High Glucose-Mediated Tyrosine Nitration of PI3-Kinase: A Molecular Switch of Survival and Apoptosis in Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
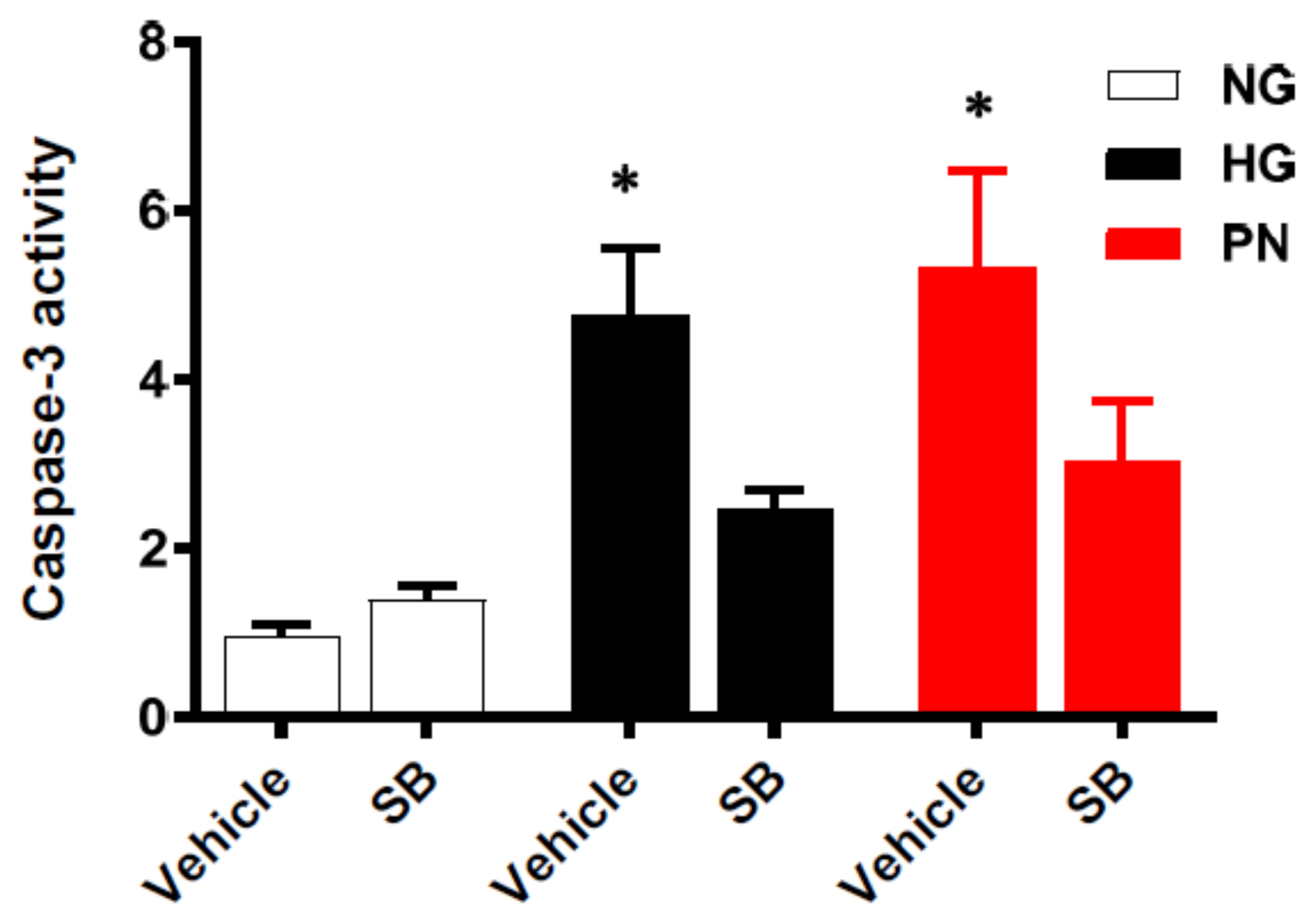
2.2. Caspase-3 Activity
2.3. TUNEL Assay
2.4. Western Blotting Analysis
2.5. Immunoprecipitation
2.6. Akt Kinase Assay
2.7. Adenoviral Constructs
2.8. Statistical Analysis
3. Results
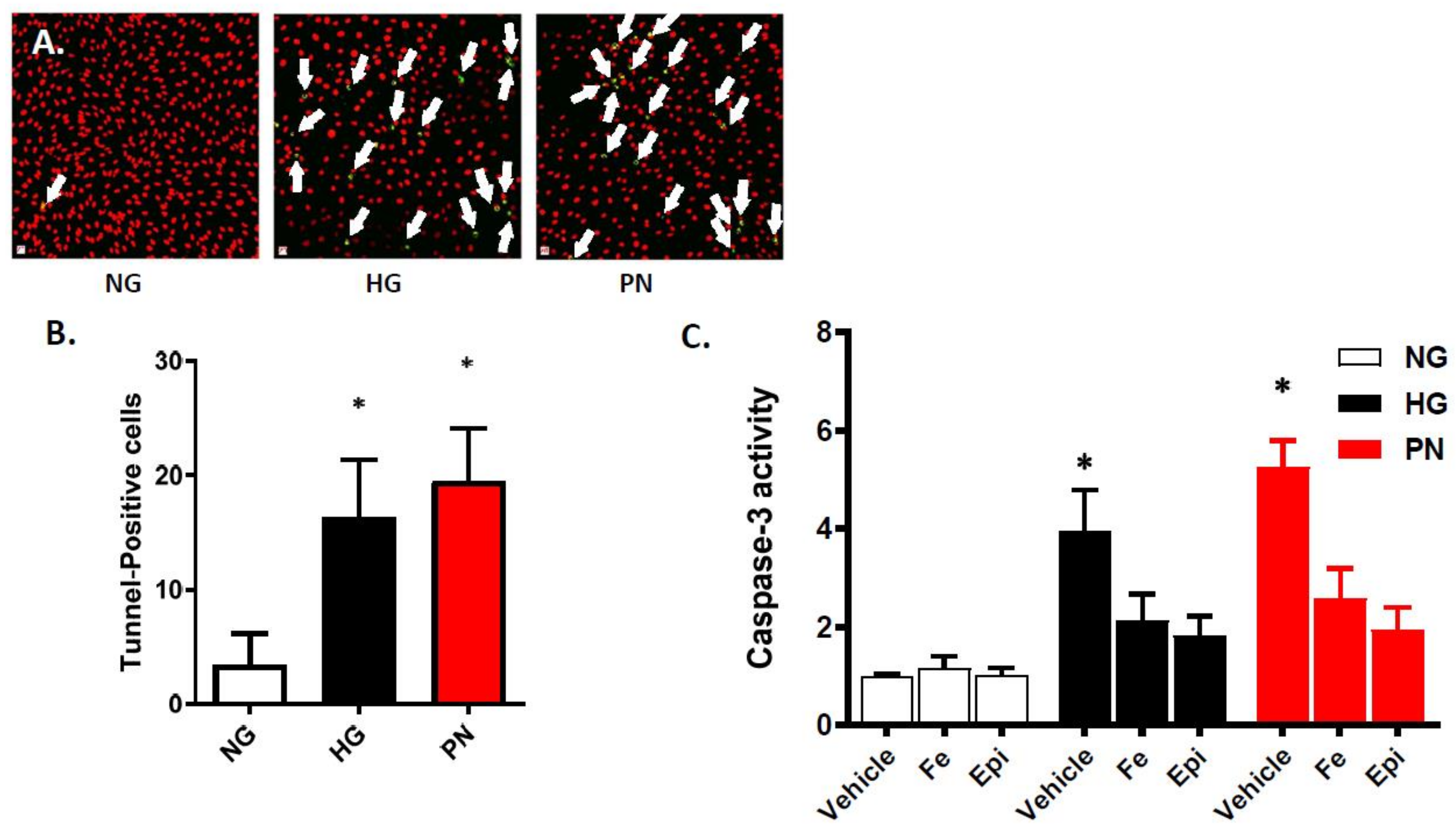
3.1. High Glucose-Induced Peroxynitrite Induces Apoptosis in Retinal Endothelial Cells
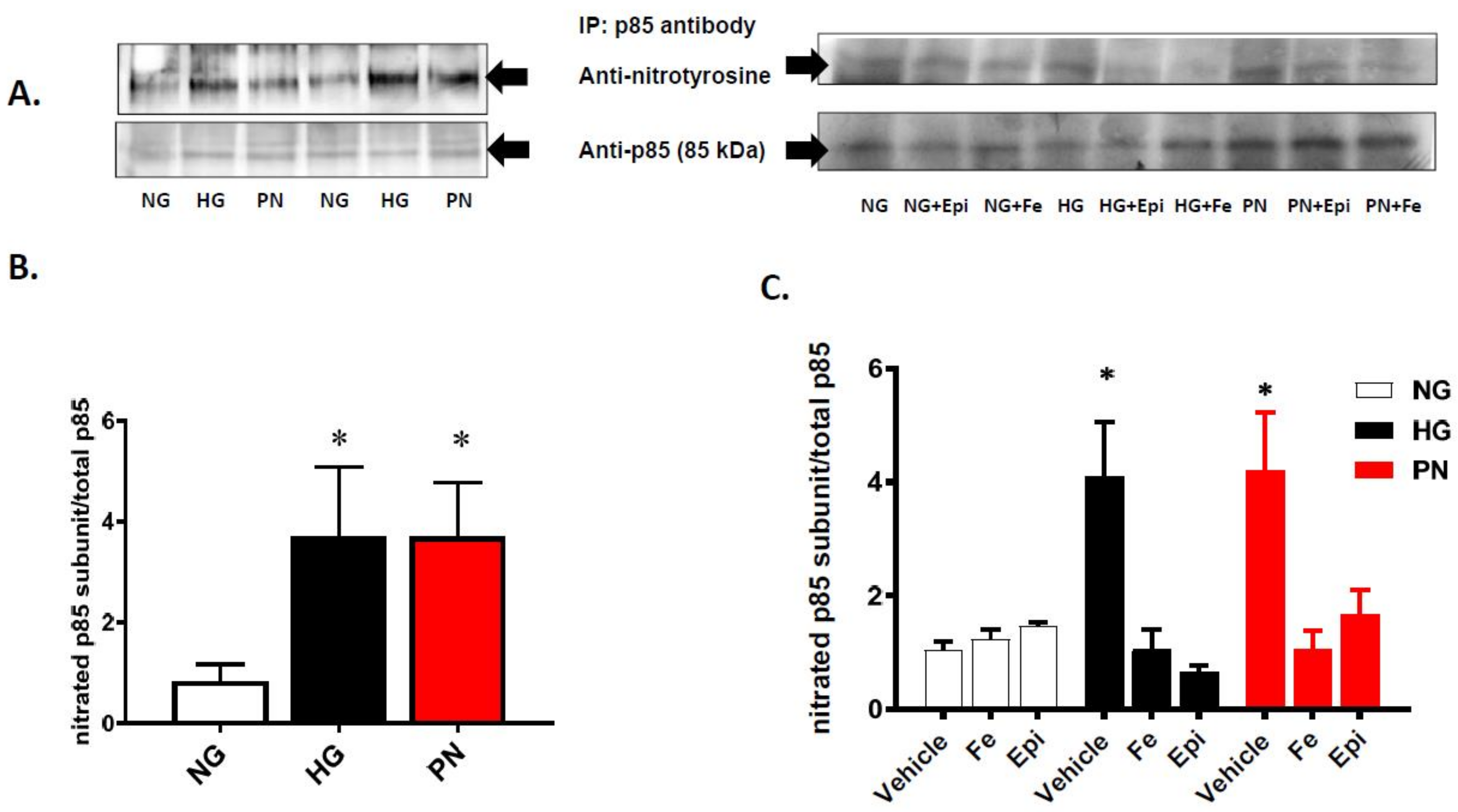
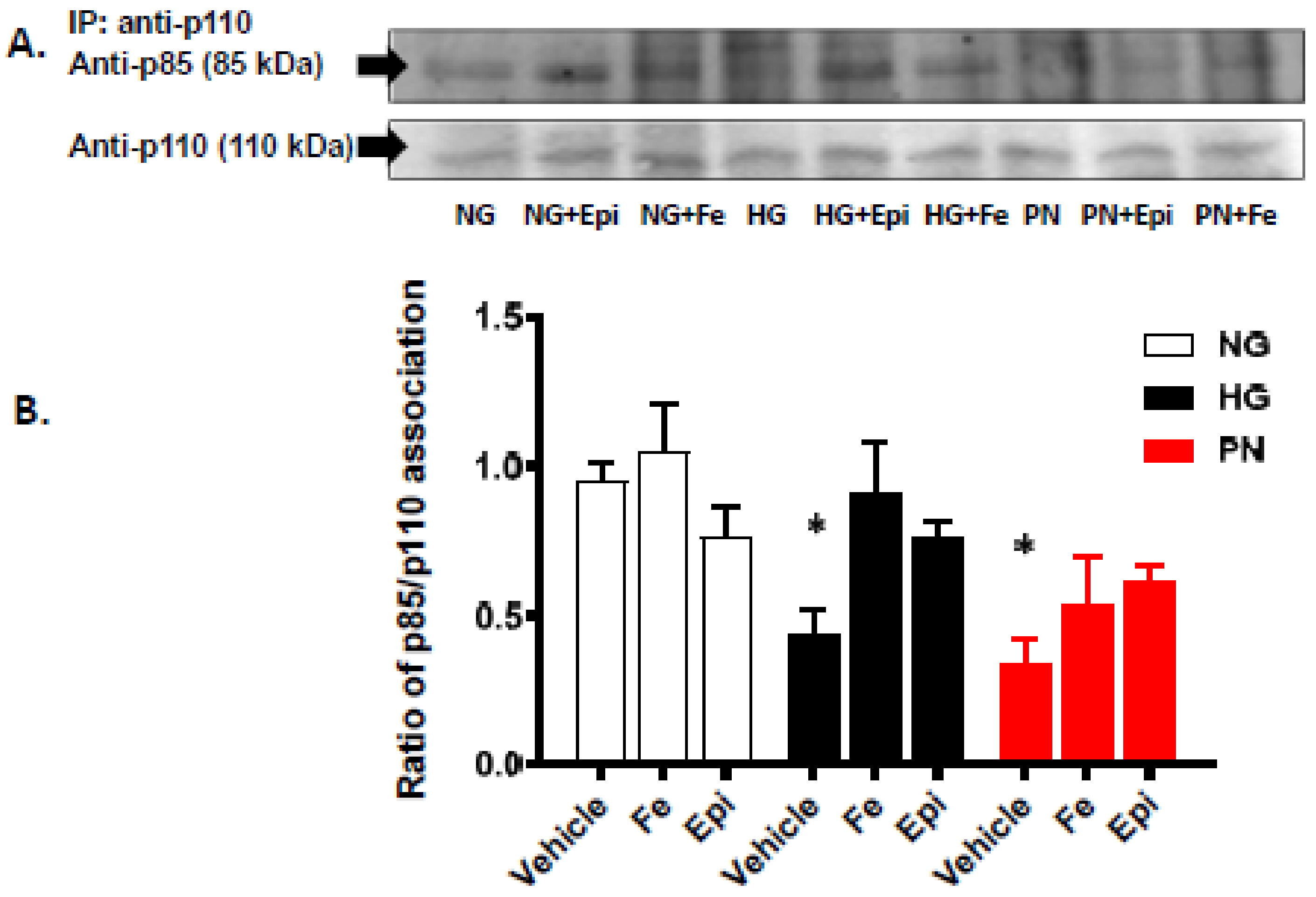
3.2. High Glucose and Peroxynitrite-Mediated Tyrosine Nitration of p85 Subunits Causes PI3-Kinase Dysfunction
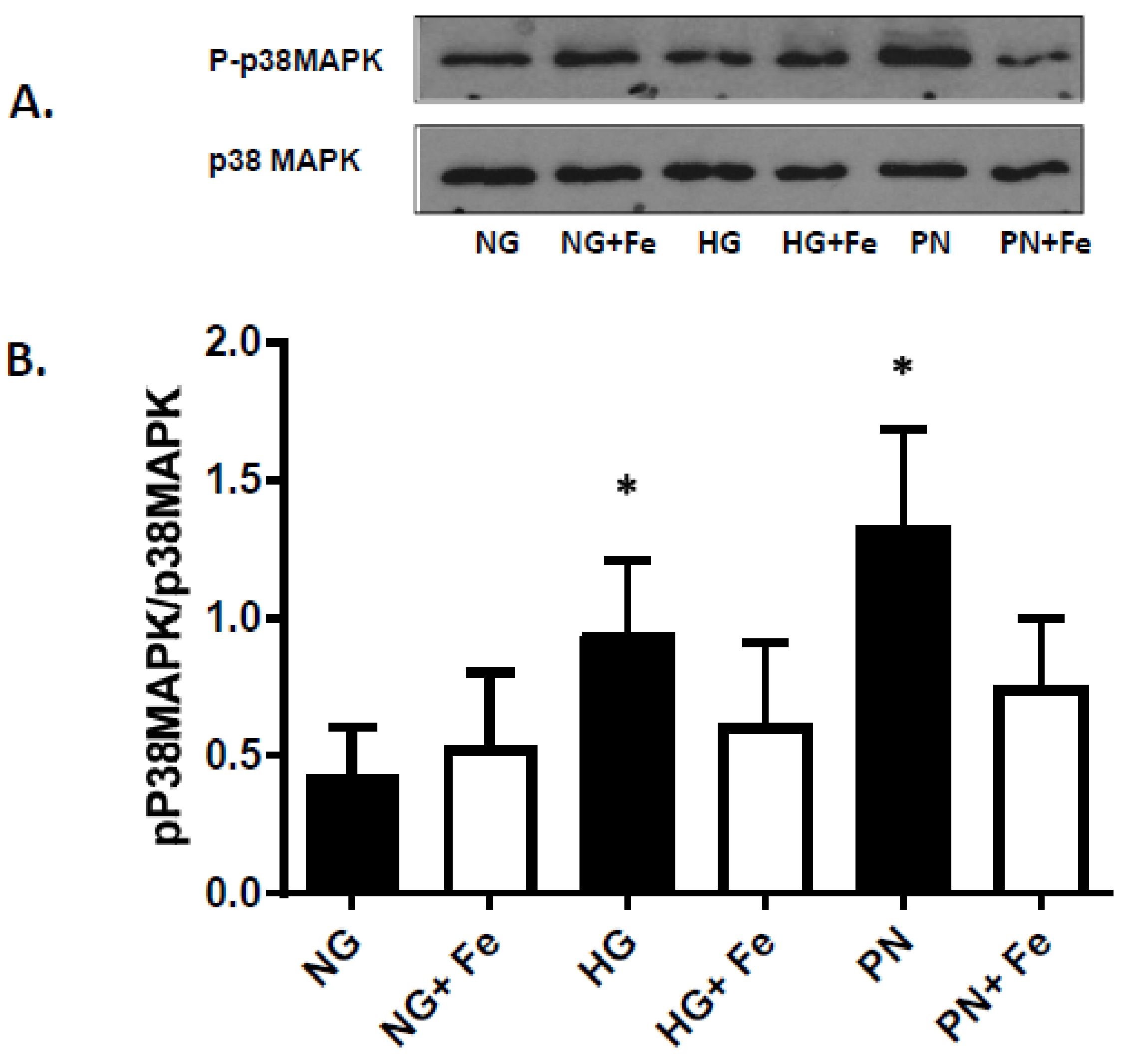
3.3. High Glucose-Induced Peroxynitrite Inhibits Akt and Activates P38 MAPK in ECs
3.4. Overexpression of Active Akt Restores EC Cultures’ Survival
3.5. Inhibition of p38 MAPK Restores Cell Survival in Retinal EC Cultures
4. Discussion
5. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- United Kingdom Prospective Diabetes Study Group. United Kingdom prospective diabetes study 24: A 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann. Int. Med. 1998, 128, 165–175. [Google Scholar]
- Pirola, L.; Balcerczyk, A.; Okabe, J.; El-Osta, A. Epigenetic phenomena linked to diabetic complications. Nat. Rev. Endocrinol. 2010, 6, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “metabolic memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.T.; El-Osta, A. Glycemic memories and the epigenetic component of diabetic nephropathy. Curr. Diab. Rep. 2013, 13, 574–581. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Abou-Mohamed, G.; Caldwell, R.W.; Caldwell, R.B. High glucose-induced tyrosine nitration in endothelial cells: Role of eNOS uncoupling and aldose reductase activation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3135–3143. [Google Scholar] [CrossRef]
- Fouda, A.Y.; Artham, S.; El-Remessy, A.B.; Fagan, S.C. Renin-angiotensin system as a potential therapeutic target in stroke and retinopathy: Experimental and clinical evidence. Clin. Sci. (Lond.) 2016, 130, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Chakravarti, D.N. Protein tyrosine nitration: Role in aging. Curr. Aging Sci. 2017, 10, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; El-Remessy, A.B.; Brooks, S.E.; Al-Shabrawey, M.; Tsai, N.T.; Caldwell, R.B. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am. J. Physiol. Cell Physiol. 2003, 285, C546–C554. [Google Scholar] [CrossRef] [PubMed]
- Abdelsaid, M.A.; Pillai, B.A.; Matragoon, S.; Prakash, R.; Al-Shabrawey, M.; El-Remessy, A.B. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J. Pharmacol. Exp. Ther. 2010, 332, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.K.; Matragoon, S.; Pillai, B.A.; Liou, G.I.; El-Remessy, A.B. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes 2008, 57, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Investig. 1996, 97, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Xi, X.; Tang, J.; Kern, T.S. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes 2002, 51, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Mecham, R.P.; Trackman, P.C.; Roy, S. Downregulation of lysyl oxidase protects retinal endothelial cells from high glucose-induced apoptosis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes 2003, 52, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xia, X.; Jiang, C.; Wu, J.; Zhang, S.; Zheng, Z.; Liu, W.; Zhang, Y.; Ren, H.; Wei, C.; et al. High glucose attenuates insulin-induced VEGF expression in bovine retinal microvascular endothelial cells. Eye (Lond.) 2010, 24, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Gratton, J.P.; Morales-Ruiz, M.; Kureishi, Y.; Fulton, D.; Walsh, K.; Sessa, W.C. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J. Biol. Chem. 2001, 276, 30359–30365. [Google Scholar] [CrossRef] [PubMed]
- Shanab, A.Y.; Mysona, B.A.; Matragoon, S.; El-Remessy, A.B. Silencing p75(NTR) prevents proNGF-induced endothelial cell death and development of acellular capillaries in rat retina. Mol. Ther. Methods Clin. Dev. 2015, 2, 15013. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papapetropoulos, A.; Sessa, W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Pannala, A.S.; Rice-Evans, C.A.; Halliwell, B.; Singh, S. Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochem. Biophys. Res. Commun. 1997, 232, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Klotz, L.O.; Sies, H. Amphiphilic properties of (−)-epicatechin and their significance for protection of cells against peroxynitrite. Biochem. Biophys. Res. Commun. 2003, 307, 69–73. [Google Scholar] [CrossRef]
- Hellberg, C.B.; Boggs, S.E.; Lapetina, E.G. Phosphatidylinositol 3-kinase is a target for protein tyrosine nitration. Biochem. Biophys. Res. Commun. 1998, 252, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Abdelsaid, M.; Prakash, R.; Li, W.; Coucha, M.; Hafez, S.; Johnson, M.H.; Fagan, S.C.; Ergul, A. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes 2015, 64, 1804–1817. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Sorensen, M.; Schmidt, F.; Schmidt, E.; Steven, S.; Kroller-Schon, S.; Daiber, A. The adverse effects of environmental noise exposure on oxidative stress and cardiovascular risk. Antioxid. Redox Signal. 2018, 28, 873–908. [Google Scholar] [CrossRef] [PubMed]
- Lassegue, B.; San Martin, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Shi, C.; Cohen, R.A. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 2002, 51, 198–203. [Google Scholar] [PubMed]
- Ceriello, A.; Mercuri, F.; Quagliaro, L.; Assaloni, R.; Motz, E.; Tonutti, L.; Taboga, C. Detection of nitrotyrosine in the diabetic plasma: Evidence of oxidative stress. Diabetologia 2001, 44, 834–838. [Google Scholar] [PubMed]
- El-Remessy, A.B.; Behzadian, M.A.; Abou-Mohamed, G.; Franklin, T.; Caldwell, R.W.; Caldwell, R.B. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am. J. Pathol. 2003, 162, 1995–2004. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Li, H.; He, C.; Lin, M.; Lyons, T.J.; Xie, Z. Tyrosine nitration of prostacyclin synthase is associated with enhanced retinal cell apoptosis in diabetes. Am. J. Pathol. 2011, 179, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wu, J.L.; Zhang, M.; Xu, J.; Zou, M.H. Endothelial nitric oxide synthase-dependent tyrosine nitration of prostacyclin synthase in diabetes in vivo. Diabetes 2006, 55, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Al-Gayyar, M.M.; Matragoon, S.; Pillai, B.A.; Ali, T.K.; Abdelsaid, M.A.; El-Remessy, A.B. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes [corrected]. Diabetologia 2011, 54, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Coucha, M.; Abdelsaid, M.; Li, W.; Johnson, M.H.; Orfi, L.; El-Remessy, A.B.; Fagan, S.C.; Ergul, A. Nox4 contributes to the hypoxia-mediated regulation of actin cytoskeleton in cerebrovascular smooth muscle. Life Sci. 2016, 163, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, R.; Gratton, J.P.; Yancopoulos, G.D.; Noseda, M.; Karsan, A.; Hussain, S.N. Angiopoietin-1 activates both anti- and proapoptotic mitogen-activated protein kinases. FASEB J. 2003, 17, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Wu, H.; Li, D.J.; Song, J.F.; Xiao, Y.D.; Liu, C.Q.; Zhou, J.Z.; Sui, Z.Q. Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. J. Agric. Food Chem. 2018, 66, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Valente, A.J.; Freeman, G.L.; Mahimainathan, L.; Mummidi, S. Interleukin-18 induces human cardiac endothelial cell death via a novel signaling pathway involving NF-kappaB-dependent PTEN activation. Biochem. Biophys. Res. Commun. 2006, 339, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Kulikova, L.; Vaskova, J.; Nagyova, A.; Mojzis, J. Fruit peel polyphenolic extract-induced apoptosis in human breast cancer cells is associated with ROS production and modulation of p38MAPK/ERK1/2 and the Akt signaling pathway. Nutr. Cancer 2017, 69, 920–931. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Franklin, T.; Ghaley, N.; Yang, J.; Brands, M.W.; Caldwell, R.B.; Behzadian, M.A. Diabetes-induced superoxide anion and breakdown of the blood-retinal barrier: Role of the VEGF/uPAR pathway. PLoS ONE 2013, 8, e71868. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, S.S.; Kim, J.C.; Kim, H.C.; Im, Y.S.; Ahn, C.W.; Lee, H.K. Serum and tear levels of nerve growth factor in diabetic retinopathy patients. Am. J. Ophthalmol. 2008, 145, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Q.; Zhang, R. Kaempferol targets estrogen-related receptor alpha and suppresses the angiogenesis of human retinal endothelial cells under high glucose conditions. Exp. Ther. Med. 2017, 14, 5576–5582. [Google Scholar] [PubMed]
- Clyne, A.M.; Zhu, H.; Edelman, E.R. Elevated fibroblast growth factor-2 increases tumor necrosis factor-alpha induced endothelial cell death in high glucose. J. Cell. Physiol. 2008, 217, 86–92. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshaer, S.L.; Lemtalsi, T.; El-Remessy, A.B. High Glucose-Mediated Tyrosine Nitration of PI3-Kinase: A Molecular Switch of Survival and Apoptosis in Endothelial Cells. Antioxidants 2018, 7, 47. https://doi.org/10.3390/antiox7040047
Elshaer SL, Lemtalsi T, El-Remessy AB. High Glucose-Mediated Tyrosine Nitration of PI3-Kinase: A Molecular Switch of Survival and Apoptosis in Endothelial Cells. Antioxidants. 2018; 7(4):47. https://doi.org/10.3390/antiox7040047
Chicago/Turabian StyleElshaer, Sally L., Tahira Lemtalsi, and Azza B. El-Remessy. 2018. "High Glucose-Mediated Tyrosine Nitration of PI3-Kinase: A Molecular Switch of Survival and Apoptosis in Endothelial Cells" Antioxidants 7, no. 4: 47. https://doi.org/10.3390/antiox7040047




