In this work, the examined aqueous mixtures can be seen in
Table 1. PDMS (Sulzer PERVAP 4060), PVA (Sulzer PERVAP 1510) and, in the first case, PEBA pervaporation membranes were investigated. The standard deviation of the experimental data was 0.05. There were a minimum of three replicates in the experiments.
2.1. Pervaporation Modelling
Modelling of traditional separation methods such as distillation is widely researched in literature [
20,
21]. The research field of distillation is heavily based on the vapour–liquid equilibrium (hence VLE). On the other hand, the mechanism of membrane separation cannot be explained by VLE. One of the most basic models that can be used for membrane separation is the diffusive or pore flow model.
In this case, the driving force of the separation is the chemical potential gradient between the two sides of the membrane. Because of the constant pressure in the membrane, the chemical potential gradient can be replaced with the concentration gradient. In this way, it is easier to define one of the most important parameters of pervaporation, the membrane flux, which can be described by Fick’s first law:
Since this is a general model for most transport processes, in this context ci is the concentration outside of the membrane.
The biggest drawback of this simplistic equation is the concentration dependency of the diffusion coefficient for non-ideal mixtures. For years, the scientific community have been working on the improvement of PV models for reliable flowsheet modelling uses.
Several pervaporation models have been brought forth as viable alternatives, such as the total solvent volume fraction model, pore-flow model and solution-diffusion model [
12,
22,
23,
24]. The solution diffusion one is quite probable theory; however, there are other theories to explain the complex phenomena of this membrane process. These theories are all only hypotheses and the authors know no experiment to prove them. One of the most widely accepted explanations is the solution-diffusion model, which is applicable for two-layered composite membranes. The model can be described by the following steps [
12,
25]:
absorption of components in the membrane;
selective diffusion of components through the length of the membrane;
desorption and consequential evaporation to vapour phase on the permeate side.
This is the model on which Rautenbach’s (1990) [
25] work is based. In his work, the driving force of the chemical potential gradient is replaced by the fugacity gradient, and the diffusion coefficient is replaced by the transport coefficient. The latter change is significant because of the lesser concentration dependency of the transport coefficient [
26].
Based on Fick’s equation (Equation (1)), the component flux can be described as follows:
where
is the geometric mean of the activity coefficient at the two sides of the composite membrane:
Introducing the transport coefficient:
Using the transport coefficient Equation (2) can be modified into:
Because the pressure at the permeate side is very low, the gas phase can be considered an ideal gas, and thus the fugacity difference can be replaced by partial pressure difference. Based on this, the partial flux can be defined as:
If Equation (5) is only determined for the active layer of the composite membrane and pressure is introduced instead of fugacity, combined with Equation (6), flux can by expressed as:
Transport coefficient can be calculated by the following Arrhenius type equation:
where
T* is the reference temperature, in this case equal to 293 K or 20 °C.
In the works of Mizsey and Valentinyi [
26,
27], Rautenbach’s equation (Equation (7)) was modified and developed into the following:
This equation is called Model I in the work of Valentinyi et al. (2013) [
27] (also, the original Rautenbach model is Equation (7)). It is generally accepted that the diffusion coefficient has an intense dependence on the feed concentration. Many authors have proposed an exponential relationship between the feed concentration and diffusion coefficient, which justifies the inserting of an exponential term into the pervaporation model [
10,
27]. This developed model is based on empirical laboratorial data [
28,
29], and it is specifically recommended for polymer and composite membranes.
Furthermore, in Mizsey’s work (2005) [
26], it was established that the first part of Equation (7) as well as Equation (9) can be ignored. The reasoning behind this is that the porous supporting layer’s permeability coefficient (
Q0) is infinitely big compared to the transport coefficient, correlating with the concept that this layer’s resistance is negligible. Thus, the Model I can be simplified as:
In this work, this equation was further improved as the following two models and researched in a similar fashion:
These models are called Model II and Model III, respectively.
2.2. Model Improvement
As mentioned, the model research is based on empirical laboratorian data [
10,
14,
15,
16,
17,
18,
19]. For the calculations, the following base parameters were needed:
mole fraction of the feed (xi1) [mole/mole];
mole fraction of the permeate (xi3) [mole/mole];
coefficients of the Wilson equation (Aij, Aji) [cal/moleK];
input temperature (T) [°C. K];
constants of the Antoine equation for both components (A, B, C, D and E) [-];
pressure on the permeate side (p3) [bar. kPa];
partial fluxes of both components (Ji) [kg/m2h].
The Antoine constants and Wilson parameters were obtained from the ChemCAD software’s database. Lower index numbers, as in the case of xi1 and p3, represent the location in the membrane module: 1 is the feed side, 2 is the intermembrane plane and 3 is the permeate side.
Based on these input parameters the following calculations can be executed. The activity coefficients can be calculated by the Wilson equation for both sides of the membrane and for both components. For example, for the component
i, the feed side activity coefficient is:
where the Λ
ij and Λ
ji coefficients were obtained by the following formulas:
where the V
i and V
j are the molar volume of pure liquid. and it can be calculated as follows:
where A, B, C and D are the constants of the Antoine equation for the i and j components, respectively.
The pure component’s partial pressures can be calculated by the Antoine equation:
where
A,
B,
C,
D and
E are the material depending constants of the Antoine equation.
Partial pressure in the feed and permeate side can be calculated by the Raoult’s law (Equation (17)) and Dalton’s law (Equation (18)), respectively:
To compare Model I, II and III, parameter fitting was used, for this purpose STATISTICA software was used. The estimated parameters were the reference transport coefficient (
). component’s activation energy (
Ei) and the added
B parameter of the new models. The estimated function derives from the combination of the respective model and Equation (8):
As this is a nonlinear estimation process, a custom loss function needs to be defined, so least squares approximation was used. The objective function (hence OF) which needed to be minimized is the following:
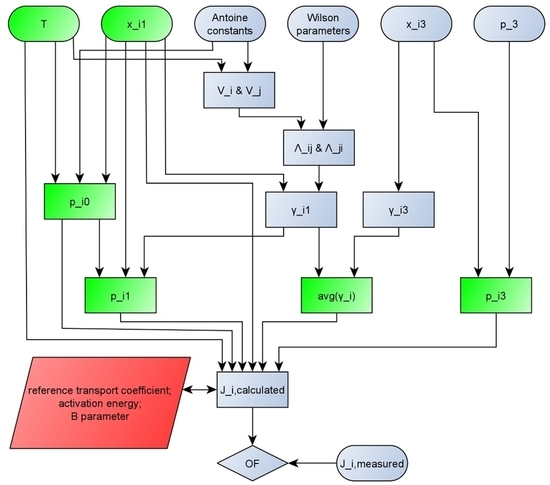
For better transparency, the calculation method that was used in this work is represented on a flowchart (
Figure 1).