Immunogenicity in African Green Monkeys of M Protein Mutant Vesicular Stomatitis Virus Vectors and Contribution of Vector-Encoded Flagellin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Animals, Vaccination and Tissue Collection
2.3. Cells
2.4. ELISA for Detection of VSV-Specific Antibody
2.5. Affinity ELISA
2.6. Neutralizing Antibody Assay
2.7. IFNγ ELISPOT Assay
2.8. Murine Vaccination Study
2.9. Statistical Analysis
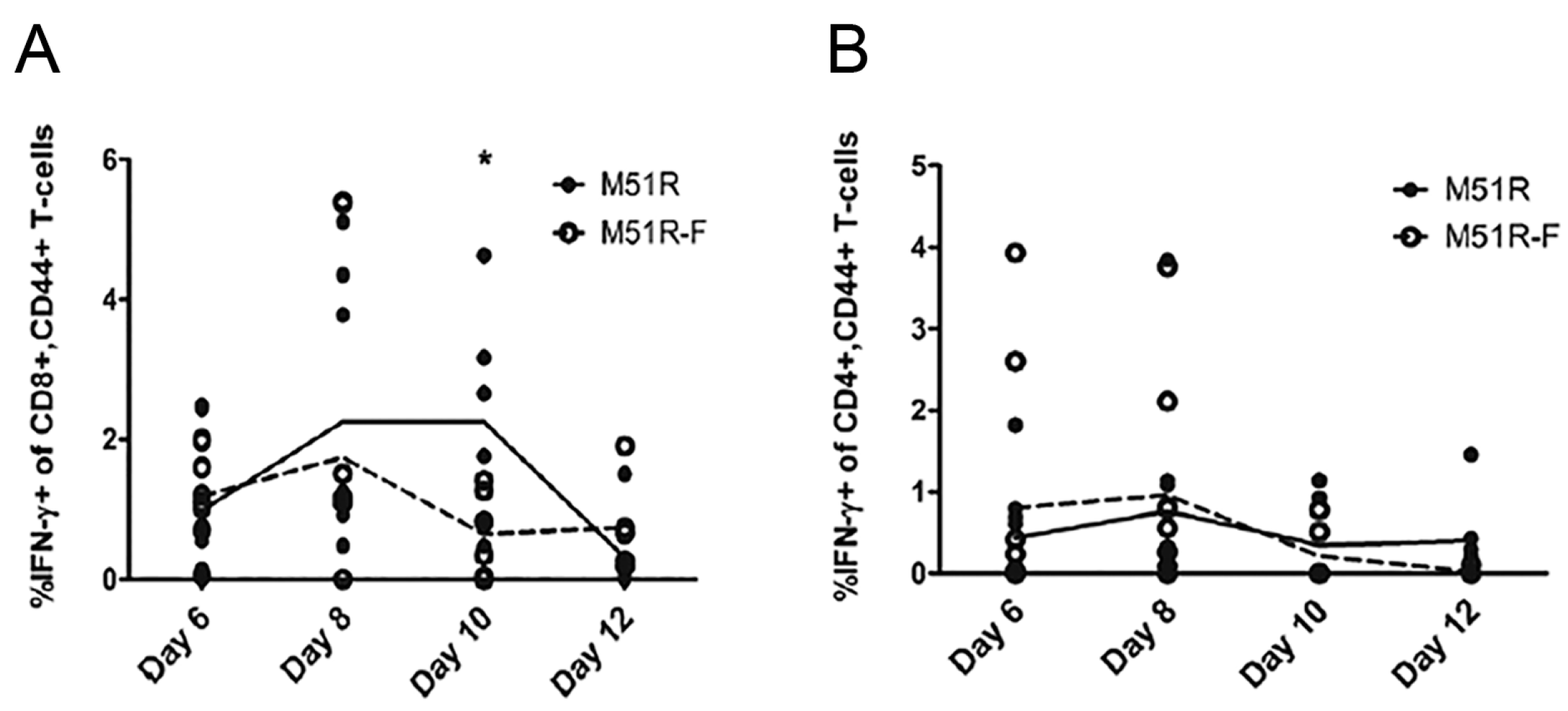
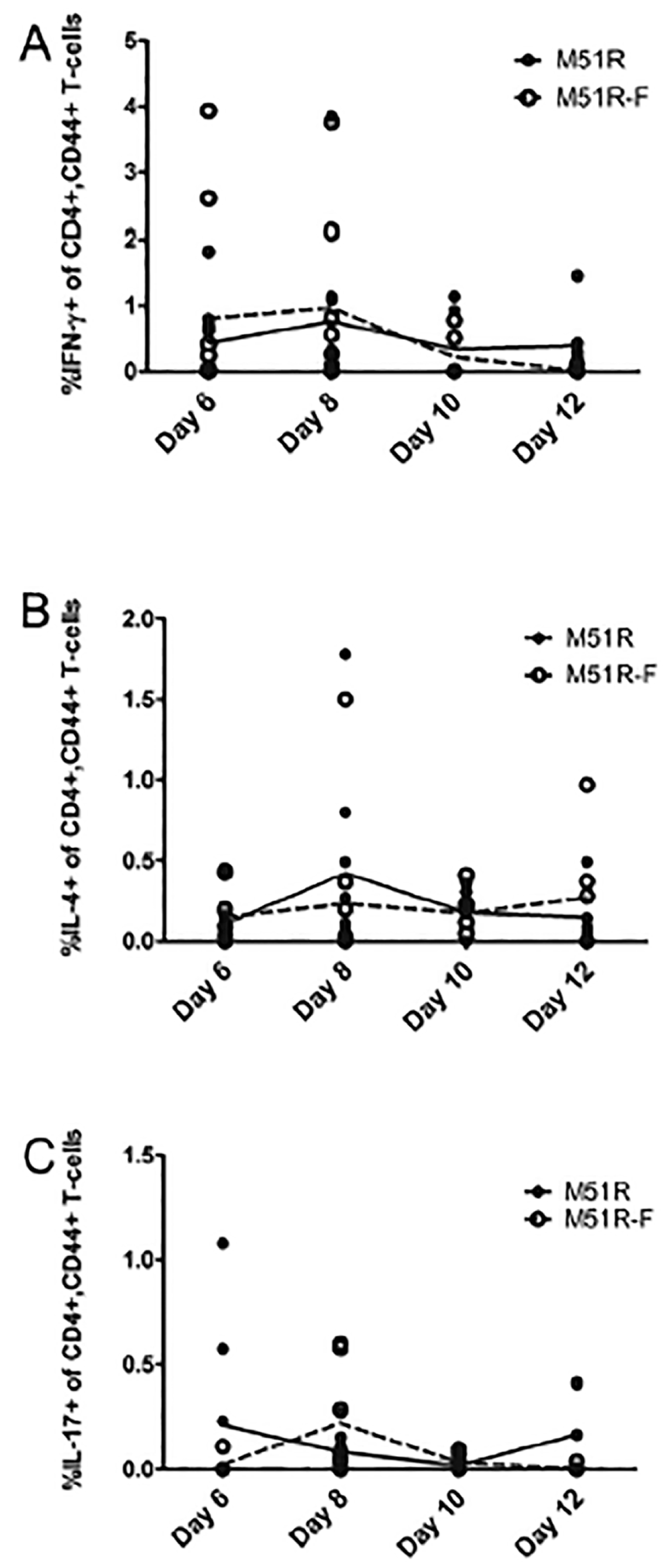
3. Results
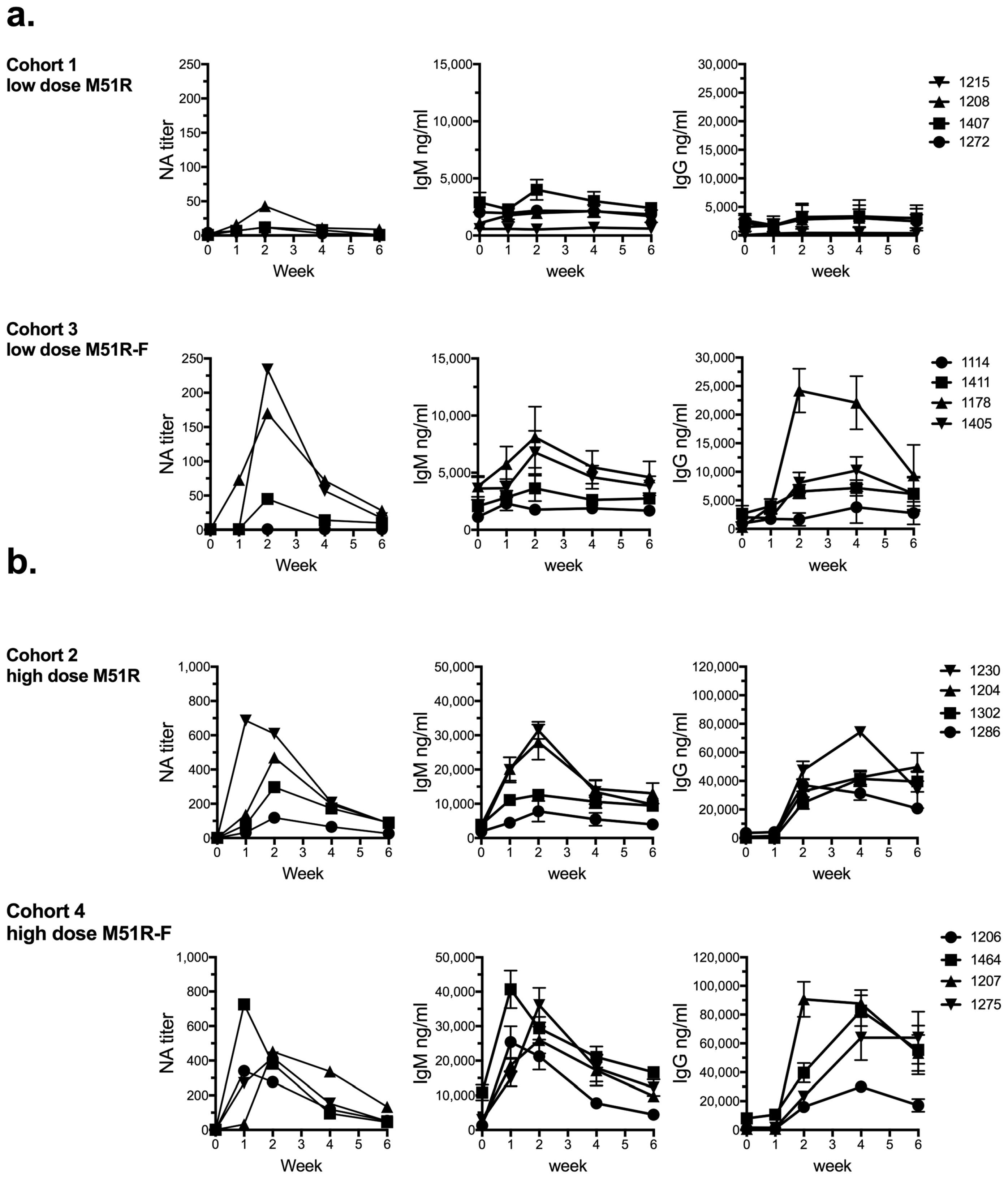
3.1. Anti-VSV Antibody Response
3.2. Anti-VSV Cellular Response
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Clarke, D.K.; Hendry, R.M.; Singh, V.; Rose, J.K.; Seligman, S.J.; Klug, B.; Kochhar, S.; Mac, L.M.; Carbery, B.; Chen, R.T.; et al. Live Virus Vaccines Based on a Vesicular Stomatitis Virus (Vsv) Backbone: Standardized Template with Key Considerations for a Risk/Benefit Assessment. Vaccine 2016, 34, 6597–6609. [Google Scholar] [CrossRef] [PubMed]
- Van den Pol, A.N.; Davis, J.N. Highly Attenuated Recombinant Vesicular Stomatitis Virus Vsv-12′gfp Displays Immunogenic and Oncolytic Activity. J. Virol. 2013, 87, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Longini, I.M.; Egger, M.; Dean, N.E.; Edmunds, W.J.; Camacho, A.; Carroll, M.W.; Doumbia, M.; Draguez, B.; Duraffour, S.; et al. Efficacy and Effectiveness of an Rvsv-Vectored Vaccine Expressing Ebola Surface Glycoprotein: Interim Results from the Guinea Ring Vaccination Cluster-Randomised Trial. Lancet 2015, 386, 857–866. [Google Scholar] [CrossRef]
- Fuchs, J.D.; Frank, I.; Elizaga, M.L.; Allen, M.; Frahm, N.; Kochar, N.; Li, S.; Edupuganti, S.; Kalams, S.A.; Tomaras, G.D.; et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 Gag Vaccine (Hvtn 090). Open Forum Infect. Dis. 2015, 2. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Feldmann, H. Recombinant Vesicular Stomatitis Virus-Based Vaccines against Ebola and Marburg Virus Infections. J. Infect. Dis. 2011, 204 (Suppl. 3), S1075–S1081. [Google Scholar] [CrossRef] [PubMed]
- Publicover, J.; Ramsburg, E.; Rose, J.K. Characterization of Nonpathogenic, Live, Viral Vaccine Vectors Inducing Potent Cellular Immune Responses. J. Virol. 2004, 78, 9317–9324. [Google Scholar] [CrossRef] [PubMed]
- Wertz, G.W.; Perepelitsa, V.P.; Ball, L.A. Gene Rearrangement Attenuates Expression and Lethality of a Nonsegmented Negative Strand Rna Virus. Proc. Natl. Acad. Sci. USA 1998, 95, 3501–3506. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.B.; Zamparo, J.M.; Ball, L.A.; Rodriguez, L.L.; Wertz, G.W. Rearrangement of the Genes of Vesicular Stomatitis Virus Eliminates Clinical Disease in the Natural Host: New Strategy for Vaccine Development. J. Virol. 2001, 75, 6107–6114. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.K.; Nasar, F.; Lee, M.; Johnson, J.E.; Wright, K.; Calderon, P.; Guo, M.; Natuk, R.; Cooper, D.; Hendry, R.M.; et al. Synergistic Attenuation of Vesicular Stomatitis Virus by Combination of Specific G Gene Truncations and N Gene Translocations. J. Virol. 2007, 81, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Wright, K.J.; Calderon, P.C.; Guo, M.; Nasar, F.; Johnson, J.E.; Coleman, J.W.; Lee, M.; Kotash, C.; Yurgelonis, I.; et al. Attenuation of Recombinant Vesicular Stomatitis Virus-Human Immunodeficiency Virus Type 1 Vaccine Vectors by Gene Translocations and G Gene Truncation Reduces Neurovirulence and Enhances Immunogenicity in Mice. J. Virol. 2008, 82, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.K.; Nasar, F.; Chong, S.; Johnson, J.E.; Coleman, J.W.; Lee, M.; Witko, S.E.; Kotash, C.S.; Abdullah, R.; Megati, S.; et al. Neurovirulence and Immunogenicity of Attenuated Recombinant Vesicular Stomatitis Viruses in Nonhuman Primates. J. Virol. 2014, 88, 6690–6701. [Google Scholar] [CrossRef] [PubMed]
- Black, B.L.; Rhodes, R.B.; McKenzie, M.; Lyles, D.S. The Role of Vesicular Stomatitis Virus Matrix Protein in Inhibition of Host-Directed Gene Expression Is Genetically Separable from Its Function in Virus Assembly. J. Virol. 1993, 67, 4814–4821. [Google Scholar] [PubMed]
- Ahmed, M.; Brzoza, K.L.; Hiltbold, E.M. Matrix Protein Mutant of Vesicular Stomatitis Virus Stimulates Maturation of Myeloid Dendritic Cells. J. Virol. 2006, 80, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Mitchell, L.M.; Puckett, S.; Brzoza-Lewis, K.L.; Lyles, D.S.; Hiltbold, E.M. Vesicular Stomatitis Virus M Protein Mutant Stimulates Maturation of Toll-Like Receptor 7 (Tlr7)-Positive Dendritic Cells through Tlr-Dependent and -Independent Mechanisms. J. Virol. 2009, 83, 2962–2975. [Google Scholar] [CrossRef] [PubMed]
- Westcott, M.M.; Ahmed, M.; Smedberg, J.R.; Rajani, K.R.; Hiltbold, E.M.; Lyles, D.S. Preservation of Dendritic Cell Function During Vesicular Stomatitis Virus Infection Reflects Both Intrinsic and Acquired Mechanisms of Resistance to Suppression of Host Gene Expression by Viral M Protein. J. Virol. 2013, 87, 11730–11740. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; McKenzie, M.O.; Puckett, S.; Hojnacki, M.; Poliquin, L.; Lyles, D.S. Ability of the Matrix Protein of Vesicular Stomatitis Virus to Suppress Beta Interferon Gene Expression Is Genetically Correlated with the Inhibition of Host Rna and Protein Synthesis. J. Virol. 2003, 77, 4646–4657. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, J.E.; Bridle, B.W.; Stephenson, K.B.; Jenkins, K.M.; Brunelliere, J.; Bramson, J.L.; Lichty, B.D.; Wan, Y. Recombinant Vesicular Stomatitis Virus Transduction of Dendritic Cells Enhances Their Ability to Prime Innate and Adaptive Antitumor Immunity. Mol. Ther. J. Am. Soc. Gene Ther. 2009, 17, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Marino, T.R.; Puckett, S.; Kock, N.D.; Lyles, D.S. Immune Response in the Absence of Neurovirulence in Mice Infected with M Protein Mutant Vesicular Stomatitis Virus. J. Virol. 2008, 82, 9273–9277. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Puckett, S.; Arimilli, S.; Braxton, C.L.; Mizel, S.B.; Lyles, D.S. Stimulation of Human Dendritic Cells by Wild-Type and M Protein Mutant Vesicular Stomatitis Viruses Engineered to Express Bacterial Flagellin. J. Virol. 2010, 84, 12093–12098. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, B.C.; D’Agostino, R.B., Jr.; Parks, G.D.; Alexander-Miller, M.A. Adjuvanting an Inactivated Influenza Vaccine with Flagellin Improves the Function and Quantity of the Long-Term Antibody Response in a Nonhuman Primate Neonate Model. Vaccine 2016, 34, 4712–4717. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kim, J.R.; Chang, T.Z.; Zhang, H.; Mohan, T.; Champion, J.A.; Wang, B.Z. Protein Nanoparticle Vaccine Based on Flagellin Carrier Fused to Influenza Conserved Epitopes Confers Full Protection against Influenza a Virus Challenge. Virology 2017, 509, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mizel, S.B.; Graff, A.H.; Sriranganathan, N.; Ervin, S.; Lees, C.J.; Lively, M.O.; Hantgan, R.R.; Thomas, M.J.; Wood, J.; Bell, B. Flagellin-F1-V Fusion Protein Is an Effective Plague Vaccine in Mice and Two Species of Nonhuman Primates. Clin. Vaccine Immunol. CVI 2009, 16, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Lottenbach, K.; Graham, I.; Anderson, E.; Bajwa, K.; May, R.C.; Mizel, S.B.; Graff, A.; Belshe, R.B. A Phase I Safety and Immunogenicity Dose Escalation Trial of Plague Vaccine, Flagellin/F1/V, in Healthy Adult Volunteers (Dmid 08-0066). Vaccine 2017, 35, 6759–6765. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The Innate Immune Response to Bacterial Flagellin Is Mediated by Toll-Like Receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Mizel, S.B.; Bates, J.T. Flagellin as an Adjuvant: Cellular Mechanisms and Potential. J. Immunol. 2010, 185, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Andersen-Nissen, E.; Warren, S.E.; Aderem, A. Tlr5 and Ipaf: Dual Sensors of Bacterial Flagellin in the Innate Immune System. Semin. Immunopathol. 2007, 29, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, J.R.; Westcott, M.M.; Ahmed, M.; Lyles, D.S. Signaling Pathways in Murine Dendritic Cells That Regulate the Response to Vesicular Stomatitis Virus Vectors That Express Flagellin. J. Virol. 2014, 88, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic Flagellin Activates Caspase-1 and Secretion of Interleukin 1beta Via Ipaf. Nat. Immunol. 2006, 7, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, Z.W.; Connor, J.H.; Lyles, D.S. Preferential Translation of Vesicular Stomatitis Virus Mrnas Is Conferred by Transcription from the Viral Genome. J. Virol. 2006, 80, 11733–11742. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, D.M.; Ishikawa, K.; Lyles, D.S. Role of De Novo Protein Synthesis in Target Cells Recognized by Cytotoxic T Lymphocytes Specific for Vesicular Stomatitis Virus. J. Virol. 1991, 65, 6856–6861. [Google Scholar] [PubMed]
- Macdonald, R.A.; Hosking, C.S.; Jones, C.L. The Measurement of Relative Antibody Affinity by Elisa Using Thiocyanate Elution. J. Immunol. Methods 1988, 106, 191–194. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Moss, B. Development and Use of a Vaccinia Virus Neutralization Assay Based on Flow Cytometric Detection of Green Fluorescent Protein. J. Virol. 2003, 77, 10684–10688. [Google Scholar] [CrossRef] [PubMed]
- Van Bleek, G.M.; Nathenson, S.G. Isolation of an Endogenously Processed Immunodominant Viral Peptide from the Class I H-2kb Molecule. Nature 1990, 348, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Burkhart, C.; Freer, G.; Rulicke, T.; Pircher, H.; Kono, D.H.; Theofilopoulos, A.N.; Ludewig, B.; Hoffmann-Rohrer, U.; Zinkernagel, R.M.; et al. Qualitative and Quantitative Requirements for Cd4+ T Cell-Mediated Antiviral Protection. J. Immunol. 1999, 162, 2867–2874. [Google Scholar] [PubMed]
- Weimer, E.T.; Lu, H.; Kock, N.D.; Wozniak, D.J.; Mizel, S.B. A Fusion Protein Vaccine Containing Oprf Epitope 8, Opri, and Type a and B Flagellins Promotes Enhanced Clearance of Nonmucoid Pseudomonas Aeruginosa. Infect. Immun. 2009, 77, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Weimer, E.T.; Ervin, S.E.; Wozniak, D.J.; Mizel, S.B. Immunization of Young African Green Monkeys with Oprf Epitope 8-Opri-Type a- and B-Flagellin Fusion Proteins Promotes the Production of Protective Antibodies against Nonmucoid Pseudomonas Aeruginosa. Vaccine 2009, 27, 6762–6769. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.M. Biotechnology Approaches to Produce Potent, Self-Adjuvanting Antigen-Adjuvant Fusion Protein Subunit Vaccines. Biotechnol. Adv. 2017, 35, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, S.Z.; Wang, K.; Cohen, J.; Paul, W.E. Il-1beta Strikingly Enhances Antigen-Driven Cd4 and Cd8 T-Cell Responses. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, S.Z.; Hogg, A.; Hu-Li, J.; Wingfield, P.; Chen, X.; Crank, M.; Caucheteux, S.; Ratner-Hurevich, M.; Berzofsky, J.A.; Nir-Paz, R.; et al. Il-1 Enhances Expansion, Effector Function, Tissue Localization, and Memory Response of Antigen-Specific Cd8 T Cells. J. Exp. Med. 2013, 210, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Lee, H.K.; Ogura, Y.; Flavell, R.; Iwasaki, A. Inflammasome Recognition of Influenza Virus Is Essential for Adaptive Immune Responses. J. Exp. Med. 2009, 206, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.K.; Ichinohe, T.; Iwasaki, A. Il-1r Signaling in Dendritic Cells Replaces Pattern-Recognition Receptors in Promoting Cd8(+) T Cell Responses to Influenza a Virus. Nat. Immunol. 2013, 14, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Al Moussawi, K.; Kazmierczak, B.I. Distinct Contributions of Interleukin-1alpha (Il-1alpha) and Il-1beta to Innate Immune Recognition of Pseudomonas Aeruginosa in the Lung. Infect. Immun. 2014, 82, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Publicover, J.; Ramsburg, E.; Rose, J.K. A Single-Cycle Vaccine Vector Based on Vesicular Stomatitis Virus Can Induce Immune Responses Comparable to Those Generated by a Replication-Competent Vector. J. Virol. 2005, 79, 13231–13238. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, N.; Khairnar, V.; Duhan, V.; Zhou, F.; Tur, R.F.; Haussinger, D.; Recher, M.; Tumanov, A.V.; Hardt, C.; Pinschewer, D.; et al. Two Separate Mechanisms of Enforced Viral Replication Balance Innate and Adaptive Immune Activation. J. Autoimmun. 2016, 67, 82–89. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.E.; Sun, J.C.; Lanier, L.L. Natural Killer Cell Memory. Immunity 2015, 43, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Liu, X.; Han, C.; Xu, S.; Xie, B.; Zhang, Q.; Gu, Y.; Hou, J.; Qian, L.; Qian, C.; et al. Identification of Ifn-Gamma-Producing Innate B Cells. Cell Res. 2014, 24, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Buonocore, L.; Price, R.; Forman, J.; Rose, J.K. Attenuated Vesicular Stomatitis Viruses as Vaccine Vectors. J. Virol. 1999, 73, 3723–3732. [Google Scholar] [PubMed]
- Le Bon, A.; Thompson, C.; Kamphuis, E.; Durand, V.; Rossmann, C.; Kalinke, U.; Tough, D.F. Cutting Edge: Enhancement of Antibody Responses through Direct Stimulation of B and T Cells by Type I Ifn. J. Immunol. 2006, 176, 2074–2078. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Lang, K.S.; Manjarrez-Orduno, N.; Junt, T.; Senn, B.M.; Holdener, M.; Akira, S.; Zinkernagel, R.M.; Hengartner, H. Early Type I Interferon-Mediated Signals on B Cells Specifically Enhance Antiviral Humoral Responses. Eur. J. Immunol. 2006, 36, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- An, H.Y.; Kim, G.N.; Wu, K.; Kang, C.Y. Genetically Modified Vsv(Nj) Vector Is Capable of Accommodating a Large Foreign Gene Insert and Allows High Level Gene Expression. Virus Res. 2013, 171, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Haglund, K.; Forman, J.; Krausslich, H.G.; Rose, J.K. Expression of Human Immunodeficiency Virus Type 1 Gag Protein Precursor and Envelope Proteins from a Vesicular Stomatitis Virus Recombinant: High-Level Production of Virus-Like Particles Containing Hiv Envelope. Virology 2000, 268, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Nasar, F.; Coleman, J.W.; Price, R.E.; Javadian, A.; Draper, K.; Lee, M.; Reilly, P.A.; Clarke, D.K.; Hendry, R.M.; et al. Neurovirulence Properties of Recombinant Vesicular Stomatitis Virus Vectors in Non-Human Primates. Virology 2007, 360, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Lichty, B.D.; tenOever, B.R.; Paterson, J.M.; Power, A.T.; Knowles, S.; Marius, R.; Reynard, J.; Poliquin, L.; Atkins, H.; et al. Vsv Strains with Defects in Their Ability to Shutdown Innate Immunity Are Potent Systemic Anti-Cancer Agents. Cancer Cell 2003, 4, 263–275. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westcott, M.M.; Smedberg, J.; Jorgensen, M.J.; Puckett, S.; Lyles, D.S. Immunogenicity in African Green Monkeys of M Protein Mutant Vesicular Stomatitis Virus Vectors and Contribution of Vector-Encoded Flagellin. Vaccines 2018, 6, 16. https://doi.org/10.3390/vaccines6010016
Westcott MM, Smedberg J, Jorgensen MJ, Puckett S, Lyles DS. Immunogenicity in African Green Monkeys of M Protein Mutant Vesicular Stomatitis Virus Vectors and Contribution of Vector-Encoded Flagellin. Vaccines. 2018; 6(1):16. https://doi.org/10.3390/vaccines6010016
Chicago/Turabian StyleWestcott, Marlena M., Jason Smedberg, Matthew J. Jorgensen, Shelby Puckett, and Douglas S. Lyles. 2018. "Immunogenicity in African Green Monkeys of M Protein Mutant Vesicular Stomatitis Virus Vectors and Contribution of Vector-Encoded Flagellin" Vaccines 6, no. 1: 16. https://doi.org/10.3390/vaccines6010016



