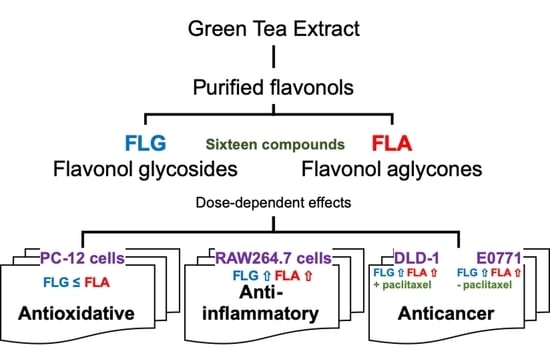
Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Cell Lines
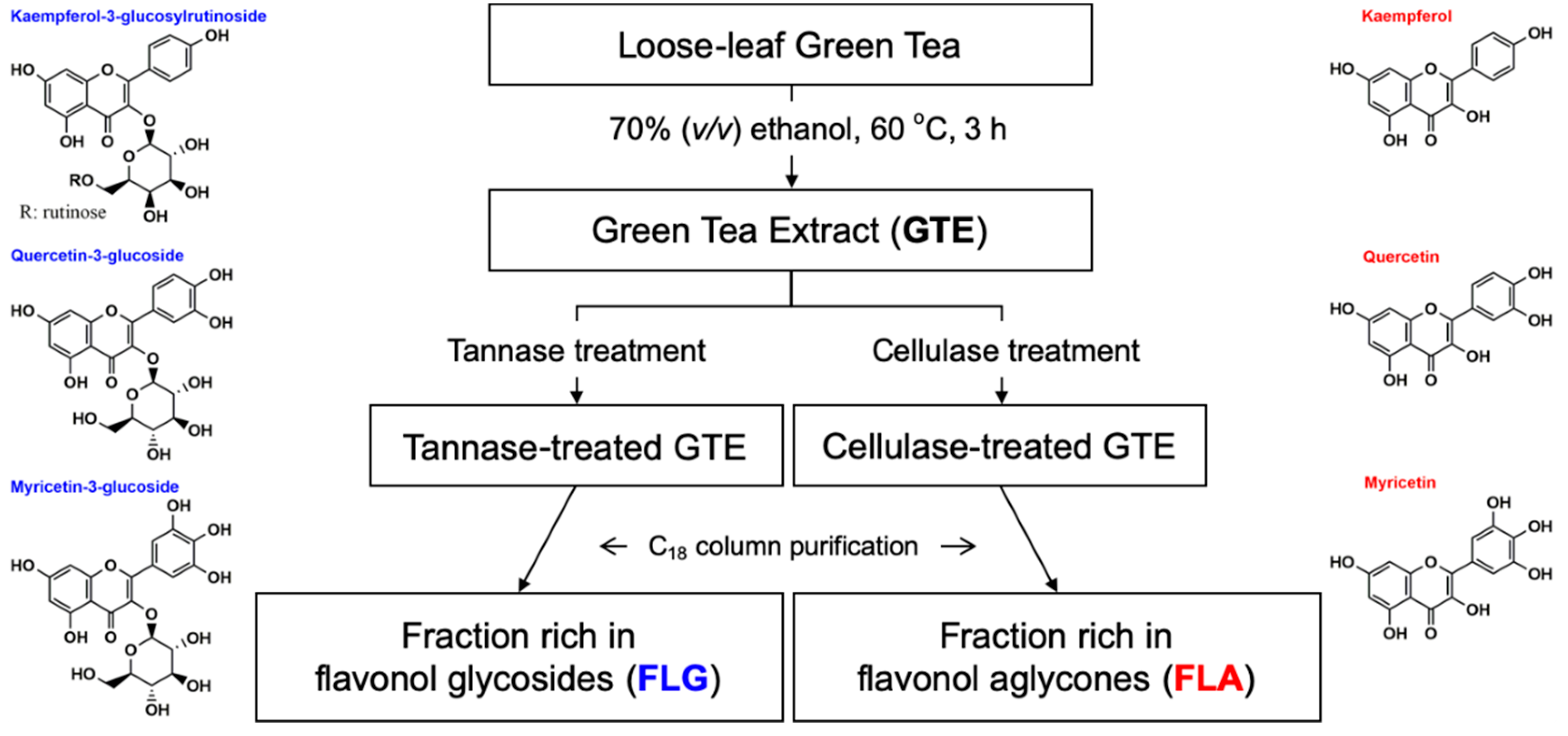
2.2. Preparation of the Purified FLG and FLA from GTE
2.3. Total Phenolic and Flavonoid Content in FLG and FLA
2.4. Quantification and Mass Identification of Flavonols
2.5. Measurements of Antioxidant Capacities of FLG and FLA
2.5.1. Antioxidant Capacity Measurements with ABTS Radicals
2.5.2. Antioxidant Capacity Measurements with DPPH Radicals
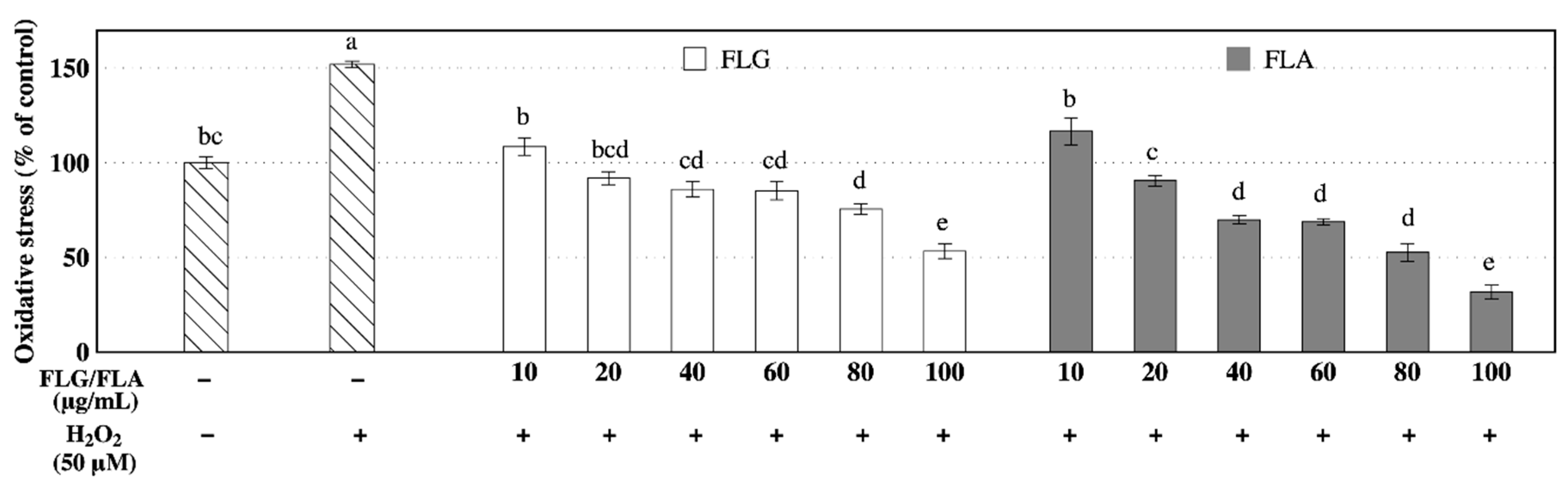
2.6. Measurements of Intracellular Oxidative Stress of FLG and FLA
2.7. Anti-Inflammatory Effects of FLG and FLA
2.8. Anticancer Effects of FLG and FLA
2.9. Statistical Analyses
3. Results
3.1. Phenolic Compositions of FLG and FLA
3.2. Antioxidant Capacities of FLG and FLA
3.3. Effects of FLG and FLA on Intracellular Oxidative Stress
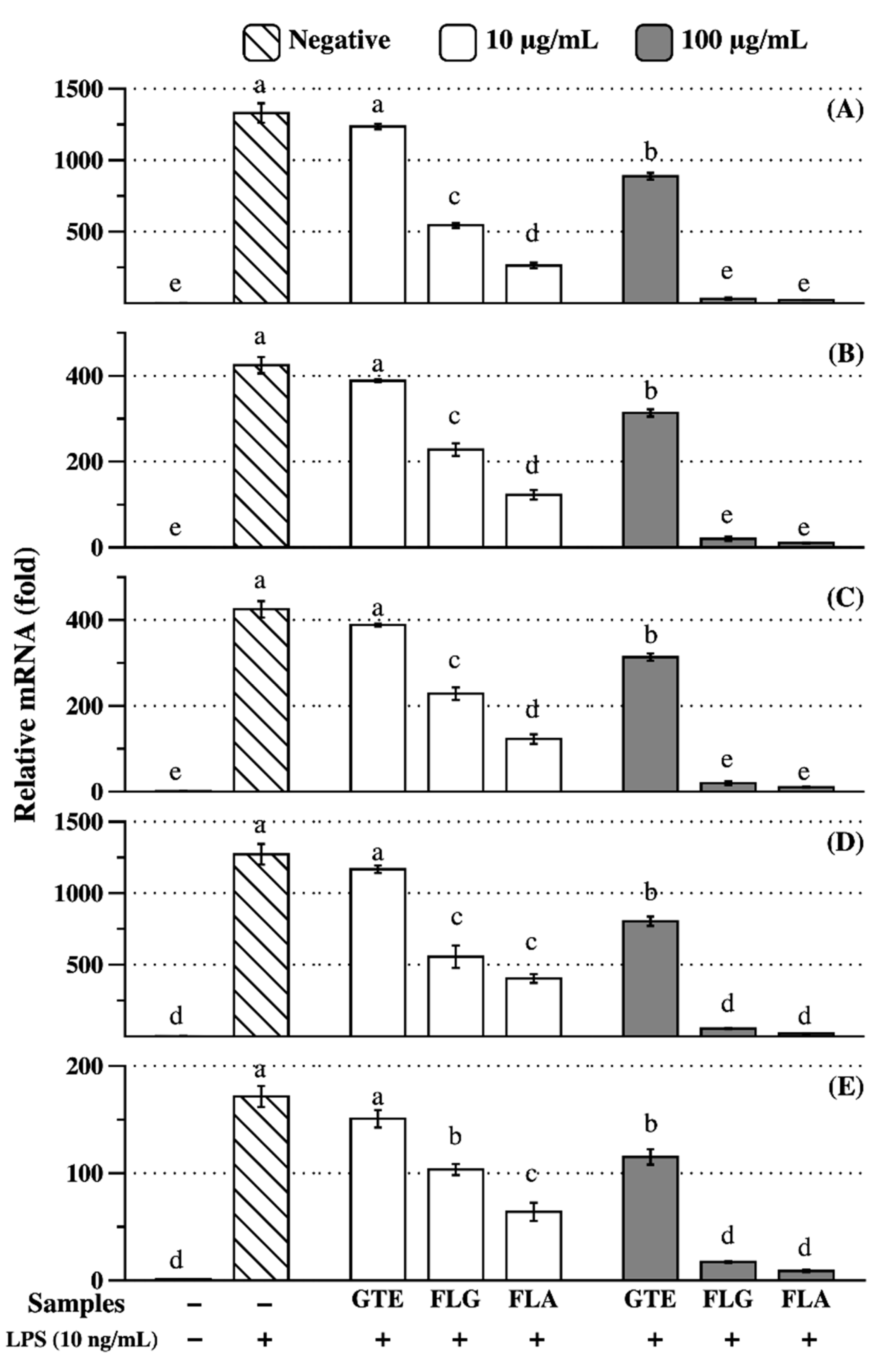
3.4. Anti-Inflammatory Effects of FLG and FLA
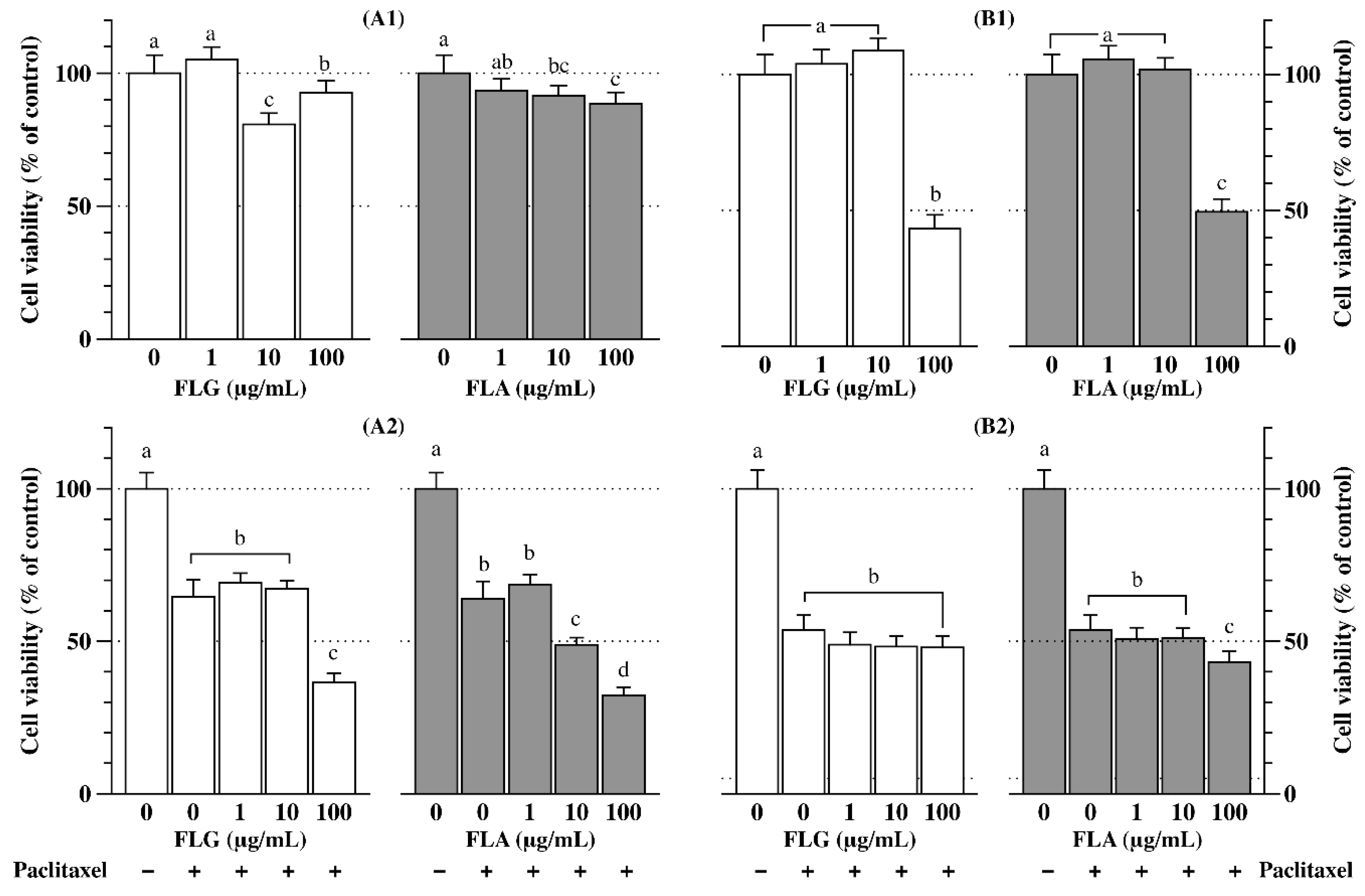
3.5. Anticancer Effects of FLG and FLA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| CE COX-2 CV DCFH-DA | catechin equivalents cyclooxygenase-2 column volume 2′,7′-dichlorofluorescein diacetate |
| DHE | dihydroethidium |
| DMSO | dimethyl sulfoxide |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DW | dry weight |
| EC | (−)-epicatechin |
| ECG | (−)-epicatechin gallate |
| EGC | (−)-epigallocatechin |
| EGCG | (−)-epigallocatechin gallate |
| FBS | fetal bovine serum |
| FLA | fraction rich in flavonol aglycones |
| FLG | fraction rich in flavonol glycosides |
| GAE GTE | gallic acid equivalents green tea extract |
| HPLC | high-performance liquid chromatography |
| HBSS HSD | Hanks’ balanced salt solution honestly significant difference |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LPS MMP9 | Lipopolysaccharide matrix metalloproteinase 9 |
| MTT | 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide |
| ODS PBS | octadecyl-silica phosphate-buffered saline |
| qPCR | quantitative real-time polymerase chain reaction |
| ROS | reactive oxygen species |
| RPMI | Roswell Park Memorial Institute |
| TLR4 | toll-like receptor 4 |
| VCE | vitamin C equivalents |
References
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. J. Food Compost. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Monobe, M.; Nomura, S.; Ema, K.; Matsunaga, A.; Nesumi, A.; Yoshida, K.; Maeda-Yamamoto, M.; Horie, H. Quercetin glycosides-rich tea cultivars (Camellia sinensis L.) in Japan. Food Sci. Technol. Res. 2015, 21, 333–340. [Google Scholar] [CrossRef]
- Wu, C.; Xu, H.; Heritier, J.; Andlauer, W. Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chem. 2012, 132, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Engelhardt, U.H.; Thrane, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.A. Flavonol regulation in tumor cells. J. Cell. Biochem. 2015, 116, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Nomura, S.; Monobe, M.; Ema, K.; Matsunaga, A.; Maeda-Yamamoto, M.; Horie, H. Effects of flavonol-rich green tea cultivar (Camellia sinensis L.) on plasma oxidized LDL levels in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 2016, 80, 360–362. [Google Scholar] [CrossRef]
- Rietveld, A.; Wiseman, S. Antioxidant effects of tea: Evidence from human clinical trials. J. Nutr. 2003, 133, 3285S–3292S. [Google Scholar] [CrossRef]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; Gonzalez-Sarrias, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef]
- Plumb, G.W.; Price, K.R.; Williamson, G. Antioxidant properties of flavonol glycosides from tea. Redox Rep. 1999, 4, 13–16. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. Adv. Exp. Clin. Endocrinol. 2007, 81, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Ferrero-Miliani, L.; Nielsen, O.; Andersen, P.; Girardin, S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, J.Y.; Chung, M.Y.; Park, Y.K.; Bower, A.M.; Koo, S.I.; Giardina, C.; Bruno, R.S. Green tea extract suppresses NF-κB activation and inflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis. J. Nutr. 2012, 142, 57–63. [Google Scholar] [CrossRef]
- Itan, Y.; Jones, B.L.; Ingram, C.J.; Swallow, D.M.; Thomas, M.G. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 2010, 10, 36. [Google Scholar] [CrossRef]
- Nemeth, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Choi, E.H.; Rha, C.S.; Balusamy, S.R.; Kim, D.O.; Shim, S.M. Impact of bioconversion of gallated catechins and flavonol glycosides on bioaccessibility and intestinal cellular uptake of catechins. J. Agric. Food. Chem. 2019, 67, 2331–2339. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kim, J.W.; Im, S.; Jeong, H.R.; Jung, Y.S.; Lee, I.; Kim, K.J.; Park, S.K.; Kim, D.O. Neuroprotective effects of Korean red pine (Pinus densiflora) bark extract and its phenolics. J. Microbiol. Biotechnol. 2018, 28, 679–687. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Chen, P.; Harnly, J.M. New phenolic components and chromatographic profiles of green and fermented teas. J. Agric. Food Chem. 2008, 56, 8130–8140. [Google Scholar] [CrossRef]
- Li, S.-S.; Wu, J.; Chen, L.-G.; Du, H.; Xu, Y.-J.; Wang, L.-J.; Zhang, H.-J.; Zheng, X.-C.; Wang, L.-S. Biogenesis of C-glycosyl flavones and profiling of flavonoid glycosides in lotus (Nelumbo nucifera). PLoS ONE 2014, 9, e108860. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, D.H.; Liu, K.-H.; Oh, T.K.; Lee, C.H. Identification of flavonoids using liquid chromatography with electrospray ionization and ion trap tandem mass spectrometry with an MS/MS library. Rapid Commun. Mass Spectrom. 2005, 19, 3539–3548. [Google Scholar] [CrossRef]
- Cho, C.H.; Jang, H.; Lee, M.; Kang, H.; Heo, H.J.; Kim, D.O. Sea Buckthorn (Hippophae rhamnoides L.) Leaf Extracts Protect Neuronal PC-12 Cells from Oxidative Stress. J. Microbiol. Biotechnol. 2017, 27, 1257–1265. [Google Scholar] [CrossRef]
- Rusak, G.; Komes, D.; Likić, S.; Horžić, D.; Kovač, M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. 2008, 110, 852–858. [Google Scholar] [CrossRef]
- Price, K.R.; Rhodes, M.J.C.; Barnes, K.A. Flavonol glycoside content and composition of tea infusions made from commercially available teas and tea products. J. Agric. Food. Chem. 1998, 46, 2517–2522. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Senanayake, S.N. Green tea extract: Chemistry, antioxidant properties and food applications–A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Ratty, A.K.; Das, N.P. Effects of flavonoids on nonenzymatic lipid peroxidation: Structure-activity relationship. Biochem. Med. Metab. Biol. 1988, 39, 69–79. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Macedo, J.A.; Battestin, V.; Ribeiro, M.; Macedo, G.A. Increasing the antioxidant power of tea extracts by biotransformation of polyphenols. Food Chem. 2011, 126, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.O.; Lee, C.Y. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Ortiz, J.; Ferruzzi, M.G.; Taylor, L.S.; Mauer, L.J. Interaction of environmental moisture with powdered green tea formulations: Effect on catechin chemical stability. J. Agric. Food. Chem. 2008, 56, 4068–4077. [Google Scholar] [CrossRef]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009, 53, S44–S53. [Google Scholar] [CrossRef]
- Dall’Asta, M.; Calani, L.; Tedeschi, M.; Jechiu, L.; Brighenti, F.; Del Rio, D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition 2012, 28, 197–203. [Google Scholar] [CrossRef]
- Clifford, M.N.; van der Hooft, J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef]
- Shim, S.-M.; Yoo, S.-H.; Ra, C.-S.; Kim, Y.-K.; Chung, J.-O.; Lee, S.-J. Digestive stability and absorption of green tea polyphenols: Influence of acid and xylitol addition. Food Res. Int. 2012, 45, 204–210. [Google Scholar] [CrossRef]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced uptake and transport of (+)-catechin and (−)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int. J. Nanomed. 2014, 9, 2157. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.; Chen, D.; Chan, T.H.; Dou, Q.P. Inhibition of catechol-O-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (−)-EGCG. Oncol. Rep. 2010, 24, 563–569. [Google Scholar] [CrossRef]
- Ortega, N.; Macià, A.; Romero, M.-P.; Reguant, J.; Motilva, M.-J. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Lun Su, Y.; Leung, L.K.; Huang, Y.; Chen, Z.-Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Ungefroren, H.; Sebens, S.; Seidl, D.; Lehnert, H.; Hass, R. Interaction of tumor cells with the microenvironment. Cell Commun. Signal. 2011, 9, 18. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Kawanishi, S.; Ohnishi, S.; Ma, N.; Hiraku, Y.; Murata, M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1808. [Google Scholar] [CrossRef]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (−)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010, 12. [Google Scholar] [CrossRef]
- Stearns, M.E.; Wang, M. Synergistic effects of the green tea extract epigallocatechin-3-gallate and taxane in eradication of malignant human prostate tumors. Transl. Oncol. 2011, 4, 147–156. [Google Scholar] [CrossRef]
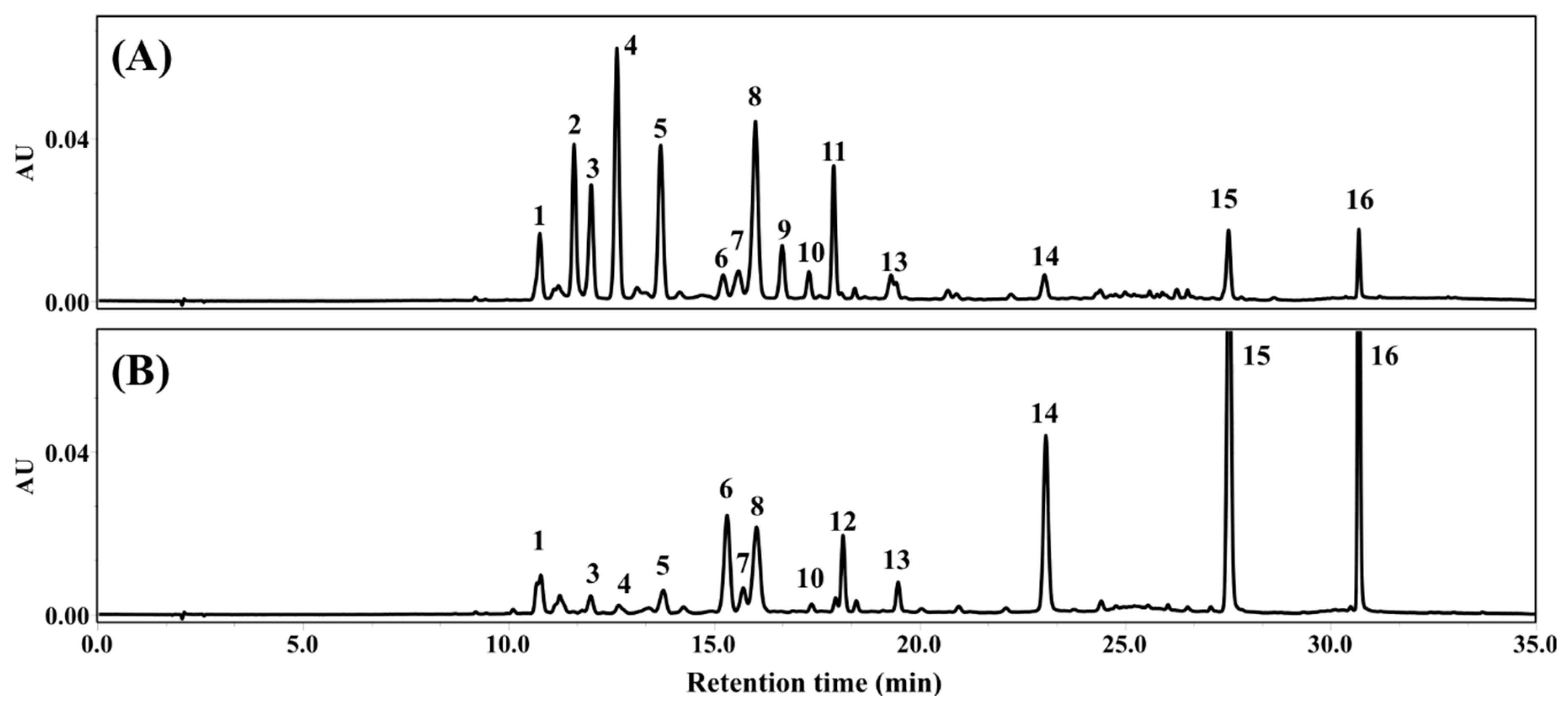
| Peak No. | Molecular ion (m/z, [M-H]−) | Formula | Fragmentation | Identification a |
|---|---|---|---|---|
| 1 | 563.14020 | C32H27O14 | 545, 473, 443, 383, 353 | Apigenin-6-C-glucosyl-8-C-arabinoside |
| 2 | 479.08228 | C21H19O13 | 316 | Myricetin-3-O-galactoside |
| 3 | 479.08267 | C21H19O13 | 316 | Myricetin-3-O-glucoside |
| 4 | 771.19812 | C33H39O21 | 301 | Quercetin-3-O-galactosylrutinoside |
| 5 | 771.19812 | C33H39O21 | 301 | Quercetin-3-O-glucosylrutinoside |
| 6 | 609.14581 | C27H29O16 | 301 | Quercetin-3-O-rhamnosylgalactoside |
| 7 | 609.14606 | C27H29O16 | 301 | Quercetin-3-O-rhamnosylglucoside |
| 8 | 431.09787 | C26H19O10 | 353, 269 | Apigenin-6-C-glucoside or isomer |
| 9 | 463.08801 | C21H19O12 | 301 | Quercetin-3-O-galactoside |
| 10 | 463.08829 | C21H19O12 | 301 | Quercetin-3-O-glucoside |
| 11 | 755.20367 | C33H39O20 | 285 | Kaempferol-3-O-glucosylrutinoside |
| 12 | 593.15009 | C27H29O15 | 285 | Kaempferol-3-O-rhamnosylgalactoside |
| 13 | 593.15131 | C27H29O15 | 285 | Kaempferol-3-O-rhamnosylglucoside |
| 14 | 317.02951 | C15H9O8 | Myricetin | |
| 15 | 301.03491 | C15H9O7 | Quercetin | |
| 16 | 285.03983 | C15H9O6 | Kaempferol |
| Myricetin a | Quercetin a | Kaempferol a | Epicatechins ab | Total Flavonoids c | Total Phenolics d | |
|---|---|---|---|---|---|---|
| FLG e | 4.15 ± 0.16 Bf | 7.72 ± 0.43 B | 5.91 ± 0.24 B | N/D g | 132.76 ± 0.98 B | 401.48 ± 1.52 B |
| FLA h | 15.94 ± 0.48 A | 38.21 ± 1.20 A | 34.71 ± 1.19 A | N/D | 174.67 ± 1.33 A | 568.89 ± 1.99 A |
| Antioxidant Capacities (mg VCE/g DW) | ||
|---|---|---|
| ABTS | DPPH | |
| GTE | 1637.06 ± 16.67 Aa | 1335.27 ± 36.35 A |
| FLG | 870.20 ± 18.60 C | 518.25 ± 32.02 C |
| FLA | 1360.80 ± 8.25 B | 864.60 ± 19.35 B |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rha, C.-S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.-O. Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants 2019, 8, 278. https://doi.org/10.3390/antiox8080278
Rha C-S, Jeong HW, Park S, Lee S, Jung YS, Kim D-O. Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants. 2019; 8(8):278. https://doi.org/10.3390/antiox8080278
Chicago/Turabian StyleRha, Chan-Su, Hyun Woo Jeong, Saitbyul Park, Siyoung Lee, Young Sung Jung, and Dae-Ok Kim. 2019. "Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea" Antioxidants 8, no. 8: 278. https://doi.org/10.3390/antiox8080278