Interplay between Oxidative Stress and Platelet Activation in Coronary Thrombus of STEMI Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Coronary Angiographic Procedure and Thrombus/Intracoronary Blood Waste Collection
2.3. Blood Samples and Laboratory Assay
2.4. Nox2-Derived Peptide (sNox2-Dp)
2.5. Oxidized Low-Density Lipoproteins
2.6. Soluble CD40 Ligand and Soluble P-Selectin
2.7. Statistical Analysis
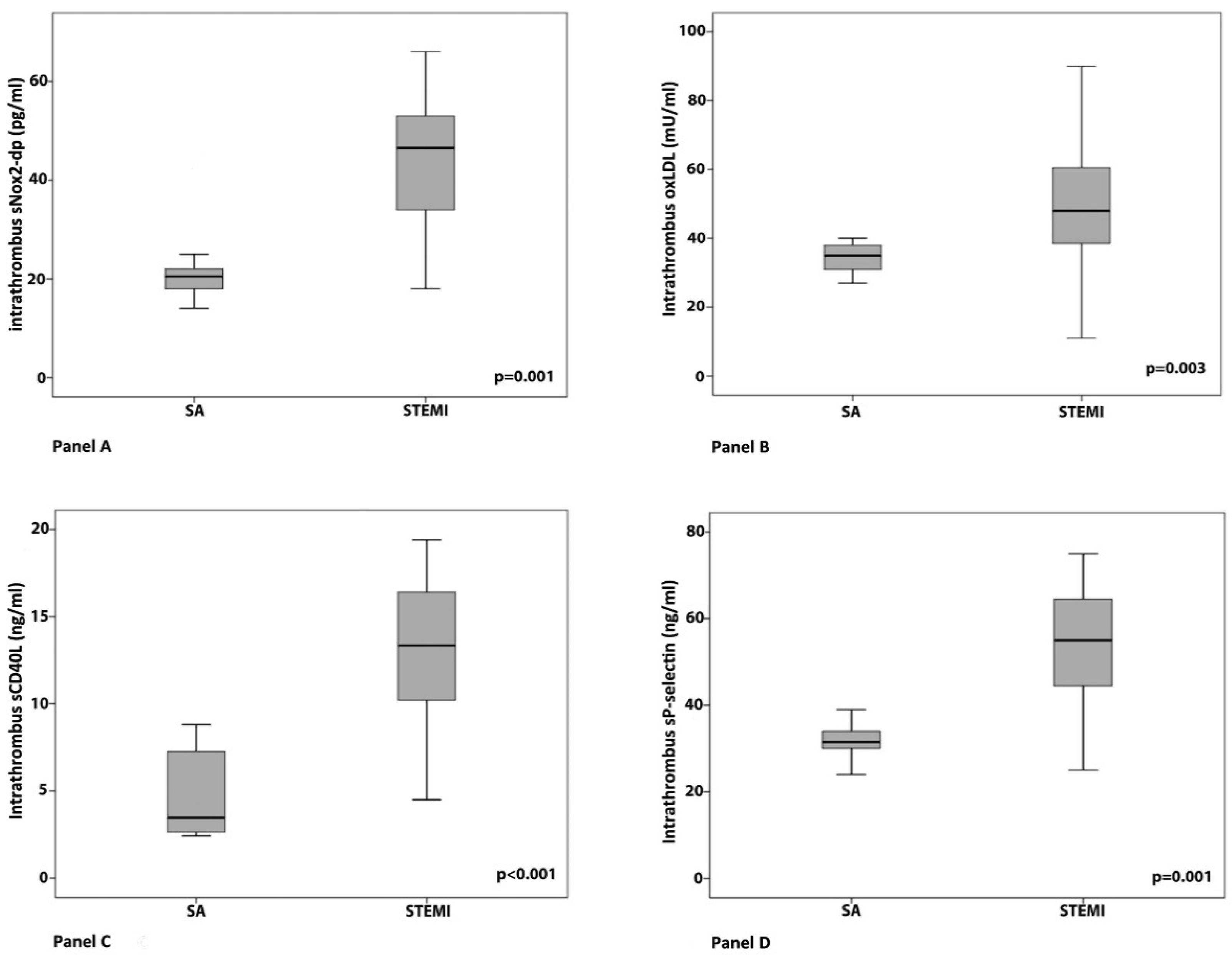
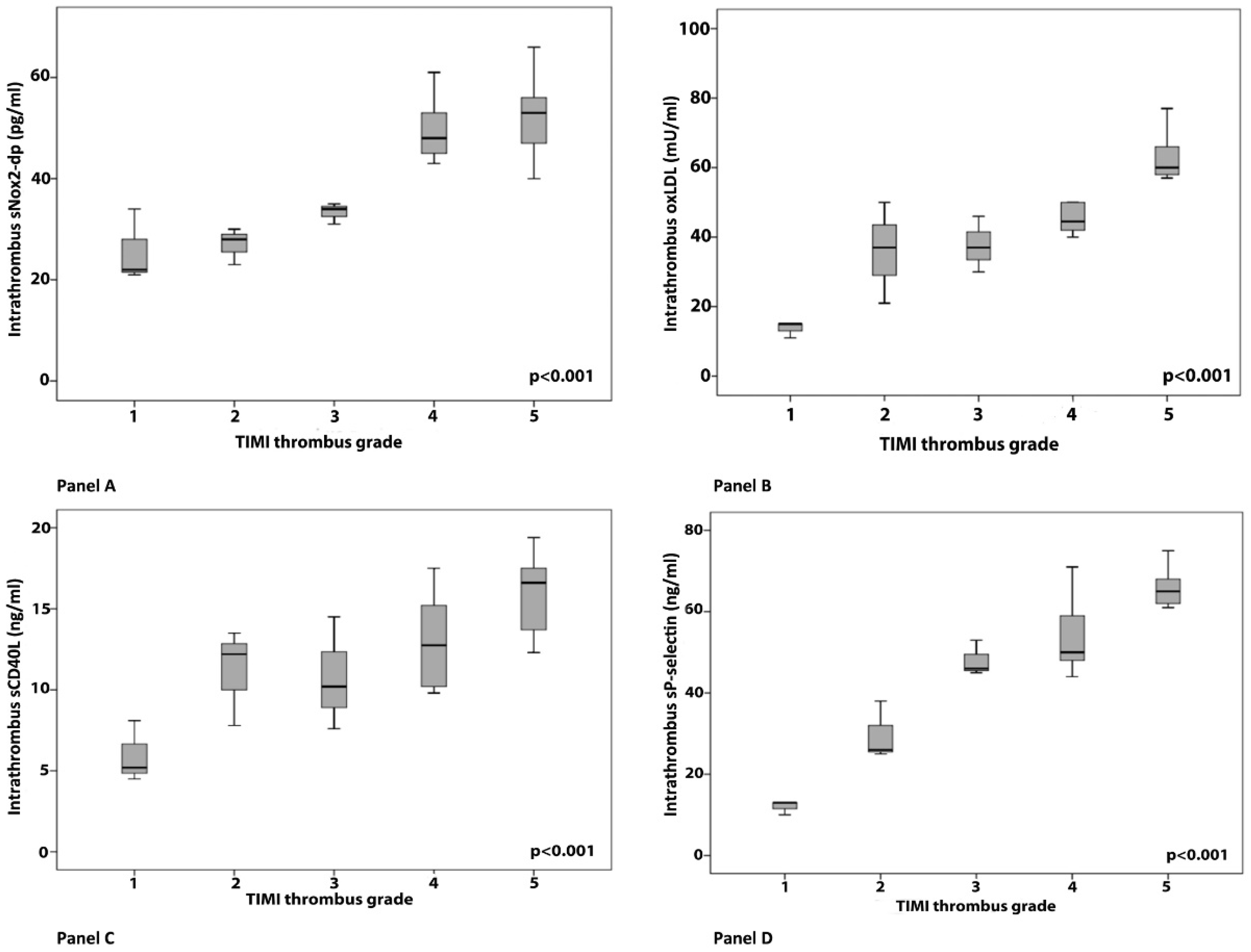
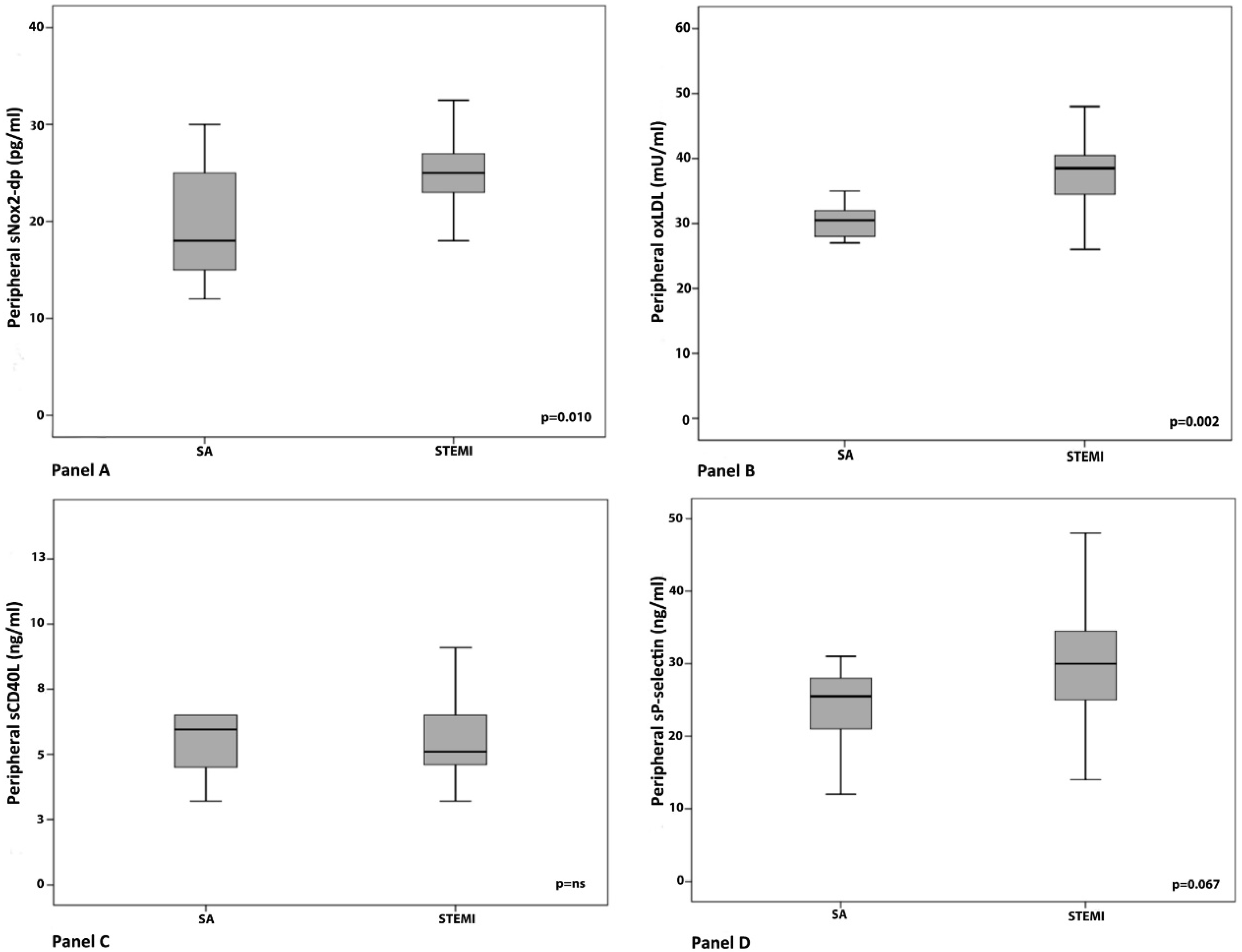
3. Results
Clinical, Angiographic and Laboratory Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Naruko, T.; Sugioka, K.; Inaba, M.; Itoh, A.; Haze, K.; Yoshiyama, M.; Ueda, M. Thrombus aspiration therapy and coronary thrombus components in patients with acute ST-elevation myocardial infarction. J. Atheroscler. Thromb. 2013, 20, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Silvain, J.; Collet, J.P.; Nagaswami, C.; Beygui, F.; Edmondson, K.E.; Bellemain-Appaix, A.; Cayla, G.; Pena, A.; Brugier, D.; Barthelemy, O.; et al. Composition of coronary thrombus in acute myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Nakano, M.; Prati, F.; Niccoli, G.; Mallus, M.T.; Ramazzotti, V.; Montone, R.A.; Kolodgie, F.D.; Virmani, R.; Crea, F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: A clinicopathological study. Circulation 2010, 122, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Naruko, T.; Sugioka, K.; Inaba, M.; Iwasa, Y.; Komatsu, R.; Itoh, A.; Haze, K.; Inoue, T.; Yoshiyama, M.; et al. Erythrocyte-rich thrombus aspirated from patients with ST-elevation myocardial infarction: Association with oxidative stress and its impact on myocardial reperfusion. Eur. Heart J. 2012, 33, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pignatelli, P.; Lenti, L.; Buchetti, B.; Sanguigni, V.; Di Santo, S.; Violi, F. LDL are oxidatively modified by platelets via GP91(phox) and accumulate in human monocytes. FASEB J. 2007, 21, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pignatelli, P. Platelet oxidative stress and thrombosis. Thromb. Res. 2012, 129, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Celestini, A.; Calvieri, C.; Cosentino, N.; Falcioni, E.; Carnevale, R.; Nocella, C.; Fracassi, F.; Roberto, M.; Antonazzo, R.P.; et al. Patients with microvascular obstruction after primary percutaneous coronary intervention show a gp91phox (NOX2) mediated persistent oxidative stress after reperfusion. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolh, P.; Windecker, S.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Juni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. J. Cardiothorac. Surg. 2014, 46, 517–592. [Google Scholar] [PubMed]
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC); Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blomstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; de Lemos, J.A.; Murphy, S.A.; Marble, S.J.; McCabe, C.H.; Cannon, C.P.; Antman, E.M.; Braunwald, E.; Group, T.S. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: A TIMI 14 substudy. Circulation 2001, 103, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Carnevale, R.; Cangemi, R.; Loffredo, L.; Sanguigni, V.; Stefanutti, C.; Basili, S.; Violi, F. Atorvastatin inhibits gp91phox circulating levels in patients with hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Zabczyk, M.; Undas, A. Coronary thrombus composition: Links with inflammation, platelet and endothelial markers. Atherosclerosis 2014, 237, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Carnevale, R.; Di Santo, S.; Bartimoccia, S.; Sanguigni, V.; Lenti, L.; Finocchi, A.; Mendolicchio, L.; Soresina, A.R.; Plebani, A.; et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.K.; Kim, K.; Estevez, B.; Xu, Z.; Stojanovic-Terpo, A.; Shen, B.; Ushio-Fukai, M.; Cho, J.; Du, X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 846–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambola, A.; Ruiz-Meana, M.; Barba, I.; Del Blanco, B.G.; Barrabes, J.A.; Lip, G.Y.; Vilardosa, U.; Sansaloni, S.; Rello, P.; Garcia-Dorado, D. Glycative and oxidative stress are associated with altered thrombus composition in diabetic patients with ST-elevation myocardial infarction. Int. J. Cardiol. 2017, 243, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Krijnen, P.A.; Meischl, C.; Hack, C.E.; Meijer, C.J.; Visser, C.A.; Roos, D.; Niessen, H.W. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J. Clin. Pathol. 2003, 56, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevale, R.; Bartimoccia, S.; Nocella, C.; Di Santo, S.; Loffredo, L.; Illuminati, G.; Lombardi, E.; Boz, V.; Del Ben, M.; De Marco, L.; et al. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 2014, 237, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Magwenzi, S.; Woodward, C.; Wraith, K.S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, B.; Koganti, S.; Lowdell, M.W.; Rakhit, R.D. Intracoronary platelet and monocyte activation status within platelet-monocyte complexes are determinants of inflammation in ST elevation myocardial infarction1. Clin. Hemorheol. Microcirc. 2016, 64, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresele, P.; Falcinelli, E.; Loffredo, F.; Cimmino, G.; Corazzi, T.; Forte, L.; Guglielmini, G.; Momi, S.; Golino, P. Platelets release matrix metalloproteinase-2 in the coronary circulation of patients with acute coronary syndromes: Possible role in sustained platelet activation. Eur. Heart J. 2011, 32, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Le, V.C.; Kim, B.H.; Shin, D.H.; Kim, J.S.; Kim, B.K.; Choi, D.; Jang, Y.; Hong, M.K. Correlations between coronary plaque tissue composition assessed by virtual histology and blood levels of biomarkers for coronary artery disease. Yonsei Med. J. 2012, 53, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Martino, F.; Carnevale, R.; Pignatelli, P.; Catasca, E.; Perri, L.; Calabrese, C.M.; Palumbo, M.M.; Baratta, F.; Del Ben, M.; et al. Obesity and hypercholesterolemia are associated with NOX2 generated oxidative stress and arterial dysfunction. J. Pediatr. 2012, 161, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Loffredo, L.; Sanguigni, V.; Plebani, A.; Rossi, P.; Pignata, C.; Martire, B.; Finocchi, A.; Pietrogrande, M.C.; Azzari, C.; et al. Different degrees of NADPH oxidase 2 regulation and in vivo platelet activation: Lesson from chronic granulomatous disease. J. Am. Heart Assoc. 2014, 3, e000920. [Google Scholar] [CrossRef] [PubMed]

| STEMI (n = 32) | SA (n = 10) | p | |
|---|---|---|---|
| Age (years) * | 64 ± 12.2 | 69.8 ± 13.0 | 0.22 |
| Males (%) ^ | 24 (75) | 9 (90) | 0.31 |
| Family history of CAD (%) ^ | 13 (41) | 3 (30) | 0.55 |
| Obesity (%) ^ | 14 (44) | 2 (20) | 0.17 |
| Arterial hypertension (%) ^ | 24 (75) | 6 (60) | 0.36 |
| Dyslipidaemia (%) ^ | 21 (66) | 50 (50) | 0.37 |
| Type II diabetes (%) ^ | 10 (31) | 3 (30) | 0.94 |
| Smoking habits (%) ^ | 16 (50) | 2 (20) | 0.09 |
| Previous myocardial infarction (%) ^ | 6 (19) | 4 (40) | 0.34 |
| Previous PCI (%) ^ | 3 (9) | 1 (10) | 0.95 |
| Cardiovascular Therapy before PCI | |||
| Antihypertensive drugs (%) ^ | 20 (62) | 4 (40) | 0.79 |
| β-blockers (%) ^ | 9 (28) | 1(10) | 0.45 |
| ASA (%) ^ | 19 (59) | 5 (50) | 0.87 |
| Statins (%) ^ | 12 (37) | 2 (20) | 0.52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvieri, C.; Tanzilli, G.; Bartimoccia, S.; Cangemi, R.; Arrivi, A.; Dominici, M.; Cammisotto, V.; Viceconte, N.; Mangieri, E.; Frati, G.; et al. Interplay between Oxidative Stress and Platelet Activation in Coronary Thrombus of STEMI Patients. Antioxidants 2018, 7, 83. https://doi.org/10.3390/antiox7070083
Calvieri C, Tanzilli G, Bartimoccia S, Cangemi R, Arrivi A, Dominici M, Cammisotto V, Viceconte N, Mangieri E, Frati G, et al. Interplay between Oxidative Stress and Platelet Activation in Coronary Thrombus of STEMI Patients. Antioxidants. 2018; 7(7):83. https://doi.org/10.3390/antiox7070083
Chicago/Turabian StyleCalvieri, Camilla, Gaetano Tanzilli, Simona Bartimoccia, Roberto Cangemi, Alessio Arrivi, Marcello Dominici, Vittoria Cammisotto, Nicola Viceconte, Enrico Mangieri, Giacomo Frati, and et al. 2018. "Interplay between Oxidative Stress and Platelet Activation in Coronary Thrombus of STEMI Patients" Antioxidants 7, no. 7: 83. https://doi.org/10.3390/antiox7070083





