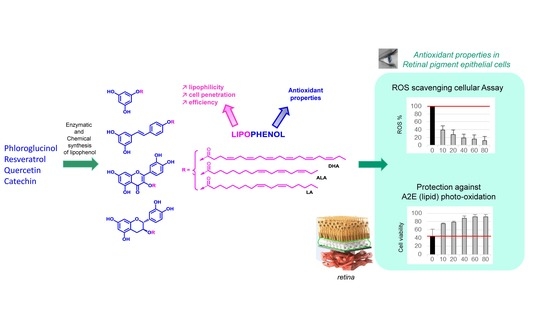
New Lipophenol Antioxidants Reduce Oxidative Damage in Retina Pigment Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PUFA Lipophenols
2.1.1. General Methods
2.1.2. NMR Characterization
2.1.3. UV Spectroscopy
2.1.4. Experimental Session
2.2. Cell Viability, Cytotoxicity and Antioxidant Activity
2.2.1. Chemicals
2.2.2. Cell Culture
2.2.3. Cell Viability
2.2.4. Cytotoxicity of Lipophenols
2.2.5. Protection of Lipophenols against ROS Production
2.2.6. Protection of Lipophenols against Photo-Oxidized A2E Toxicity
2.2.7. Statistical Analysis
3. Results
3.1. Chemical and Enzymatic Synthesis of PUFA Lipophenols
3.1.1. Phloroglucinol and Resveratrol Conjugates
Synthesis of Phloroglucinol-LA
Synthesis of Resveratrol-4′-LA
3.1.2. Catechin and Quercetin Conjugates
Synthesis of Catechin-3-LA
Synthesis of Quercetin-3-LA, 3-ALA, 3-DHA and 7-ALA
3.2. Toxicity of Lipophenols in ARPE-19 Cells
3.3. Inhibition of ROS Production by Lipophenol Pre-Treatment in ARPE-19 Cells
3.4. Lipophenols Protected RPE Cells from A2E Photo-Oxidation Toxicity
4. Discussion
4.1. Chemical and Enzymatic Synthesis of Lipophenols
4.2. How Does PUFA Introduction Influence Toxicity of Phenols in ARPE-19 Cells?
4.3. Antioxidant Properties of Lipophenols: ROS Scavenging and Protection against Oxidized A2E
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta 2017, 1862, 430–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, J.R.; Fishkin, N.; Zhou, J.; Cai, B.; Jang, Y.P.; Krane, S.; Itagaki, Y.; Nakanishi, K. A2E, a byproduct of the visual cycle. Vis. Res. 2003, 43, 2983–2990. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The Bisretinoids of Retinal Pigment Epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jockusch, S.; Itagaki, Y.; Turro, N.J.; Sparrow, J.R. Mechanisms involved in A2E oxidation. Exp. Eye Res. 2008, 86, 975–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Semchyshyn, H.M. Reactive Carbonyl Species In Vivo: Generation and Dual Biological Effects. Sci. World J. 2014, 2014, 417842. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Różanowska, M.; Sarna, T. Zeaxanthin in combination with ascorbic acid or α-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med. 2004, 36, 1094–1101. [Google Scholar] [CrossRef]
- Zhou, J.; Jang, Y.P.; Chang, S.; Sparrow, J.R. OT-674 Suppresses Photooxidative Processes Initiated by an RPE Lipofuscin Fluorophore. Phytochem. Photobiol. 2008, 84, 75–80. [Google Scholar] [CrossRef]
- Kang, J.-H.; Choung, S.-Y. Protective effects of resveratrol and its analogs on age-related macular degeneration in vitro. Arch. Pharm. Res. 2016, 39, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Hanneken, A.; Lin, F.-F.; Johnson, J.; Maher, P. Flavonoids Protect Human Retinal Pigment Epithelial Cells from Oxidative-Stress-Induced Death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3164. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Raju, R.; Nagineni, K.K.; Kommineni, V.K.; Cherukuri, A.; Kutty, R.K.; Hooks, J.J.; Detrick, B. Resveratrol Suppresses Expression of VEGF by Human Retinal Pigment Epithelial Cells: Potential Nutraceutical for Age-related Macular Degeneration. Aging Dis. 2014, 5, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef]
- Cia, D.; Cubizolle, A.; Crauste, C.; Jacquemot, N.; Guillou, L.; Vigor, C.; Angebault, C.; Hamel, C.P.; Vercauteren, J.; Brabet, P. Phloroglucinol protects retinal pigment epithelium and photoreceptor against all-trans-retinal–induced toxicity and inhibits A2E formation. J. Cell. Mol. Med. 2016, 20, 1651–1663. [Google Scholar] [CrossRef]
- McKay, T.B.; Karamichos, D. Quercetin and the ocular surface: What we know and where we are going. Exp. Biol. Med. 2017, 242, 565–572. [Google Scholar] [CrossRef]
- Joshi, D.; Field, J.; Murphy, J.; Abdelrahim, M.; Schönherr, H.; Sparrow, J.R.; Ellestad, G.; Nakanishi, K.; Zask, A. Synthesis of Antioxidants for Prevention of Age-Related Macular Degeneration. J. Nat. Prod. 2013, 76, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Liu, M.; Tuo, J.; Shen, D.; Chan, C.-C. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp. Eye Res. 2010, 91, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jin, H.L.; Jang, D.S.; Jeong, K.W.; Choung, S.-Y. Quercetin-3-O-α-l-arabinopyranoside protects against retinal cell death via blue light-induced damage in human RPE cells and Balb-c mice. Food Funct. 2018, 9, 2171–2183. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Mainini, F.; Contini, A.; Nava, D.; Corsetto, P.A.; Rizzo, A.M.; Agradi, E.; Pini, E. Synthesis, Molecular Characterization and Preliminary Antioxidant Activity Evaluation of Quercetin Fatty Esters. J. Am. Oil Chem. Soc. 2013, 90, 1751–1759. [Google Scholar] [CrossRef]
- Georgiou, T.; Neokleous, A.; Nicolaou, D.; Sears, B. Pilot study for treating dry age-related macular degeneration (AMD) with high-dose omega-3 fatty acids. PharmaNutrition 2014, 2, 8–11. [Google Scholar] [CrossRef]
- Crauste, C.; Vigor, C.; Brabet, P.; Picq, M.; Lagarde, M.; Hamel, C.; Durand, T.; Vercauteren, J. Synthesis and Evaluation of Polyunsaturated Fatty Acid-Phenol Conjugates as Anti-Carbonyl-Stress Lipophenols. Eur. J. Org. Chem. 2014, 2014, 4548–4561. [Google Scholar] [CrossRef]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Pavlickova, T.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol-Linoleate protects from exacerbated endothelial permeability via a drastic inhibition of the MMP-9 activity. Biosci. Rep. 2018, 38, BSR20171712. [Google Scholar] [CrossRef] [PubMed]
- Crauste, C.; Rosell, M.; Durand, T.; Vercauteren, J. Omega-3 polyunsaturated lipophenols, how and why? Biochimie 2016, 120, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.A.; Hashimoto, M.; Nakanishi, K.; Dillon, J.; Sparrow, J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 1998, 95, 14609–14613. [Google Scholar] [CrossRef] [Green Version]
- Vlachogianni, I.C.; Fragopoulou, E.; Kostakis, I.K.; Antonopoulou, S. In vitro assessment of antioxidant activity of tyrosol, resveratrol and their acetylated derivatives. Food Chem. 2015, 177, 165–173. [Google Scholar] [CrossRef]
- Zhu, Q.; Zheng, Z.-P.; Cheng, K.-W.; Wu, J.-J.; Zhang, S.; Tang, Y.S.; Sze, K.-H.; Chen, J.; Chen, F.; Wang, M. Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal. Chem. Res. Toxicol. 2009, 22, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Liu, S. Targeted acylation for all the hydroxyls of (+)-catechin and evaluation of their individual contribution to radical scavenging activity. Food Chem. 2016, 197, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Hiipakka, R.A.; Zhang, H.-Z.; Dai, W.; Dai, Q.; Liao, S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Lin, S.F.; Lin, Y.-H.; Lin, M.; Kao, Y.-F.; Wang, R.-W.; Teng, L.-W.; Chuang, S.-H.; Chang, J.-M.; Yuan, T.-T.; Fu, K.C.; et al. Synthesis and structure–activity relationship of 3-O-acylated (–)-epigallocatechins as 5α-reductase inhibitors. Eur. J. Med. Chem. 2010, 45, 6068–6076. [Google Scholar] [CrossRef] [PubMed]
- Uesato, S.; Taniuchi, K.; Kumagai, A.; Nagaoka, Y.; Seto, R.; Hara, Y.; Tokuda, H.; Nishino, H. Inhibitory effects of 3-O-acyl-(+)-catechins on Epstein-Barr virus activation. Chem. Pharm. Bull. 2003, 51, 1448–1450. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Park, Y.S.; Cho, S.J.; Sun, W.S.; Kim, S.H.; Jung, D.H.; Kim, J.H. Antimicrobial Activity of 3-O-Acyl-(-)-epicatechin and 3-O-Acyl-(+)-catechin derivatives. Planta Med. 2004, 70, 272–276. [Google Scholar] [CrossRef]
- Viskupicova, J.; Ondrejovi, M.; Šturdík, E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 2008, 47, 151–162. [Google Scholar]
- Gatto, M.T.; Falcocchio, S.; Grippa, E.; Mazzanti, G.; Battinelli, L.; Nicolosi, G.; Lambusta, D.; Saso, L. Antimicrobial and Anti-Lipase Activity of Quercetin and its C2-C16 3-O-Acyl-Esters. Bioorg. Med. Chem. 2002, 10, 269–272. [Google Scholar] [CrossRef]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970; ISBN 978-3-642-88460-3. [Google Scholar]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of Hydroxyl Groups: An Overlooked Influence on Antiradical Properties of Flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tao, Z.; Jin, Y.; Yuan, Y.; Dong, T.T.X.; Tsim, K.W.K.; Zhou, Z. Flavonoids, a Potential New Insight of Leucaena leucocephala Foliage in Ruminant Health. J. Agric. Food Chem. 2018, 66, 7616–7626. [Google Scholar] [CrossRef]
- Maeda, A.; Golczak, M.; Chen, Y.; Okano, K.; Kohno, H.; Shiose, S.; Ishikawa, K.; Harte, W.; Palczewska, G.; Maeda, T.; et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 2011, 8, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, K.C.; Aotaki-keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, A Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Kim, S.R.; Wu, Y. Experimental Approaches to the Study of A2E, a Bisretinoid Lipofuscin Chromophore of Retinal Pigment Epithelium. Methods Mol. Biol. 2010, 652, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Conte, R.; Calarco, A.; Napoletano, A.; Valentino, A.; Margarucci, S.; Di Cristo, F.; Di Salle, A.; Peluso, G. Polyphenols Nanoencapsulation for Therapeutic Applications. J. Biomol. Res. Ther. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef] [Green Version]
- Hytti, M.; Piippo, N.; Salminen, A.; Honkakoski, P.; Kaarniranta, K.; Kauppinen, A. Quercetin alleviates 4-hydroxynonenal-induced cytotoxicity and inflammation in ARPE-19 cells. Exp. Eye Res. 2015, 132, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Aiello, F. Quercetin-3-oleate. Molbank 2018, 2018, M1006. [Google Scholar] [CrossRef]
- Cialdella-Kam, L.; Nieman, D.; Knab, A.; Shanely, R.; Meaney, M.; Jin, F.; Sha, W.; Ghosh, S. A Mixed Flavonoid-Fish Oil Supplement Induces Immune-Enhancing and Anti-Inflammatory Transcriptomic Changes in Adult Obese and Overweight Women—A Randomized Controlled Trial. Nutrients 2016, 8, 277. [Google Scholar] [CrossRef]
- Liu, A.; Lin, Y.; Terry, R.; Nelson, K.; Bernstein, P.S. Role of long-chain and very-long-chain polyunsaturated fatty acids in macular degenerations and dystrophies. Clin. Lipidol. 2011, 6, 593–613. [Google Scholar] [CrossRef] [Green Version]
- Johansson, I.; Monsen, V.T.; Pettersen, K.; Mildenberger, J.; Misund, K.; Kaarniranta, K.; Schønberg, S.; Bjørkøy, G. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells. Autophagy 2015, 11, 1636–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahn, U.; Galano, J.-M.; Durand, T. Beyond Prostaglandins—Chemistry and Biology of Cyclic Oxygenated Metabolites Formed by Free-Radical Pathways from Polyunsaturated Fatty Acids. Angew. Chem. Int. Ed. 2008, 47, 5894–5955. [Google Scholar] [CrossRef] [PubMed]
- Balas, L.; Durand, T. Dihydroxylated E,E,Z-docosatrienes. An overview of their synthesis and biological significance. Prog. Lipid Res. 2016, 61, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Shahidi, F. Lipophilization of Resveratrol and Effects on Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 8617–8625. [Google Scholar] [CrossRef] [PubMed]
- Mbatia, B.; Kaki, S.S.; Mattiasson, B.; Mulaa, F.; Adlercreutz, P. Enzymatic synthesis of lipophilic rutin and vanillyl esters from fish byproducts. J. Agric. Food Chem. 2011, 59, 7021–7027. [Google Scholar] [CrossRef] [PubMed]
- Viskupicova, J.; Maliar, T. Rutin fatty acid esters: From synthesis to biological health effects and application. J. Food Nutr. Res. 2017, 56, 232–243. [Google Scholar]
- Bhullar, K.S.; Warnakulasuriya, S.N.; Rupasinghe, H.P.V. Biocatalytic synthesis, structural elucidation, antioxidant capacity and tyrosinase inhibition activity of long chain fatty acid acylated derivatives of phloridzin and isoquercitrin. Bioorg. Med. Chem. 2013, 21, 684–692. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef]
- Jin, G.; Yoshioka, H. Synthesis of lipophilic poly-lauroyl-(+)-catechins and radical-scavenging activity. Biosci. Biotechnol. Biochem. 2005, 69, 440–447. [Google Scholar] [CrossRef]
- Perera, N.; Ambigaipalan, P.; Shahidi, F. Epigallocatechin gallate (EGCG) esters with different chain lengths fatty acids and their antioxidant activity in food and biological systems. J. Food Bioact. 2018, 1, 124–133. [Google Scholar] [CrossRef]
- Fontaine, V.; Monteiro, E.; Brazhnikova, E.; Lesage, L.; Balducci, C.; Guibout, L.; Feraille, L.; Elena, P.-P.; Sahel, J.-A.; Veillet, S.; et al. Norbixin Protects Retinal Pigmented Epithelium Cells and Photoreceptors against A2E-Mediated Phototoxicity In Vitro and In Vivo. PLoS ONE 2016, 11, e0167793. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, E.H.; Kim, S.R.; Jang, Y.P. Anti-apoptotic effects of Curcuma longa L. extract and its curcuminoids against blue light-induced cytotoxicity in A2E-laden human retinal pigment epithelial cells. J. Pharm. Pharmacol. 2017, 69, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef] [PubMed]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]








| Compound | δ (H-6) | δ (H-8) | δ (H-2′) | δ (H-5′) | δ (H-6′) |
|---|---|---|---|---|---|
| quer | 6.18 | 6.39 | 7.73 | 6.89 | 7.63 |
| quer-3-ALA (14) | 6.23 | 6.42 | 7.33 | 6.88 | 7.27 |
| quer-7-ALA (18) | 6.46 | 6.81 | 7.77 | 6.88 | 7.65 |
| Compound | δ (2-C) | δ (3-C) | δ (4-C) | δ (4a-C) | δ (6-C) | δ (7-C) | δ (8-C) |
|---|---|---|---|---|---|---|---|
| quer | 148.03 | 137.20 | 177.33 | 104.50 | 99.28 | 165.73 | 94.45 |
| quer-3-ALA (14) | 158.42 | 131.45 | 177.21 | 105.29 | 100.14 | 166.25 | 95.04 |
| quer-7-ALA (18) | 149.31 | 138.02 | 177.62 | 108.49 | 104.82 | 157.19 | 101.86 |
| Entry | Compound | ARPE-19 CC50 1 | ROS production IC50 2 |
|---|---|---|---|
| 1 | Phloroglucinol | >160 | 10.25 ± 3.44 |
| 2 | Phloro-LA (3) | >160 | >80 |
| 3 | Resveratrol | >160 | 7.40 ± 0.51 |
| 4 | Resv-4′-LA (8) | >160 | >>80 |
| 5 | (+)-catechin | >160 | 5.93 ± 0.06 |
| 6 | Cat-3-LA (9) | 148.63 ± 6.61 | 10.61 ± 4.41 ◆◆◆ |
| 7 | Quercetin | 111.45 ± 4.92 | 6.82 ± 0.73 |
| 8 | Quer-3-LA (13) | >160 | 52.25 ± 10.84 *** |
| 9 | Quer-3-DHA (15) | 134.10 ± 8.09 *; °°° | 59.42 ± 18.80 *** |
| 10 | Quer-3-ALA (14) | 69.44 ± 3.46 *** | 61.72 ± 7.43 *** |
| 11 | Quer-7-ALA (18) | >160 | 9.44 ± 2.37 **; ### |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moine, E.; Brabet, P.; Guillou, L.; Durand, T.; Vercauteren, J.; Crauste, C. New Lipophenol Antioxidants Reduce Oxidative Damage in Retina Pigment Epithelial Cells. Antioxidants 2018, 7, 197. https://doi.org/10.3390/antiox7120197
Moine E, Brabet P, Guillou L, Durand T, Vercauteren J, Crauste C. New Lipophenol Antioxidants Reduce Oxidative Damage in Retina Pigment Epithelial Cells. Antioxidants. 2018; 7(12):197. https://doi.org/10.3390/antiox7120197
Chicago/Turabian StyleMoine, Espérance, Philippe Brabet, Laurent Guillou, Thierry Durand, Joseph Vercauteren, and Céline Crauste. 2018. "New Lipophenol Antioxidants Reduce Oxidative Damage in Retina Pigment Epithelial Cells" Antioxidants 7, no. 12: 197. https://doi.org/10.3390/antiox7120197