Avian Stress-Related Transcriptome and Selenotranscriptome: Role during Exposure to Heavy Metals and Heat Stress
Abstract
:1. Introduction
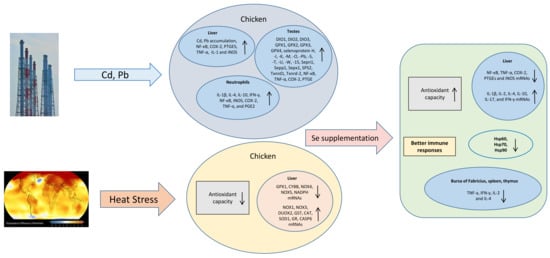
2. Avian Transcriptome Response to Cadmium Toxicity and the Benefits of Selenium Supplementation
2.1. Inflammation Transcripts, Apoptotic Factors and Selenotranscriptome
2.2. Selenium Supplementation
3. Avian Transcriptome Response to Lead Toxicity and the Benefits of Selenium Supplementation
Inflammatory Response
4. Heat Stress and Avian Transcriptome
4.1. An Overview of Heat Stress
4.2. Avian Transcriptome Response to Heat Stress
4.3. Antioxidant Supplements during Heat Stress and Avian Antioxidant Transcriptome Response
5. Bibliometric Evaluation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATF4 | Activating transcription factor 4 |
| AvUcp | Avian uncoupling protein |
| Bak | BCL2 antagonist/killer 1 |
| Bcl-2 | B-cell lymphoma 2 gene |
| Bcl-xL | B-cell lymphoma-extra large |
| BNIP3 | Adenovirus E1B 19 kDa protein-interacting protein 3 |
| CaM | Calmodulin protein gene |
| CHOP | CCAAT-enhancer-binding protein homologous protein |
| COX-2 | Cyclooxygenase 2 |
| DIO | Iodothyronine deiodinase |
| eIF2α | Eukaryotic Initiation Factor 2 |
| GPX | Glutathione peroxidase |
| GRP78 | Unfolded protein response regulator |
| GSH | Glutathione |
| GST | Glutathione S-transferases |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| iNOS | Inducible NO synthase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| p53 | Tumor protein p53 gene |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| PGE2 | Prostaglandin E2 |
| PTGES | prostaglandin E synthase |
| SECIS | Selenocysteine insertion sequence |
| SEPHS2 | Selenophosphate Synthetase 2 gene |
| SEPP1 | Selenoprotein P |
| Sepx1 | Methionine-R-sulfoxide reductase B1 |
| SOD | Superoxide dismutase |
| TGF-β4 | Transforming Growth Factor-β |
| TNF-α | Tumor necrosis factor alfa |
| TNR-α | Tumor necrosis receptor alfa |
| TXNRD | Thioredoxin reductase |
References
- Schomburg, L.; Schweizer, U.; Köhrle, J. Selenium and selenoproteins in mammals: Extraordinary, essential, enigmatic. Cell. Mol. Life Sci. 2004, 61, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Wang, W.-H.; Wang, C.-X.; Wang, Z.-J.; Rui, H.-F.; Wang, W.-Z.; Yang, Z.-W. The role of humic substances in drinking water in Kashin-Beck disease in China. Environ. Health Perspect. 1999, 107, 293. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Böck, A.; Forchhammer, K.; Heider, J.; Leinfelder, W.; Sawers, G.; Veprek, B.; Zinoni, F. Selenocysteine: The 21st amino acid. Mol. Microbiol. 1991, 5, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Zinoni, F.; Birkmann, A.; Leinfelder, W.; Böck, A. Cotranslational insertion of selenocysteine into formate dehydrogenase from Escherichia coli directed by a UGA codon. Proc. Natl. Acad. Sci. USA 1987, 84, 3156–3160. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Frampton, J.; Goldfarb, P.; Affara, N.; McBain, W.; Harrison, P.R. The structure of the mouse glutathione peroxidase gene: The selenocysteine in the active site is encoded by the ‘termination’codon, TGA. EMBO J. 1986, 5, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Bo, A.; Forchhammer, K.; Heider, J.; Baron, C. Selenoprotein synthesis: An expansion of the genetic code. Trends Biochem. Sci. 1991, 16, 463–467. [Google Scholar]
- Berry, M.J.; Banu, L.; Chen, Y.; Mandel, S.J.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature 1991, 353, 273. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Tang, J.; Jia, G.; Liu, G.; Chen, X.; Cai, J.; Shang, H.; Zhao, H. Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. PLoS ONE 2017, 12, e0182079. [Google Scholar] [CrossRef]
- Thompson, J.; Scott, M. Role of selenium in the nutrition of the chick. J. Nutr. 1969, 97, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Li, D.-L.; Zhao, H.; Sun, L.-H.; Xia, X.-J.; Wang, K.-N.; Luo, X.; Lei, X.G. The Selenium Deficiency Disease Exudative Diathesis in Chicks Is Associated with Downregulation of Seven Common Selenoprotein Genes in Liver and Muscle–3. J. Nutr. 2011, 141, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Ren, F.-Z.; Jiang, Y.-Y.; Lei, X. Characterization of selenoprotein M and its response to selenium deficiency in chicken brain. Biol. Trace Elem. Res. 2016, 170, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Ren, F.-Z.; Jiang, Y.-Y.; Xiao, C.; Lei, X.G. Selenoproteins protect against avian nutritional muscular dystrophy by metabolizing peroxides and regulating redox/apoptotic signaling. Free Radic. Biol. Med. 2015, 83, 129–138. [Google Scholar] [CrossRef]
- Scheuhammer, A.M. The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: A review. Environ. Pollut. 1987, 46, 263–295. [Google Scholar]
- Al-Waeli, A.; Zoidis, E.; Pappas, A.; Demiris, N.; Zervas, G.; Fegeros, K. The role of organic selenium in cadmium toxicity: Effects on broiler performance and health status. Animal 2013, 7, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Marettová, E.; Maretta, M.; Legáth, J.; Košutzká, E. The Retention of Cadmium and Selenium Influence in Fowl and Chickens of F 1 Generation. Biol. Trace Elem. Res. 2012, 147, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jia, T.; Cui, Y.; Lin, H.; Li, S. The Protective Effect of Selenium on the Chicken Pancreas against Cadmium Toxicity via Alleviating Oxidative Stress and Autophagy. Biol. Trace Elem. Res. 2018, 184, 240–246. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Wang, C.; Zhao, P.; Liu, H.; Li, J.; Bao, J. Ameliorative Effects of Dietary Selenium Against Cadmium Toxicity Is Related to Changes in Trace Elements in Chicken Kidneys. Biol. Trace Elem. Res. 2017, 176, 391–400. [Google Scholar] [CrossRef]
- Al-Waeli, A.; Pappas, A.; Zoidis, E.; Georgiou, C.; Fegeros, K.; Zervas, G. The role of selenium in cadmium toxicity: Interactions with essential and toxic elements. Br. Poult. Sci. 2012, 53, 817–827. [Google Scholar] [CrossRef]
- Liu, S.; Xu, F.; Fu, J.; Li, S. Protective Roles of Selenium on Nitric Oxide and the Gene Expression of Inflammatory Cytokines Induced by Cadmium in Chicken Splenic Lymphocytes. Biol. Trace Elem. Res. 2015, 168, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Bulat, Z.P.; Djukić-Ćosić, D.; Maličević, Ž.; Bulat, P.; Matović, V. Zinc or magnesium supplementation modulates Cd intoxication in blood, kidney, spleen, and bone of rabbits. Biol. Trace Elem. Res. 2008, 124, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Papadomichelakis, G.; Zoidis, E.; Pappas, A.C.; Danezis, G.; Georgiou, C.A.; Fegeros, K. Dietary organic selenium addition and accumulation of toxic and essential trace elements in liver and meat of growing rabbits. Meat Sci. 2018, 145, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Risso-de Faverney, C.; Orsini, N.; De Sousa, G.; Rahmani, R. Cadmium-induced apoptosis through the mitochondrial pathway in rainbow trout hepatocytes: Involvement of oxidative stress. Aquat. Toxicol. 2004, 69, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.; Zoidis, E.; Fegeros, K.; Surai, P.; Zervas, G. Relation of cadmium to other elements and the antioxidant system. In Cadmium in the Environment; Nova Science Publishers: New York, NY, USA, 2010; pp. 263–295. [Google Scholar]
- Buchet, J.-P.; Lauwerys, R.; Roels, H.; Bernard, A.; Bruaux, P.; Claeys, F.; Ducoffre, G.; De Plaen, P.; Amery, A.; Lijnen, P. Renal effects of cadmium body burden of the general population. Lancet 1990, 336, 69–702. [Google Scholar] [CrossRef]
- Kamaraju, S.; Ramasamy, K. Effect of cadmium chloride on glycogen content in gill, liver and kidney of edible exotic fish Hypophthalmichthys molitrix. Int. J. Curr. Res. 2011, 3, 53–57. [Google Scholar]
- Wang, X.; Bao, R.; Fu, J. The Antagonistic Effect of Selenium on Cadmium-Induced Damage and mRNA Levels of Selenoprotein Genes and Inflammatory Factors in Chicken Kidney Tissue. Biol. Trace Elem. Res. 2018, 181, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Amdur, M.O. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill: New York, NY, USA, 2013; Volume 1236. [Google Scholar]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Mubeen, S.; Yuana, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Dietert, R.R.; Piepenbrink, M.S. Lead and immune function. Crit. Rev. Toxicol. 2006, 36, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Jin, X.; Wang, J.; Shi, Q.; Cai, J.; Xu, S. The Antagonistic Effect of Selenium on Lead-Induced Immune Dysfunction via Recovery of Cytokine and Heat Shock Protein Expression in Chicken Neutrophils. Biol. Trace Elem. Res. 2018, 185, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Y.; An, Y.; Tian, Y.; Li, S.; Teng, X. Selenium for the mitigation of toxicity induced by lead in chicken testes through regulating mRNA expressions of HSPs and selenoproteins. Environ. Sci. Pollut. Res. 2017, 24, 14312–14321. [Google Scholar] [CrossRef] [PubMed]
- Gasparik, J.; Venglarcik, J.; Slamecka, J.; Kropil, R.; Smehyl, P.; Kopecky, J. Distribution of lead in selected organs and its effect on reproduction parameters of pheasants (Phasianus colchicus) after an experimental per oral administration. J. Environ. Sci. Health Part A Environ. Sci. Eng. 2012, 47, 1267–1271. [Google Scholar] [CrossRef]
- Butkauskas, D.; Sruoga, A. Effect of lead and chromium on reproductive success of Japanese quail. Environ. Toxicol. Int. J. 2004, 19, 412–415. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, H.-W.; Song, W.-J.; Jeon, E.Y.; Bang, B.; Shim, E.-J.; Moon, H.-G.; Kim, Y.-K.; Kang, H.-R.; Min, K.-U. TNF-α enhance Th2 and Th17 immune responses regulating by IL23 during sensitization in asthma model. Cytokine 2016, 79, 23–30. [Google Scholar] [CrossRef]
- de Andrés, M.C.; Takahashi, A.; Oreffo, R.O. Demethylation of an NF-κB enhancer element orchestrates iNOS induction in osteoarthritis and is associated with altered chondrocyte cell cycle. Osteoarthr. Cartil. 2016, 24, 1951–1960. [Google Scholar] [CrossRef]
- Tan, S.; Chi, Q.; Liu, T.; Sun, Z.; Min, Y.; Zhang, Z.; Li, S. Alleviation mechanisms of selenium on cadmium-spiked neutrophil injury to chicken. Biol. Trace Elem. Res. 2017, 178, 301–309. [Google Scholar] [CrossRef]
- Liu, L.-L.; Li, C.-M.; Zhang, Z.-W.; Zhang, J.-L.; Yao, H.-D.; Xu, S.-W. Protective effects of selenium on cadmium-induced brain damage in chickens. Biol. Trace Elem. Res. 2014, 158, 176–185. [Google Scholar] [CrossRef]
- Låg, M.; Refsnes, M.; Lilleaas, E.M.; Holme, J.A.; Becher, R.; Schwarze, P.E. Role of mitogen activated protein kinases and protein kinase C in cadmium-induced apoptosis of primary epithelial lung cells. Toxicology 2005, 211, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, S.; Li, S. Effects of selenium and cadmium on changes in the gene expression of immune cytokines in chicken splenic lymphocytes. Biol. Trace Elem. Res. 2015, 165, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yi, R.; Bi, Y.; Xing, L.; Bao, J.; Li, J. The effect of selenium on the Cd-induced apoptosis via NO-mediated mitochondrial apoptosis pathway in chicken liver. Biol. Trace Elem. Res. 2017, 178, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pan, T.; Wan, N.; Sun, Z.; Zhang, Z.; Li, S. Cadmium-induced endoplasmic reticulum stress in chicken neutrophils is alleviated by selenium. J. Inorg. Biochem. 2017, 170, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Xing, L.; Zhao, N.; Zheng, Q.; Li, J.; Bao, J. The protective role of selenium against cadmium-induced hepatotoxicity in laying hens: Expression of Hsps and inflammation-related genes and modulation of elements homeostasis. Ecotoxicol. Environ. Saf. 2018, 159, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Fan, R.; Yang, J.; Jin, X.; Hamid, S.; Xu, S. Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere 2018, 194, 396–402. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Z.; Yin, H.; Min, Y.; Li, S. Alleviation mechanisms of selenium on cadmium-spiked in chicken ovarian tissue: Perspectives from autophagy and energy metabolism. Biol. Trace Elem. Res. 2018, 186, 521–528. [Google Scholar] [CrossRef]
- Amantana, A.; Vorachek, W.; Butler, J.; Costa, N.; Whanger, P. Effect of copper, zinc and cadmium on the promoter of selenoprotein W in glial and myoblast cells. J. Inorg. Biochem. 2002, 91, 356–362. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, W.; Chen, X.; Zhu, Y.; Zhang, Z.; Yao, H.; Xu, S. Four endoplasmic reticulum resident selenoproteins may be related to the protection of selenium against cadmium toxicity in chicken lymphocytes. Biol. Trace Elem. Res. 2014, 161, 328–333. [Google Scholar] [CrossRef]
- Yao, H.; Fan, R.; Zhao, X.; Zhao, W.; Liu, W.; Yang, J.; Sattar, H.; Zhao, J.; Zhang, Z.; Xu, S. Selenoprotein W redox-regulated Ca2+ channels correlate with selenium deficiency-induced muscles Ca2+ leak. Oncotarget 2016, 7, 57618–57632. [Google Scholar] [CrossRef]
- Liu, S.; Xu, F.-P.; Yang, Z.-J.; Li, M.; Min, Y.-H.; Li, S. Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: Mechanisms of oxidative stress and apoptosis. Biol. Trace Elem. Res. 2014, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Fan, R.; Cao, C.; Yao, H.; Xu, S. Selenium antagonizes cadmium-induced apoptosis in chicken spleen but not involving Nrf2-regulated antioxidant response. Ecotoxicol. Environ. Saf. 2017, 145, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-D.; Wu, Q.; Zhang, Z.-W.; Li, S.; Wang, X.-L.; Lei, X.-G.; Xu, S.-W. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- El-Sharaky, A.; Newairy, A.; Badreldeen, M.; Eweda, S.; Sheweita, S. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 2007, 235, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Banni, M.; Said, L.; Said, K.; Kerkeni, A. Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem.-Biol. Interact. 2010, 188, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Speyer, C.L.; Neff, T.A.; Warner, R.L.; Guo, R.-F.; Sarma, J.V.; Riedemann, N.C.; Murphy, M.E.; Murphy, H.S.; Ward, P.A. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am. J. Pathol. 2003, 163, 2319–2328. [Google Scholar] [CrossRef]
- Kanuri, G.; Spruss, A.; Wagnerberger, S.; Bischoff, S.C.; Bergheim, I. Role of tumor necrosis factor α (TNFα) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 2011, 22, 527–534. [Google Scholar] [CrossRef]
- Sehnert, B.; Burkhardt, H.; Wessels, J.T.; Schröder, A.; May, M.J.; Vestweber, D.; Zwerina, J.; Warnatz, K.; Nimmerjahn, F.; Schett, G. NF-κB inhibitor targeted to activated endothelium demonstrates a critical role of endothelial NF-κB in immune-mediated diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 16556–16561. [Google Scholar] [CrossRef]
- Avadhesh, K.; Chauhan, R.S.; Singh, N.P. Effects of sub chronic lead intoxication on macrophage functions of chickens. J. Clin. Immunol. Immunopathol. Res. 2000, 2, 71–72. [Google Scholar]
- Sun, G.X.; Chen, Y.; Liu, C.P.; Li, S.; Fu, J. Effect of Selenium Against Lead-Induced Damage on the Gene Expression of Heat Shock Proteins and Inflammatory Cytokines in Peripheral Blood Lymphocytes of Chickens. Biol. Trace Elem. Res. 2016, 172, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lei, Z.; Huang, A.; Lu, Q.; Wang, X.; Ahmed, S.; Awais, I.; Yuan, Z. Mechanisms of the Testis Toxicity Induced by Chronic Exposure to Mequindox. Front. Pharmacol. 2017, 8, 679. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Yang, K.; An, Y.; Teng, X.; Teng, X. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ. Sci. Pollut. Res. Int. 2017, 24, 7555–7564. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; An, Y.; Jiao, W.; Wang, J.; Li, S.; Teng, X. CHOP/caspase-3 signal pathway involves in mitigative effect of selenium on lead-induced apoptosis via endoplasmic reticulum pathway in chicken testes. Environ. Sci. Pollut. Res. 2018, 25, 18838–18845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Huang, H.; Gu, X.; Teng, X. Alleviative effect of selenium on inflammatory damage caused by lead via inhibiting inflammatory factors and heat shock proteins in chicken testes. Environ. Sci. Pollut. Res. 2017, 24, 13405–13413. [Google Scholar] [CrossRef]
- Jin, X.; Xu, Z.; Zhao, X.; Chen, M.; Xu, S. The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 2017, 180, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; An, Y.; Jiao, W.; Zhang, Z.; Han, H.; Gu, X.; Teng, X. Selenium protects against lead-induced apoptosis via endoplasmic reticulum stress in chicken kidneys. Biol. Trace Elem. Res. 2018, 182, 354–363. [Google Scholar] [CrossRef]
- Li, X.; Xing, M.; Chen, M.; Zhao, J.; Fan, R.; Zhao, X.; Cao, C.; Yang, J.; Zhang, Z.; Xu, S. Effects of selenium-lead interaction on the gene expression of inflammatory factors and selenoproteins in chicken neutrophils. Ecotoxicol. Environ. Saf. 2017, 139, 447–453. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X. Selenium Antagonizes the Lead-Induced Apoptosis of Chicken Splenic Lymphocytes In Vitro by Activating the PI3K/Akt Pathway. Biol. Trace Elem. Res. 2018, 182, 119–129. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiao, X.; An, Y.; Li, S.; Teng, X. Selenium against lead-induced apoptosis in chicken nervous tissues via mitochondrial pathway. Oncotarget 2017, 8, 108130. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, X.; Teng, X.; Gu, X.; Teng, X. Antagonistic effect of selenium on lead-induced inflammatory injury through inhibiting the nuclear factor-κB signaling pathway and stimulating selenoproteins in chicken hearts. RSC Adv. 2017, 7, 24878–24884. [Google Scholar] [CrossRef]
- Gao, H.; Liu, C.P.; Song, S.Q.; Fu, J. Effects of Dietary Selenium Against Lead Toxicity on mRNA Levels of 25 Selenoprotein Genes in the Cartilage Tissue of Broiler Chicken. Biol. Trace Elem. Res. 2016, 172, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, Y.; Yang, J.; Liu, Q.; Zhao, R.; Hamid, S.; Wang, H.; Xu, S.; Zhang, Z. Antagonistic effects of selenium against necroptosis injury via adiponectin-necrotic pathway induced by cadmium in heart of chicken. RSC Adv. 2017, 7, 44438–44446. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhu, Y.H.; Cheng, X.Y.; Zhang, Z.W.; Xu, S.W. The protection of selenium against cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules (Basel) 2012, 17, 14565–14572. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Daghir, N.J. Poultry Production in Hot Climates; Cabi: Wallingford, UK, 2008. [Google Scholar]
- Mashaly, M.; Hendricks, G., 3rd.; Kalama, M.; Gehad, A.; Abbas, A.; Patterson, P. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.; Rostagno, M. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Sohail, M.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Zaneb, K.; Aleem, M.; Rehman, H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010, 89, 1934–1938. [Google Scholar]
- Novero, R.; Beck, M.; Gleaves, E.; Johnson, A.; Deshazer, J. Plasma progesterone, luteinizing hormone concentrations, and granulosa cell responsiveness in heat-stressed hens. Poult. Sci. 1991, 70, 2335–2339. [Google Scholar] [CrossRef]
- Maak, S.; Melesse, A.; Schmidt, R.; Schneider, F.; Von Lengerken, G. Effect of long-term heat exposure on peripheral concentrations of heat shock protein 70 (Hsp70) and hormones in laying hens with different genotypes. Br. Poult. Sci. 2003, 44, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Rozenboim, I.; Tako, E.; Gal-Garber, O.; Proudman, J.; Uni, Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007, 86, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Zahraa, H.; Ghamdi, A. Effects of commutative heat stress on immunoresponses in broiler chickens reared in closed system. Int. J. Poult. Sci. 2008, 7, 964–968. [Google Scholar]
- Syafwan, S.; Kwakkel, R.; Verstegen, M. Heat stress and feeding strategies in meat-type chickens. World’s Poult. Sci. J. 2011, 67, 653–674. [Google Scholar] [CrossRef] [Green Version]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.; Sakai, M.; Sá, L.R.M.D.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; Pan, X.J.; Peng, Z.Q. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 2009, 88, 1078–1084. [Google Scholar] [CrossRef]
- Sahin, K.; Kucuk, O. Zinc supplementation alleviates heat stress in laying Japanese quail. J. Nutr. 2003, 133, 2808–2811. [Google Scholar] [CrossRef] [PubMed]
- Kelman, K.R.; Pannier, L.; Pethick, D.W.; Gardner, G.E. Selection for lean meat yield in lambs reduces indicators of oxidative metabolism in the longissimus muscle. Meat Sci. 2014, 96, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- El-Kholy, M.S.; El-Hindawy, M.M.; Alagawany, M.; Abd El-Hack, M.E.; El-Sayed, S. Dietary Supplementation of Chromium Can Alleviate Negative Impacts of Heat Stress on Performance, Carcass Yield, and Some Blood Hematology and Chemistry Indices of Growing Japanese Quail. Biol. Trace Elem. Res. 2017, 179, 148–157. [Google Scholar] [CrossRef]
- S. El-Kholy, M.; M. El-Hindawy, M.; Alagawany, M.; Abd El-Hack, M.; El-Sayed, S. Use of acetylsalicylic acid as an allostatic modulator in the diets of growing Japanese quails exposed to heat stress. J. Therm. Biol. 2018, 74, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.; Frouel, S.; Awati, A.; Owusu-Asiedu, A.; Amerah, A.; Patridge, G.; Dunshea, F. Betaine mitigates intestinal permeability in growing pigs induced by heat stress. In Proceedings of the Manipulating Pig Production XIV, Melbourne, Australia, 24–27 November 2013; Pluske, J.R., Pluske, J.M., Eds.; Australian Pig Science Association: Melbourne, Australia, 2013; Volume 85. [Google Scholar]
- Adomako, K.; Habashy, W.; Milfort, M.; Fuller, A.; Rekaya, R.; Aggrey, S. Transcriptome analysis of genes in the protein biosynthesis and ubiquitin-proteosome pathways in meat-type chickens under heat stress. In Proceedings of the 25th World’s Poultry Congress September, Beijing, China, 5–9 September 2016; pp. 5–9. [Google Scholar]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca(2+) dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 11497. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, H.; Gu, Z.; Liu, Z.; Geng, Y.; Liu, Y.; Tong, H.; Tang, Y.; Qiu, J.; Su, L. Heat stress induces apoptosis through a Ca(2)(+)-mediated mitochondrial apoptotic pathway in human umbilical vein endothelial cells. PLoS ONE 2014, 9, e111083. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Kronfeld, D.; Hess, T.; Saker, K.; Waldron, J.; Crandell, K.; Hoffman, R.; Harris, P. Antioxidant supplementation and subsequent oxidative stress of horses during an 80-km endurance race. J. Anim. Sci. 2004, 82, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; De Vos, D.; Decuypere, E.; Buyse, J. Dynamic changes in parameters of redox balance after mild heat stress in aged laying hens (Gallus gallus domesticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 2009, 87, E101–E108. [Google Scholar] [CrossRef] [PubMed]
- Skrivan, M.; Dlouha, G.; Mašata, O.; Ševčíková, S. Effect of dietary selenium on lipid oxidation, selenium and vitamin E content in the meat of broiler chickens. Czech. J. Anim. Sci. 2008, 53, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.Z.; Kumbhar, S.; Hamid, M.; Afzal, S.; Parveen, F.; Liu, Y.; Shu, H.; Mengistu, B.M.; Huang, K. Effects of Selenium-Enriched Probiotics on Heart Lesions by Influencing the mRNA Expressions of Selenoproteins and Heat Shock Proteins in Heat Stressed Broiler Chickens. Pak. Vet. J. 2016, 36, 460–464. [Google Scholar]
- Özdemir, D.; Akşit, M.; Özkan, S.; Yalçin, S.; Metin, K. Effects of Temperature During Rearing and Crating on Stress Parameters and Meat Quality of Broilers. Poult. Sci. 2006, 85, 1867–1874. [Google Scholar]
- Chan, J.T.; Omana, D.A.; Betti, M. Functional and rheological properties of proteins in frozen turkey breast meat with different ultimate pH. Poult. Sci. 2011, 90, 1112–1123. [Google Scholar] [CrossRef]
- Slawinska, A.; Hsieh, J.C.; Schmidt, C.J.; Lamont, S.J. Heat Stress and Lipopolysaccharide Stimulation of Chicken Macrophage-Like Cell Line Activates Expression of Distinct Sets of Genes. PLoS ONE 2016, 11, e0164575. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, W.; Huang, Y.; He, J.; Tian, Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz. (PAMK) on immune response in chicken spleen under heat stress. Biol. Trace Elem. Res. 2014, 160, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lu, Q.; Hu, Z.; Yu, Y.; Chen, Q.; Wang, Q.K. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 2017, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, W.; Li, B.; Tian, Y.; Huang, Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz.(PAMK) on endoplasmic reticulum stress and apoptosis in chicken spleen induced by heat stress. RSC Adv. 2017, 7, 7519–7525. [Google Scholar] [CrossRef]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol. Biol. Rep. 2018, 45, 389–394. [Google Scholar] [CrossRef]
- Banfi, B.; Clark, R.A.; Steger, K.; Krause, K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003, 278, 3510–3513. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Ueno, N.; Takeya, R.; Miyano, K.; Kikuchi, H.; Sumimoto, H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: Its regulation by oxidase organizers and activators. J. Biol. Chem. 2005, 280, 23328–23339. [Google Scholar] [CrossRef]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 1502–1504. [Google Scholar] [CrossRef]
- Banfi, B.; Tirone, F.; Durussel, I.; Knisz, J.; Moskwa, P.; Molnár, G.Z.; Krause, K.-H.; Cox, J.A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J. Biol. Chem. 2004, 279, 18583–18591. [Google Scholar]
- Banfi, B.; Molnar, G.; Maturana, A.; Steger, K.; Hegedus, B.; Demaurex, N.; Krause, K.H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Du, R.; Gu, X.H.; Li, F.C.; Zhang, Z.Y. A Study on the Plasma Biochemical Indices of Heat-Stressed Broilers. Asian-Australas. J. Anim. Sci. 2000, 13, 1210–1218. [Google Scholar] [CrossRef]
- Hu, Y.; Rosen, D.G.; Zhou, Y.; Feng, L.; Yang, G.; Liu, J.; Huang, P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: Role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005, 280, 39485–39492. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Kim, H.J.; Yun, J.; Lee, J.; Hong, H.; Jeong, J.; Kim, E.; Bae, Y.S.; Lee, K.J. SUMO1 attenuates stress-induced ROS generation by inhibiting NADPH oxidase 2. Biochem. Biophys. Res. Commun. 2011, 410, 555–562. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Azad, M.A.; Kikusato, M.; Maekawa, T.; Shirakawa, H.; Toyomizu, M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 401–406. [Google Scholar] [CrossRef]
- Min, J.Y.; Lim, S.O.; Jung, G. Downregulation of catalase by reactive oxygen species via hypermethylation of CpG island II on the catalase promoter. FEBS Lett. 2010, 584, 2427–2432. [Google Scholar] [CrossRef] [Green Version]
- Quan, X.; Lim, S.O.; Jung, G. Reactive oxygen species downregulate catalase expression via methylation of a CpG island in the Oct-1 promoter. FEBS Lett. 2011, 585, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.-N.; Yadav, P.K.; Adamec, J.; Banerjee, R. S-glutathionylation enhances human cystathionine β-synthase activity under oxidative stress conditions. Antioxid. Redox Signal. 2015, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Habashy, W.S.; Milfort, M.C.; Adomako, K.; Attia, Y.A.; Rekaya, R.; Aggrey, S.E. Effect of heat stress on amino acid digestibility and transporters in meat-type chickens. Poult. Sci. 2017, 96, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Prigge, J.R.; Talago, E.A.; Arnér, E.S.J.; Schmidt, E.E. Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nat. Commun. 2015, 6, 6479. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Z.; Sharma, R.; Yang, Y.; Singhal, S.S.; Sharma, A.; Saini, M.K.; Singh, S.V.; Zimniak, P.; Awasthi, S.; Awasthi, Y.C. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J. Biol. Chem. 2001, 276, 41213–41223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Calkins, M.J.; Johnson, D.A.; Johnson, J.A. Role of Nrf2-dependent ARE-driven antioxidant pathway in neuroprotection. Methods Mol. Biol. (Clifton, N.J.) 2007, 399, 67–78. [Google Scholar]
- Al-Zghoul, M.B.; Sukker, H.; Ababneh, M.M. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult. Sci. 2019, 98, 991–1001. [Google Scholar] [CrossRef]
- Kumbhar, S.; Khan, A.Z.; Parveen, F.; Nizamani, Z.A.; Siyal, F.A.; El-Hack, M.E.A.; Gan, F.; Liu, Y.; Hamid, M.; Nido, S.A.; et al. Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature. AMB Express 2018, 8, 112. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Ringuet, M.; Furness, J.B.; Dunshea, F.R. Betaine and Antioxidants Improve Growth Performance, Breast Muscle Development and Ameliorate Thermoregulatory Responses to Cyclic Heat Exposure in Broiler Chickens. Animals 2018, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Kumbhar, S.; Liu, Y.; Hamid, M.; Pan, C.; Nido, S.A.; Parveen, F.; Huang, K. Dietary Supplementation of Selenium-Enriched Probiotics Enhances Meat Quality of Broiler Chickens (Gallus gallus domesticus) Raised Under High Ambient Temperature. Biol. Trace Elem. Res. 2018, 182, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Safdari-Rostamabad, M.; Hosseini-Vashan, S.J.; Perai, A.H.; Sarir, H. Nanoselenium Supplementation of Heat-Stressed Broilers: Effects on Performance, Carcass Characteristics, Blood Metabolites, Immune Response, Antioxidant Status, and Jejunal Morphology. Biol. Trace Elem. Res. 2017, 178, 105–116. [Google Scholar] [CrossRef] [PubMed]
- El-Hack, M.E.A.; Mahrose, K.; Askar, A.A.; Alagawany, M.; Arif, M.; Saeed, M.; Abbasi, F.; Soomro, R.N.; Siyal, F.A.; Chaudhry, M.T. Single and combined impacts of vitamin A and selenium in diet on productive performance, egg quality, and some blood parameters of laying hens during hot season. Biol. Trace Elem. Res. 2017, 177, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Mahrose, K.; Arif, M.; Chaudhry, M.T.; Saadeldin, I.M.; Saeed, M.; Soomro, R.N.; Abbasi, I.H.; Rehman, Z.U. Alleviating the environmental heat burden on laying hens by feeding on diets enriched with certain antioxidants (vitamin E and selenium) individually or combined. Environ. Sci. Pollut. Res. Int. 2017, 24, 10708–10717. [Google Scholar] [CrossRef] [PubMed]
- Organic Forms of Zinc, Selenium and Chromium on Performance, Anti-Oxidant and Immune Responses in Broiler Chicken Reared in Tropical Summer. Biol. Trace. Elem. Res. 2016, 172, 511–520. [CrossRef]
- Habibian, M.; Ghazi, S.; Moeini, M.M. Effects of Dietary Selenium and Vitamin E on Growth Performance, Meat Yield, and Selenium Content and Lipid Oxidation of Breast Meat of Broilers Reared Under Heat Stress. Biol. Trace Elem. Res. 2016, 169, 142–152. [Google Scholar] [CrossRef]
- Xu, D.; Tian, Y. Selenium and Polysaccharides of Atractylodes macrocephala Koidz Play Different Roles in Improving the Immune Response Induced by Heat Stress in Chickens. Biol. Trace Elem. Res. 2015, 168, 235–241. [Google Scholar] [CrossRef]
- Habibian, M.; Ghazi, S.; Moeini, M.M.; Abdolmohammadi, A. Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int. J. Biometeorol. 2014, 58, 741–752. [Google Scholar] [CrossRef]
- Liao, X.; Lu, L.; Li, S.; Liu, S.; Zhang, L.; Wang, G.; Li, A.; Luo, X. Effects of selenium source and level on growth performance, tissue selenium concentrations, antioxidation, and immune functions of heat-stressed broilers. Biol. Trace Elem. Res. 2012, 150, 158–165. [Google Scholar] [CrossRef]
- Ghazi Harsini, S.; Habibiyan, M.; Moeini, M.M.; Abdolmohammadi, A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012, 148, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, A.; Kermanshahi, H.; Riasi, A.; Farhangfar, H.; Sarir, H.; Ziaie, H. Effects of sodium selenite and turmeric powder on thyroid hormones and plasma lipids of broiler chickens reared under heat stress condition. Glob. Vet. 2011, 6, 237–240. [Google Scholar]
- Rey, A.I.; López-Bote, C.J.; Litta, G. Effects of dietary vitamin E (DL-α-tocopheryl acetate) and vitamin C combination on piglets oxidative status and immune response at weaning. J. Anim. Feed Sci. 2017, 26, 226–235. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [Green Version]
- Gao, J.; Lin, H.; Wang, X.J.; Song, Z.G.; Jiao, H.C. Vitamin E supplementation alleviates the oxidative stress induced by dexamethasone treatment and improves meat quality in broiler chickens. Poult. Sci. 2010, 89, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Kucuk, O. Effects of vitamin C and vitamin E on performance, digestion of nutrients and carcass characteristics of Japanese quails reared under chronic heat stress (34 degrees C). J. Anim. Physiol. Anim. Nutr. 2001, 85, 335–341. [Google Scholar] [CrossRef]
- Hosseini-Mansoub, N.; Chekani-Azar, S.; Tehrani, A.; Lotfi, A.; Manesh, M. Influence of dietary vitamin E and zinc on performance, oxidative stability and some blood measures of broiler chickens reared under heat stress (35 °C). J. Agrobiol. 2010, 27, 103–110. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants (Basel) 2018, 7, E66. [Google Scholar] [CrossRef]
- Hu, C.H.; Li, Y.L.; Xiong, L.; Zhang, H.M.; Song, J.; Xia, M.S. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim. Feed Sci. Technol. 2012, 177, 204–210. [Google Scholar] [CrossRef]
- Rao, S.V.; Prakash, B.; Raju, M.V.; Panda, A.K.; Poonam, S.; Murthy, O.K. Effect of supplementing organic selenium on performance, carcass traits, oxidative parameters and immune responses in commercial broiler chickens. Asian-Australas. J. Anim. Sci. 2013, 26, 247–252. [Google Scholar] [CrossRef]




| Type of Supplementation | Heavy Metal | Tissue | Inflammation Factors and Other Proteins | Heat Stress Proteins | Cell Death Regulation Proteins | Selenoproteins | Other Results | Analytical Method | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Na2SeO3 2 mg/Κg | CdCl2 150 mg/Kg | Liver (in vivo) | Se/Cd alleviation of increased mRNA levels of NF-κB, COX-2, PTGES, TNF-α, and IL-1 in relation to Cd treatment | Alleviation of increased mRNA/protein levels of HSP60, HSP70, HSP90 in relation to Cd treatment | No | No | Decrease of Cd induction (decrease of Li, B, Ca, Fe, Ti, Cu, Mo, Cd, Cr, Se, Sr, Ba, and Hg concentrations) | RT-PCR, Western blot | [47] |
| Na2O3Se 1 mg/Kg | Pb(CH3COO)2 350 mg/Kg | Neutrophils (in vivo) | Decrease of (IL-1β, IL-1R, IL-4, IL-8, IL-10, IL-12, TGF-β4) increased the mRNA expression of IL-2 and IFN-γ | Decrease of protein HSP27, -40, -60, -70, -90 and mRNA of HSP60 and -70 in relation to Pb treatment | No | No | No | RT-PCR, Western blot | [35] |
| Na2SeO3 2 mg/Kg | CdCl2 150 mg/Kg | Pancreas (in vivo) | No | No | No | No | Se/Cd treatment alleviated the mRNA increase of T-SOD, CAT, GSH-Px, T-AOC caused by Cd toxicity in relation to control | ICP-MS, RT-PCR | [18] |
| Na2SeO3 1 mg/Kg Se | Pb(CH3COO)2 350 mg/L | Testes (in vivo) | No | No | Se/Pb: downregulation of caspase-3, caspase-12 in relation to Pb treatment | GPX upregulation in Se treatment and alleviation of GPX downregulation induced by Pb in Se/Pb treatment | No | RT-PCR | [66] |
| Na2SeO3 2 mg/Kg | CdCl2 218.44 mg/Kg | Ovary (in vivo) | Se/Cd treatment alleviated the mRNA increase of HK2, PK, SDH, PbHX, LC3, Atg5, Beclin 1, Dynein, Lc3-I, Lc3-ll, mTOR caused by Cd toxicity in relation to control | No | No | No | No | q-PCR, Western Blot | [49] |
| Na2SeO3 1mg/Kg | Pb(CH3COO)2 350 mg/L | Kidney (in vivo) | No | No | caspase-3, caspase-12, Bcl-2 increase in Pb group and alleviation of increase in Pb/Se group | No | No | RT-PCR, Western Blot | [69] |
| No | CdCl2 10 mg/Kg | Spleen (in vivo) | AKT and mTOR decrease | HSP70 decrease | No | No | [Ca, Cr, Se, Sr, Sn, Ba decrease and Na, Mg, V, Fe, Mo, Cu, Zn, Cd increase] LC3-I, LC3-II, Beclin-1,NF-kB, p-JNK/JNK increased | ICP-MS, qRT-PCR, Western Blot | [48] |
| Na2SeO3 2 mg/Kg | CdCl2 150 mg/Kg | Kidney (in vivo) | Cd group: increase in mRNA levels of COX-2, NF-κB, PTGES, and TNF-α Se/Cd group: alleviation of mRNA level increase of NF-kB and TNF-α. COX-2 and PTGES were not influenced | No | No | (Decrease in the mRNA levels of GPX2, GPX3, DIO3, selenoprotein K, -N, -O, -Pb, -S, -T, -U, and -W between the Cd group and control, not in the mRNA levels of the GPX1, GPX4, DIO1, DIO2, Txnard1, -2, -3, selenoprotein H, -I, -M, Sep15, Sepp1, Sepx1, SPS2) AND (between Cd/Se group and control GPX2, GPX3, selenoprotein T, -U, and -W smaller decrease) | No | RT-PCR | [28] |
| Na2SeO3 0.02 mg/L | Pb(CH3COO)2 12 mg/L | Spleen (in vitro) | No | No | Se/Pb: increase due to Pb exposure of p53, Bak, caspace-3, caspase-9, Cyt-c and decrease of PL3K, Akt, Bcl2 alleviated via Se supplementation | No | Se alleviated the increase of MDA levels due to Pb and alleviated the decrease in antioxidant enzyme activity (GPX, SOD, and CAT) due to Pb Additionally, ROS levels in the control group and the Se group were not significantly different. Se alleviated the increase of ROS levels due to Pb | RT-PCR, Flow cytometry, Western Blot | [71] |
| Na2SeO3 2 mg/Kg | CdCl2 150 mg/Kg | Spleen (in vivo) | No | No | Se/Cd: caspase-3, caspase-9 small alleviation of mRNA increase due to Cd treatment but extensive alleviation of increase of caspase-3 protein levels | Se/Cd treatment alleviate the decrease of TrxR1, GPX1 due to Cd treatment | Cd increased H2O2 and MDA and SOD but T-AOC, CAT decreased Bax, Cyt-c, Bak alleviation of increase due to Cd | ICP-MS, Western Blot, RT-PCR | [54] |
| Na2SeO3 2 mg/Kg | CdCl2 150 mg/Kg | Liver (in vivo) | Se/Cd: iNOS alleviaton of increase of mRNA levels due to Cd similarly in protein levels | No | Se/Cd: caspase-3, caspase-9, p53 alleviation of increase of mRNA levels due to Cd protein levels | No | Se/Cd: Cyt-c alleviation of increase due to Cd | RT-PCR, Western Blot, TUNEL assay | [45] |
| Na2SeO3 2 mg/Kg | CdCl2 150 mg/Kg | Neutrophils (in vivo) | Se/Cd: alleviated increase of mRNA levels of COX-2 and the decrease of TNF-α due to Cd | Se/Cd: HSP 40, HSP 70, HSP 90 alleviation of increase of mRNA levels due to Cd but in HSP 60 is the same with Cd group | Se/Cd: NF-κB, IL-2, IL-4, IL-17, IFN-γ alleviation of mRNA levels increase due to Cd and IL-10, IL-1β, iNOS alleviation of decrease of mRNA levels due to Cd | No | No | RT-PCR | [41] |
| Na2SeO3 1mg/Kg | Pb(CH3COO)2 350 mg/Kg | Testes (in vivo) | No | Se/Pb: alleviation of increase of HSP27, -40, -60, -70, -90 mRNA levels caused by Pb toxicity in relation to control | No | DIO1, DIO2, DIO3, GPX1, GPX2, GPX3, GPX4, selenoprotein H, -I, -K, -M, -O, -Pb, -S, -T, -U, -W, -15, Sepn1, Sepp1, Sepx1, SPS2, Txnrd1, -2 and -3 increase of mRNA expression in Se group and alleviation of increase in Se/Cd group | No | qRT-PCR | [36] |
| Na2SeO3 1mg/L | Pb(CH3COO)2 350 mg/L | Neutrophils (in vivo) | Se treatment slightly increased TNF-α 3, Cox-2, iNOS, NF-κB mRNA levels in relation to control while Pb increased TNF-α3, Cox-2, iNOS, NF-κB mRNA levels in relation to control and Se/Pb treatment alleviated aforementioned increase of mRNA levels | No | GPX2, GPX3, GPX4, DIO1, DIO2, DIO3, Txnrd1, Txnrd2, Txnrd3, SPS2, Sepx1, Sepp1, selenoprotein S, -K, -O, -U, -H, -15, and -M, significantly higher in Se group than in control and slightly higher in Pb treatment in relation to control. Se/Pb treatment intensified the increase in Pb treatment in relation to control | No | No | RT-PCR, Western Blot | [70] |
| Na2SeO3 0.02 mg/L | CdCl2 0.2 mg/L | Neutrophils (in vitro) | Se/Cd treatment alleviated the increase of mRNA levels of IL-1β,IL-4, IL-10, IFN-γ, NF-κB, iNOS, COX-2, TNF-α, and PGE2 due to Cd present and also alleviated the mRNA levels decrease of IL-17 due to Cd toxicity in relation to control | No | No | No | No | TUNEL assay, RT-PCR, Western blot | [46] |
| Na2SeO3 1mg/L | Pb(CH3COO)2 350 mg/L | Testes (in vivo) | Se/Pb treatment alleviated the increase of NF-κB, TNF-α, COX-2, PTGE mRNA, and NF-κB protein levels due to Pb toxicity in relation to control | Se/Pb treatment alleviated the mRNA levels increase of HSP60, -70, -90 due to Pb toxicity in relation to control | No | No | Se/Pb treatment alleviated the 90 days Pb accumulation in testes | qRT-PCR, Western Blot | [67] |
| Na2SeO3 1mg/L | Pb(CH3COO)2 350 mg/L | Bursa of Fabricius (in vivo) | Se/Pb alleviated the mRNA increase of IL-2, IL-4, IL-6, IL-12β, IL-17, and the mRNA decrease of IFN-γ caused by Pb toxicity in relation to control | No | No | No | T-AOC, GPX, GST, SOD, and CAT activities increase in Se treatment in relation to control, in Pb treatment T-AOC, GPX, GST, SOD, and CAT activities decreased in relation to control and Se/Pb treatment alleviated this decrease | qRT-PCR | [65] |
| Na2SeO3 1mg/L | Pb(CH3COO)2 350 mg/L | Nervous Tissues (in vivo) | No | No | Se/Pb treatment alleviate the decrease of Bcl2 protein/ mRNA levels while alleviate the increase of protein/mRNA levels in p53, Bax, Cyt-c, caspases-3 due to Pb toxicity | No | No | qRT-PCR | [72] |
| Na2SeO3 1 mg/L | Pb(CH3COO)2 350 mg/L | Heart (in vivo) | Se/Pb treatment alleviated the increase of NF-kB, TNF-a, COX-2 and PTGEs mRNA levels due to Pb toxicity | No | No | Se/Pb treatment alleviated the decrease of mRNA levels of GPX1, -2, -3, and -4, Txnrd1, -2, -3, DIO1, -2, -3, selenoprotein N1, -K, -S, -T, -O, -H, -M, -15, -U, -Pb, Sepp1, Sepn1, Sepw1, Sepx1, SPS2 due to Pb toxicity in relation to control | No | qRT-PCR | [73] |
| Na2SeO3 2 mg/Kg | CdCl2 150 mg/Kg | Heart (in vivo) | No | No | Se/Cd treatment alleviated the increase of JNK, AMPK and PPARα due to Cd exposure and alleviated the decrease of P-JNK | No | No | qRT-PCR, Western Blot | [75] |
| Na2SeO3 1 mg/L | Pb(CH3COO)2 350 mg/L | Kidney (in vivo) | No | No | Se/Pb treatment alleviated the decrease of mRNA levels of mfn1, drp1, opa1, mff, mfn2 due to Pb toxicity | No | Se/Pb treatment alleviated the decrease of Cpx, SOD, MDA, ATPase activities, Mitochondrial complex V, -II, -I activities due to Pb toxicity | RT-PCR, Western Blot, TUNEL assay | [68] |
| Na2SeO3 1 mg/L | Pb(CH3COO)2 350 mg/L | Lymphocytes (in vivo) | Se/Pb treatment alleviated the mRNA increase of iNOS, TNF-a, COX-2, NF-KB due to Pb in relation to control | Se/Pb treatment alleviated the increase of mRNA levels of HSP27, -40, -60, -70, -90 due to Pb toxicity in relation to control | No | No | No | RT-PCR | [63] |
| Na2SeO3 1 mg/L | Pb(CH3COO)2 350 mg/L | Cartilage (in vivo) | No | No | No | Se alleviated the downtrend of the expression of GPX1, -2, -4, Txnrd2, Txnrd3, DIO1, DIO2, selenoprotein I, -U, Sepx1, selenoprotein K, -W, -O, -M, Sep15, Sepnn1, selenoprotein S, and -T induced by Pb in relation to control | Se/Pb treatment alleviated the concentration of Pb in sword cartilage tissue | qRT-PCR, ICP-MS | [74] |
| Na2SeO3 1 mg/L | Pb(CH3COO)2 350 mg/L | Liver (in vivo) | Se/Pb treatment alleviated the increase of mRNA levels of NF-κB, TNF-α, COX-2, PTGEs, and iNOS due to Pb toxicity in relation to control | Se/Pb treatment alleviate the increase of mRNA levels of HSP27, -40, -60, -70, -90 caused by Pb toxicity in relation to control | No | No | No | qRT-PCR | [31] |
| Na2SeO3 0.02 mg/L | CdCl2 0.2 mg/L | Splenic Lymphocytes (in vitro) | Se/Pb treatment alleviated the decrease of IL-1β, -2, -4, -10, -17, and IFN-γ mRNA levels due to Cd toxicity in relation to control | No | No | No | No | qRT-PCR | [44] |
| Na2SeO3 0.02 mg/L | CdCl2 0.2 mg/L | Lymphocytes (in vitro) | No | No | No | Se/Cd treatment alleviated the decrease of selenoprotein K, -N, -T, -S mRNA levels caused by Cd toxicity in relation to control | No | qRT-PCR | [51] |
| Na2SeO3 10 mg/Kg | CdCl2 150 mg/Kg | Immune organs (serum, thymus, spleen, Bursa of Fabricius) (in vivo) | Se/Pb treatment alleviated the increase of iNOS activity and NO production caused by Pb in relation to control | No | Se/Pb treatment alleviated the mRNA increase of p53 and apoptotic rates while alleviated the mRNA decrease of Bcl2 in relation to control | No | No | qRT-PCR, TUNEL assay | [42] |
| Na2SeO3 10 mg/Kg | CdCl2 150 mg/Kg | Cerebrum and Cerebellum (in vivo) | Se/Cd treatment alleviated the increase of iNOS mRNA/protein levels and NO activity induced by Cd toxicity in relation to control | No | No | Se/Cd treatment alleviated the GPX mRNA levels decrease caused by Cd toxicity in relation to control | Se/Cd treatment alleviated Pb accumulation | qRT-PCR, FAAS | [42] |
| Na2SeO3 0.02 mg/L | CdCl2 0.2 mg/L | Splenic Lymphocytes (in vitro) | No | No | Se/Cd treatment alleviated the mRNA increase of Bak, caspase-3, -9, p53 and Cyt-c and alleviated the mRNA decrease of Bcl-x, Bcl-2, CaM induced by Cd toxicity in relation to control | No | No | DCF, TUNEL Assay, qRT-PCR | [53] |
| Na2SeO3 0.02 mg/L | CdCl2, 0.2 mg/L | Splenic Lymphocytes (in vitro) | No | Se/Cd treatment alleviated the mRNA levels increase of HSP27, -40, -60, -70, -90 induced by Cd toxicity in relation to control | No | No | No | qRT-PCR | [76] |
| Type of Supplementation | Tissue | Selenoproteins | Heat Stress Proteins | Antioxidant Capacity | Other Results | Analytical Techniques/Methods | References |
|---|---|---|---|---|---|---|---|
| Na2SeO3 0.2 mg/Kg, Vit E 250 mg | Breast muscles | Se/Vit E: upregulation of Gpx1, Gpx4 and selenoprotein P in relation to control and Se group | HSP60, -70, -90 small mRNA decrease in Se group, no differences in Se/Vit E group | Se/Vit E and Se group: increase in concentration of CAT, SOD, GSH-P and MDA especially in Se/Vit E group in relation to control | RT-PCR | [134] | |
| BET 1g/Kg, Vit E 250 mg/Kg, Se 0.8 mg/Kg | Breast muscle | BET, Vit E and Se: increased GPx activity | BET reduced respiratory rate | GPx Assay | [135] | ||
| No | Liver | Gpx1 mRNA decrease | Heat stress treatment: increase NOX1, NOX3, DUOX2, GST, CAT, SOD1, GR, CASP6 mRNAs and decrease of CYBB, NOX4, NOX5, NADPH mRNAs | RT-PCR | [110] | ||
| Na2SeO3 0.30 mg/Kg, Se-yeast 0.30 mg/Kg | Breast muscles | upregulation of Gpx1, Gpx4 in both Se treatments | Downregulation of HSP70 in inorganic Se group, Se-yeast group showed a further downregulation HSP70 mRNA levels compared to control and inorganic Se group | Improved organoleptic meat characteristics (meat drip loss, water holding capacity, and shear force) | qRT-PCR, HG-AFS | [136] | |
| Νano-selenium 1.2 mg/Kg | Jejunal tissue | Decreased the plasma concentrations of LDL-C and AST, but linearly increased that of HDL-C before heat exposure. Moreover, the cholesterol concentration was lower in broilers fed diets supplemented with 0.6 mg/kg Nano-Se than that in the control ones. Heat stress decreased the plasma total protein concentration, but increased the AST activity | Enzymatic Kits | [137] | |||
| Vit A 16.000 IU/kg, Na2SeO3 0.50 mg/kg | Se/Vit E group: no significant change in egg quality in relation to control but significant changes in hen performance | [138] | |||||
| DL-α-tocopherole acetate 500 mg/Kg, Na2SeO3 0.5 mg/Kg | Se/Vit E group: synergistic effect between Se and Vit E in alleviation of heat stress | [139] | |||||
| Na2SeO3 1.5 mg/Kg, PAMK 200 mg/Kg | Spleen | Alleviation of increase of mRNA expression of HSP90, GRP-78 caused by heat stress | Se/PAMK group: alleviation of increase of expression of Bcl-2, caspase-3, ATF4, ATF6, IRE due to heat stress | qRT-PCR, Western Blot | [106] | ||
| Organic Se 0.3mg/Kg, Cr 2 mg/Kg, Zn 40 mg/Kg | Blood | Improved performance and antioxidant responses (reduced LP and increased superoxide dismutase) | [140] | ||||
| SeMet 1 mg/Kg α-tocopherol acetate 250 mg/Kg | Breast | Se/Vit E group: growth performance was not improved but improved lipid oxidation of breast meat | AAS, MDA determination | [141] | |||
| Se 3 mg/Kg, PAMK 200 mg/Kg | Bursa of Fabricius, spleen, thymus | Se/PAMK: Improved alleviation of mRNA increase of HSP60, -70, -90 | Se/PAMK group: Alleviation of mRNA increase of TNF-a, IFN-γ, IL2 and IL4 caused by heat stress | qRT-PCR | [142] | ||
| Se 3 mg/Kg, PAMK 200 mg/Kg | Endoplasmic reticulum of Spleen tissue | Higher alleviation of HSP27 and -70 increase in Se/PAMK group | qRT-PCR, Western Blot Analysis | [108] | |||
| Se 1mg/Kg, Vit E 250 mg/Kg | Better immune responses | Enzymatic methods | [143] | ||||
| Na2SeO3 0.028 mg/Kg | Liver, Breast muscle | GSH-Px activity increase due to Se supplementation | Enzymatic methods | [144] | |||
| Vit E 250 mg/kg, Se 1 mg/kg | Pectoralis muscle | SOD and Gpx activity increase | There was not a significant interaction in broiler growth performance between dietary treatments and environmental temperature | Enzymatic methods | [145] | ||
| Se 0.3 g/Kg, TP 10 g/Kg | Blood | Se/TP significantly reduced plasma triglycerides no significant effects on plasma hormones T | Enzymatic Methods | [146] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seremelis, I.; Danezis, G.P.; Pappas, A.C.; Zoidis, E.; Fegeros, K. Avian Stress-Related Transcriptome and Selenotranscriptome: Role during Exposure to Heavy Metals and Heat Stress. Antioxidants 2019, 8, 216. https://doi.org/10.3390/antiox8070216
Seremelis I, Danezis GP, Pappas AC, Zoidis E, Fegeros K. Avian Stress-Related Transcriptome and Selenotranscriptome: Role during Exposure to Heavy Metals and Heat Stress. Antioxidants. 2019; 8(7):216. https://doi.org/10.3390/antiox8070216
Chicago/Turabian StyleSeremelis, Isidoros, Georgios P. Danezis, Athanasios C. Pappas, Evangelos Zoidis, and Kostas Fegeros. 2019. "Avian Stress-Related Transcriptome and Selenotranscriptome: Role during Exposure to Heavy Metals and Heat Stress" Antioxidants 8, no. 7: 216. https://doi.org/10.3390/antiox8070216