering. Version 4.0. Software Reference Manual; M86-E00005-0600; Bruker AXS Inc.: Fitchburg, WI, USA, 2000. [Google Scholar]
- Konarev, P.V.; Volkov, V.V.; Sokolova, A.V.J.; Koch, M.H.; Svergun, D.I. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003, 36, 1277–1282. [Google Scholar] [CrossRef]
- Software SASView UMD, UTK, NIST, ORNL, ISIS, ESS and ILL. 2009–2013. Available online: https://www.sasview.org/ (accessed on 23 March 2020).
- Davletbaeva, I.M.; Akhmetshina, A.I.; Davletbaev, R.S.; Zaripov, I.I.; Gumerov, A.M.; Sharifullin, R.R. Optically transparent mesoporous polymers based on anionic macroinitiators and 2,4-toluene diisocyanate. Polym. Sci. Ser. B 2014, 56, 814–821. [Google Scholar] [CrossRef]
- Bellami, L. New Data on IR Spectra of Complex Molecules; Inostr. Lit.: Moskva, Russia, 1971. [Google Scholar]
- Agarwal, C.; Pandey, A.K.; Das, S.; Sharma, M.K.; Pattyn, D.; Ares, P.; Goswami, A. Neck-size distributions of through-pores in polymer membranes. J. Membr. Sci. 2012, 415–416, 608–615. [Google Scholar] [CrossRef]
- Guinier, A.; Fournet, G. Small-angle Scattering of X-rays; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Vorotyntsev, I.V.; Shablykin, D.N.; Drozdov, P.N.; Trubyanov, M.M.; Petukhov, A.N.; Battalov, S.V. Separation of ammonia-containing gas mixtures in a one-compressor multistage membrane apparatus. Pet. Chem. 2017, 57, 172–181. [Google Scholar] [CrossRef]
- Robb, W.L. Thin silicone membranes-their permeation properties and some applications. Ann. N. Y. Acad. Sci. 1968, 146, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.A.; Bhide, B.D. Permeability of silicone polymers to ammonia and hydrogen sulfide. J. Appl. Polym. Sci. 1989, 38, 2131–2147. [Google Scholar] [CrossRef]
- Modigell, M.; Schumacher, M.; Teplyakov, V. A membrane contactor for efficient CO2 removal in biohydrogen production. Desalination 2008, 224, 186–190. [Google Scholar] [CrossRef]
- Phillip, W.A.; Martono, E.; Chen, L.; Hillmyer, M.A.; Cussler, E.L. Seeking an ammonia selective membrane based on nanostructured sulfonated block copolymers. J. Memb. Sci. 2009, 337, 39–46. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]

















 ] groups (E/K).
] groups (E/K).| [Gl-POSS], wt% | 0 | 0.1 | 0.4 | 0.5 | 1.0 | 2.0 | 5.0 | 8.0 | 10.0 | 15.0 | 20.0 |
| E/K, g/eq | 0 | 0.19 | 0.75 | 0.92 | 1.87 | 3.71 | 7.2 9.17 | 15.0 | 18.75 | 27.92 | 37.08 |
| [Gl-POSS] wt% | 0 | 0.1 | 0.5 | 1.0 | 2.0 | 5.0 | 8.0 | |
|---|---|---|---|---|---|---|---|---|
| Gas | ||||||||
| N2 | 3.1 ± 0.4 | 1.5 ± 0.2 | 1.8 ± 0.3 | 1.9 ± 0.3 | 95.2 ± 8.6 | 101.6 ± 8.13 | 120 ± 7.2 | |
| H2 | 4.7 ± 0.6 | 8.1 ± 0.9 | 32.6 ± 3.3 | 48.5 ± 4.6 | 42.1 ± 4.2 | 53.6 ± 4.8 | 120 ± 8.4 | |
| NH3 | 489 ± 19.6 | 716 ± 21.5 | 841 ± 25.2 | 1032 ± 20.6 | 434 ± 17.4 | 528 ± 21.12 | 210 ± 10.5 | |
| [Gl-POSS], wt% | NH3/H2 | NH3/N2 |
|---|---|---|
| 0 | 104 ± 13.2 | 157.7 ± 21.5 |
| 0.1 | 88.4 ± 10.08 | 477.3 ± 73.02 |
| 0.5 | 25.8 ± 2.7 | 467.2 ± 71.5 |
| 1.0 | 21.3 ± 2.07 | 543.2 ± 76.8 |
| 2.0 | 10.3 ± 1.11 | 4.6 ± 0.4 |
| 5.0 | 9.9 ± 0.9 | 5.2 ± 0.5 |
| 8.0 | 1.8 ± 0.15 | 1.7 ± 0.14 |
| [Gl-POSS] wt% | 0 | 0.1 | 0.5 | 1.0 | 2.0 | 5.0 | 8.0 | |
|---|---|---|---|---|---|---|---|---|
| Gas | ||||||||
| N2 | 5.4 ± 0.7 | 1.9 ± 0.3 | 1.5 ± 0.2 | 4.2 ± 0.6 | 6 ± 0.6 | 3.7 ± 0.3 | 5.7 ± 0.4 | |
| H2 | 6.6 ± 0.8 | 2.2 ± 0.2 | 2 ± 0.2 | 5.5 ± 0.5 | 8.2 ± 0.8 | 6.6 ± 0.6 | 6.1 ± 0.4 | |
| NH3 | 8.1 ± 0.4 | 3.9 ± 0.13 | 3.8 ± 0.13 | 5.9 ± 0.15 | 3.8 ± 0.16 | 7.8 ± 0.3 | 6.4 ± 0.3 | |
| [Gl-POSS] wt% | 0 | 0.1 | 0.5 | 1.0 | 2.0 | 5.0 | 8.0 | |
|---|---|---|---|---|---|---|---|---|
| Gas | ||||||||
| N2 | 0.2 ± 0.04 | 0.3 ± 0.06 | 0.4 ± 0.09 | 0.2 ± 0.03 | 5.3 ± 0.7 | 9.4 ± 1.11 | 7 ± 0.6 | |
| H2 | 0.2 ± 0.04 | 1.2 ± 0.2 | 5.4 ± 0.8 | 3 ± 0.4 | 1.7 ± 0.2 | 2.8 ± 0.4 | 6.6 ± 0.7 | |
| NH3 | 20.2 ± 1.2 | 61 ± 2.8 | 74 ± 3.4 | 58 ± 1.9 | 38 ± 2.2 | 22.6 ± 1.3 | 11.1 ± 0.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaripov, I.I.; Davletbaeva, I.M.; Faizulina, Z.Z.; Davletbaev, R.S.; Gubaidullin, A.T.; Atlaskin, A.A.; Vorotyntsev, I.V. Synthesis and Characterization of Novel Nanoporous Gl-POSS-Branched Polymeric Gas Separation Membranes. Membranes 2020, 10, 110. https://doi.org/10.3390/membranes10050110
Zaripov II, Davletbaeva IM, Faizulina ZZ, Davletbaev RS, Gubaidullin AT, Atlaskin AA, Vorotyntsev IV. Synthesis and Characterization of Novel Nanoporous Gl-POSS-Branched Polymeric Gas Separation Membranes. Membranes. 2020; 10(5):110. https://doi.org/10.3390/membranes10050110
Chicago/Turabian StyleZaripov, Ilnaz I., Ilsiya M. Davletbaeva, Zulfiya Z. Faizulina, Ruslan S. Davletbaev, Aidar T. Gubaidullin, Artem A. Atlaskin, and Ilya V. Vorotyntsev. 2020. "Synthesis and Characterization of Novel Nanoporous Gl-POSS-Branched Polymeric Gas Separation Membranes" Membranes 10, no. 5: 110. https://doi.org/10.3390/membranes10050110