Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals
Abstract
:1. Introduction
2. Mechanisms of Photoaging and Photocarcinogenesis
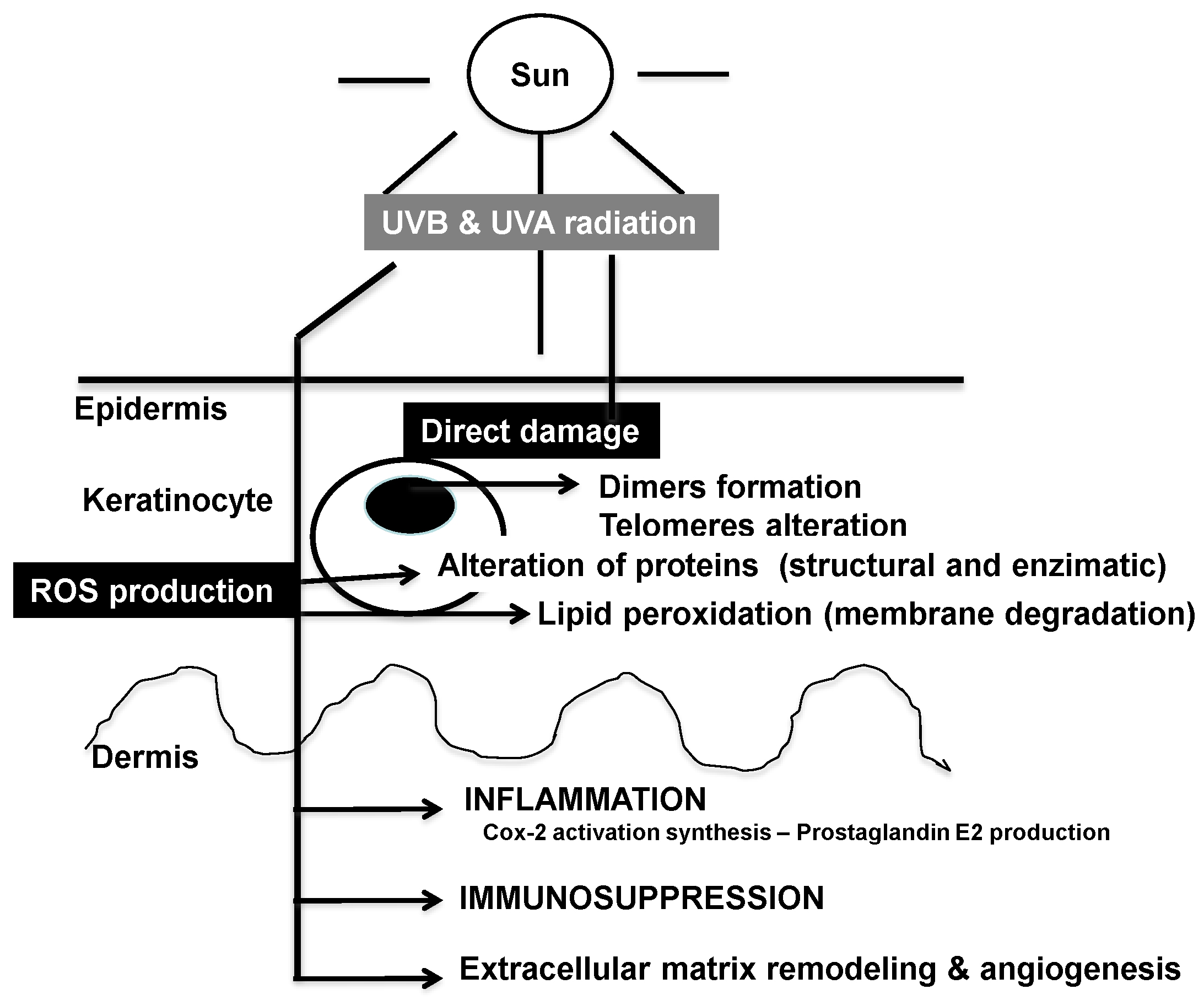
2.1. Effects of Solar Ultraviolet Radiation
2.2. DNA and Cellular Homeostasis
2.3. Signal Transduction Pathways
2.4. Role of Mitochondria and Cellular Bioenergetics
2.5. Inflammation Cascade
2.6. Immunosuppression

2.7. Extracellular Remodeling: Collagen, Elastin and Matrix Metalloproteinases Network
3. Photoprotective Strategies

3.1. Blockade of UV Photon Incidence
3.2. DNA Repair Systems
3.3. Antioxidant Activity
3.4. Anti-Inflammatory Action
3.5. Immunomodulatory Action
3.6. Inhibitory Activity of ECM Remodeling
4. Photoprotective Activity of Phytochemical Derivatives
4.1. Polyphenols
4.1.1. Flavonoids
| Polyphenol | Major Sources |
|---|---|
| Flavonoids | |
| Catechins: catechin, epicatechin, galactocatechin, epicatechingallate, epigallocatechin-3-gallate | Tea |
| Isoflavones: Genistein | Soy |
| Sylimarin | Thistle |
| Proanthocyanidins (tannins) | Grapeseed |
| Anthocyanins | Pomegranate |
| Non-flavonoids | |
| Phenolic acids | Grape & derivatives |
| Benzoic acids: Galic acid | Tea |
| Cinnamic acids | Polypodium leucotomos |
| Stilbene | Grape & derivatives |
| Resveratrol | Nuts, peanuts |
4.1.2. Non-Flavonoids
4.1.3. Natural Sources of Polyphenols
4.2. Non-Polyphenols with Photoprotective Sctivity
| Substance and Origin | Activity | Reference |
|---|---|---|
| Topical “Baicalin” | Inhibition of Ki67, PCNA and COX-2 expression | [118] |
| Genus Scutellaria | ||
| Oral “Flavangenol” | Reduction of Ki-67, and (8-OHdG)-positive cells and VEGF expression | [119] |
| French maritime pine bark extract | ||
| Topical black raspberry extract | Reduction of edema, p53 levels and neutrophil activation | [120] |
| Topical Photomorphe umbellata extract | Inhibition of the hyperplastic reaction and p53-positive cells | [121] |
| Oral and topical Brown algae polyphenols | Inhibition of ciclooxygenase-2 activity and cell proliferation | [122] |
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Uliasz, A.; Spencer, J.M. Chemoprevention of skin cancer and photoaging. Clin. Dermatol. 2004, 22, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Sivamani, R.K.; Fazel, N. Botanical agents for the treatment of nonmelanoma skin cancer. Dermatol. Res. Pract. 2013, 2013. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S; Voorhes, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. 1996, 379, 335–339. [Google Scholar]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Bachelor, M.A.; Bowden, G.T. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Sem. Cancer Biol. 2004, 14, 131–138. [Google Scholar] [CrossRef]
- Dreher, D.; Junod, A.F. Role of oxygen free radicals in cancer development. Eur. J. Cancer 1996, 32A, 30–38. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of oxidative stress and antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Perchellet, J.P.; Perchellet, E.M.; Gali, H.U.; Gao, X.M. Oxidant stress and multistage skin carcinogenesis. In Skin Cancer: Mechanisms and Human Relevance; CRC Press: Boca Raton, FL, USA, 1995; pp. 145–180. [Google Scholar]
- Hofseth, L.J.; Hussain, S.P.; Wogan, G.N.; Harris, C.C. Nitric oxide in cancer and chemoprevention. Free Radic. Biol. Med. 2003, 34, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Hiraku, Y.; Oikawa, S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat. Res. 2001, 488, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Lloyd, J.R.; Bickers, D.R.; Mukhtar, H. Malignant conversion of UV radiation and chemically induced mouse skin benign rumors by free-radical-generating compounds. Carcinogenesis 1989, 10, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Lelkes, P.I.; Hahn, K.L.; Sukovich, D.A.; Karmiol, S.; Schmidt, D.H. On the possible role of reactive oxygen species in angiogenesis. Adv. Exp. Med. Biol. 1998, 454, 295–310. [Google Scholar] [PubMed]
- Lippke, J.A.; Gordon, L.K.; Brash, D.E.; Haseltine, W.A. Distribution of UV light-induced damage in a defined sequence of human DNA: Detection of alkaline-sensitive lesions at pyrimidine nucleoside-cytidine sequences. Proc. Natl. Acad. Sci. USA 1981, 78, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.L.; Nairn, R.S. The biology of the (6–4) photoproduct. Photochem. Photobiol. 1989, 49, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C. Cellular aspects of photocarcinogenesis. Photochem. Photobiol. Sci. 2006, 5, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Einspahr, J.G.; Alberts, D.S.; Warneke, J.A.; Bozzo, P.; Basye, J.; Grogan, T.M.; Nelson, M.A.; Bowden, G.T. Relationship of p53 mutations to epidermal cell proliferation and apoptosis in human UV-induced skin carcinogenesis. Neoplasia 1999, 1, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.M.; Sanchez, C.A.; Morgan, C.A.; Schimke, M.K.; Ramel, S.; Idzerda, R.L.; Raskind, W.H.; Reid, B.J. A p53-dependent mouse spindle checkpoint. Science 1995, 267, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Hashimoto, T. Ultraviolet B-induced cell death in four cutaneous cell lines exhibiting different enzymatic antioxidant defences: Involvement of apoptosis. J. Dermatol. Sci. 1998, 17, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Scott, M.P.; Bretscher, A. Molecular Cell Biology, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Magné, N.; Toillon, R.A.; Bottero, V.; Didelot, C.; Houtte, P.V.; Gérard, J.P.; Peyron, J.F. NF-κB modulation and ionizing radiation: Mechanisms and future directions for cancer treatment. Cancer Lett. 2006, 231, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Swalwell, H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis 2010, 25, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Shimmura, S.; Tsubota, K. Ultraviolet B-induced mitochondrial dysfunction is associated with decreased cell detachment of corneal epithelial cells in vitro. Investig. Ophthalmol. Vis. Sci. 1997, 38, 620–626. [Google Scholar]
- Denning, M.F.; Wang, Y.; Tibudan, S.; Alkan, S.; Nickoloff, B.J.; Qin, J.Z. Caspaseactivation and disruption of mitochondrial membrane potential during UV radiation induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002, 9, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Gniadecki, R.; Thorn, T.; Vicanova, J.; Petersen, A.; Wulf, H.C. Role of mitochondria in ultraviolet-induced oxidative stress. J Cell Biochem. 2000, 80, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancerstrategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.M.; Wilhelm, K.P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Int. J. Cosmet. Sci. 2008, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Tulah, A.S.; Birch-Machin, M.A. Stressed out mitochondria: The role of mitochondria in ageing and cancer focussing on strategies and opportunities in human skin. Mitochondrion 2013, 13, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.; Leffell, D.J. The molecular basis of nonmelanoma skin cancer: New understanding. Arch. Dermatol. 1997, 133, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Hofseth, L.J.; Saito, S.; Hussain, S.P.; Espey, M.G.; Miranda, K.M.; Araki, Y.; Jahppan, C.; Higashimoto, Y.; He, P.; Linke, S.P.; et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc. Natl. Acad. Sci. USA 2003, 100, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; An, K.P.; Morel, K.D.; Kim, A.L.; Aszterbaum, M.; Longley, J. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: An immunohistochemical study. Biochem. Biophys. Res. Commun. 2001, 280, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Einspahr, J.G.; Xu, M.J.; Warneke, J.; Saboda, K.; Ranger-Moore, J.; Bozzo, P. Reproducibility and expression of skin bio markers in sun-damaged skin and actinic keratoses. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1841–1848. [Google Scholar] [CrossRef]
- Maeda, H.; Akaike, T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry 1998, 637, 854–865. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammatory cells and cancer: solid think different! J. Exp. Med. 2001, 193, F23–F26. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.E. Mechanisms underlying UV-induced immune suppression. Mutat. Res. 2005, 571, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Tigelaar, R.E.; Bergstresser, P.R.; Edelbaum, D.; Cruz, P.D., Jr. Ultraviolet B radiation converts Langerhans cells from immunogenic to tolergenic antigen-presenting cells. J. Immunol. 1991, 146, 485–491. [Google Scholar] [PubMed]
- Norval, M.; Simpson, T.J.; Ross, J.A. Urocanic acid and immunosuppression. Photochem. Photobiol. 1989, 50, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Noonan, F.P.; de Fabo, E.C. Immunosuppression by ultraviolet B radiation: Initiation by urocanic acid. Immunol. Today 1992, 13, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, I.; and Streilein, J.W. Deleterious effects of cis-urocanic acid and UVB radiation on Langerhans cells and on induction of contact hypersensitivity are mediated by tumor necrosis factor-alpha. J. Investig. Dermatol. 1992, 99, 69S–70S. [Google Scholar] [CrossRef] [PubMed]
- Kruger-Krasagakes, S.; Krasagakis, K.; Garbe, C.; Schmitt, E.; Huls, C.; Blankenstein, T.; Diamantstein, T. Expression of interleukin 10 in human melanoma. Br. J. Cancer 1994, 70, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Baadsgaard, O.; Fox, D.A.; Cooper, K.D. Human epidermal cells from ultraviolet light-exposed skin preferentially activate autoreactive CD4+2H4+ suppressor- inducer lymphocytes and CD8+ suppressor/cytotoxic lymphocytes. J. Immunol. 1988, 140, 1738–1744. [Google Scholar] [PubMed]
- Simon, J.C.; Cruz, P.D., Jr.; Bergstresser, P.R.; Tigelaar, R.E. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+ cells of the T helper 2 subset. J. Immunol. 1990, 145, 2087–2091. [Google Scholar] [PubMed]
- Schmitt, D.A.; Owen-Schaub, L.; Ullirch, S.E. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J. Immunol. 1995, 154, 5114–5120. [Google Scholar] [PubMed]
- Wondrak, G.T.; Roberts, M.J.; Cervantes-Laurean, D.; Jacobson, M.K.; Jacobson, E.L. Proteins of the extracellular matrix are sensitizers of photo-oxidative stress in human skin cells. J. Investig. Dermatol. 2003, 121, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.; Griffiths, C.E.; Craven, N.M.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-rich microfibrils are reduced in photo aged skin. Distribution at the dermal-epidermal junction. J. Investig. Dermatol. 1999, 112, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Burchill, D.; O’Donoghue, D.; Keller, T.; Gonzalez, S. Identification of benzene metabolites in dermal fibroblasts as nonphenolic: Regulation of cell viability, apoptosis, lipid peroxidation and expression of matrix metalloproteinase 1 and elastin by benzene metabolites. Skin Pharmacol. Physiol. 2004, 17, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interleukin-8 genes in dermal, fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Tuason, M.; Chang, T.; Lin, Y.; Tahir, M.; Rodriguez, S.G. Differential effects of ceramide on cell viability and extracellular matrix remodeling in keratinocytes and fibroblasts. Skin Pharmacol. Physiol. 2009, 22, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Herouy, Y. Matrix metalloproteinases in skin pathology (Review). Int. J. Mol. Med. 2001, 7, 3–12. [Google Scholar] [PubMed]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase gene expression. J. Cell. Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar] [PubMed]
- Kligman, L.H.; Akin, F.J.; Kligman, A.M. Sunscreens prevent ultraviolet photocarcinogenesis. J. Am. Acad. Dermatol. 1980, 3, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Forbes, P.D. Photocarcinogenesis: An overview. J. Investig. Dermatol. 1981, 77, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Anderson, C.Y. Sunscreens and photocarcinogenesis: An objective assessment. Photochem. Photobiol. 1996, 63, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Stege, H. Effect of xenogenic repair enzymes on photoimmunology and photocarcinogenesis. J. Photochem. Photobiol. 2001, 65, 105–108. [Google Scholar] [CrossRef]
- Emanuele, E.; Altabas, V.; Altabas, K.; Berardesca, E. Topical application of preparations containing DNA repair enzymes prevents ultraviolet-induced telomere shortening and c-FOS proto-oncogene hyperexpression in human skin: An experimental pilot study. J. Drugs Dermatol. 2013, 12, 1017–1021. [Google Scholar] [PubMed]
- Weber, S. Light-driven enzymatic catalysis of DNA repair: A review of recent biophysical studies on photolyase. Biochimica et Biophysica Acta 2005, 1707, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Brownell, I. Repairing DNA damage in xeroderma pigmentosum: T4N5 lotion and gene therapy. J. Drugs Dermatol. 2008, 7, 405–408. [Google Scholar] [PubMed]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Cutaneous oxidative stress. J. Cosmet. Dermatol. 2012, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M.; Pathak, M.A.; Parrish, J.; Fitzpatrick, T.B.; Kass, E.H.; Toda, K.; Clemens, W. A clinical trial of the effects of oral β-carotene on the responses of human skin to solar radiation. J. Investig. Dermatol. 1972, 59, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.R.; Baron, J.A.; Stukel, T.A.; Stevens, M.M.; Mandel, J.S.; Spencer, S.K.; Elisa, P.M.; Lowe, N.; Nierenberg, D.W.; Bsyrd, G. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. N. Eng. J. Med. 1990, 323, 789–795. [Google Scholar] [CrossRef]
- Werninghaus, K.; Meydani, M.; Bhawan, J.; Margolis, R.; Blumberg, J.B.; Gilchrest, B.A. Evaluation of the photoprotective effect of oral vitamin E supplementation. Arch. Dermatol. 1994, 130, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monterio-Rivire, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J. Potentials and limitations of the natural antioxidants RRR-α-tocopherol, l-ascorbic acid and β-carotene in cutaneous photoprotection. Free Radic. Biol. Med. 1998, 25, 848–873. [Google Scholar] [CrossRef] [PubMed]
- De Gálvez, M.V. Antioxidantes en fotoprotección, ¿realmente funcionan? Actas Dermo Sifiliogr. 2010, 101, 197–200. [Google Scholar] [CrossRef]
- Kraemer, K.H.; DiGiovanna, J.J.; Moshell, A.N.; Tarone, R.E.; Peck, G.L. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N. Eng. J. Med. 1988, 318, 1633–1637. [Google Scholar] [CrossRef]
- De Graaf, Y.G.; Euvrard, S.; Bouwes Bavinck, J.N. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. Dermatol. Surg. 2004, 304, 656–661. [Google Scholar]
- Kalil, C.L.; Fachinello, F.Z.; Lamb, F.M.; Comunello, L.N. Use of oral isotretinoin in photoaging therapy. Skinmed 2008, 7, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Marks, F.; Furstenberger, G. Cancer chemoprevention through interruption of multistage carcinogenesis. The lessons learnt by comparing mouse skin carcinogenesis and human large bowel cancer. Eur. Cancer 2000, 36, 314–329. [Google Scholar] [CrossRef]
- Peterson, S.R.; Goldberg, L.H. New and emerging treatments for nonmelanomas and actinic keratoses. J. Drugs Dermatol. 2003, 2, 429–432. [Google Scholar] [PubMed]
- Tripp, C.S.; Blomme, E.A.; Chinn, K.S.; Hardy, M.M.; LaCelle, P.; Pentland, A.P. Epidermal COX-2 induction following ultraviolet irradiation: Suggested mechanism for the role of COX-2 inhibition in photoprotection. J. Investig. Dermatol. 2003, 121, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Viner, J.L.; Pentland, A.P.; Cantrell, W.; Lin, H.Y.; Bailey, H. Chemoprevention of nonmelanoma skin cancer with celecoxib: A randomized, double-blind, placebo-controlled trial. J. Natl. Cancer Inst. 2010, 102, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Mukhtar, H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukoc. Biol. 2001, 69, 719–726. [Google Scholar] [PubMed]
- Sharma, S.D.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm. Res. 2010, 27, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.L.; Williams, T.; Huang, A.C.; Jacobson, E.L. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr. Cancer 1999, 34, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Mulero, M.; Rodriguez-Yanes, E.; Nogues, M.R.; Giralt, M.; Romeu, M.; Gonzalez, S. Polypodium leucotomos extract inhibits glutathione oxidation and prevents Langerhans cell depletion induced by UVB/UVA radiation in a hairless rat model. Exp. Dermatol. 2008, 17, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Conte, J.; Chen, Y.J.; Natrajan, P.; Taw, M.; Keller, T. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-beta by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini reviews in medicinal chemistry. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mnich, C.D.; Hoek, K.S.; Virkki, L.V.; Farkas, A.; Dudli, C.; Laine, E.; Urosevik, M.; Dummer, R. Green tea extract reduces induction of p53 and apoptosis in UVB-irradiated human skin independent of transcriptional controls. Exp. Dermatol. 2009, 18, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [PubMed]
- Katiyar, S.K.; Meleth, S.; Sharma, S.D. Silymarin, a flavonoid from milk thistle (Silybum marianum L.), inhibits UV-induced oxidative stress through targeting infiltrating CD11b+ cells in mouse skin. Photochem. Photobiol. 2008, 84, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Elmets, C.A.; Katiyar, S.K. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice:relationship to decreased fat and lipid peroxidation. Carcinogenesis 2003, 24, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 22, 1117–1120. [Google Scholar] [PubMed]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Reagan-Shaw, S.; Wu, J.; Longley, B.J.; Ahmad, N. Chemoprevention of skin cancer by grape constituent resveratrol: Relevance to human disease. FASEB J. 2005, 19, 1193–1195. [Google Scholar] [PubMed]
- Caddeo, C.; Teskac, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Singh, D.; Tubesing, K.; Matsui, M.; Katiyar, S.; Mukhtar, H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. Am. Acad. Dermatol. 2001, 44, 425–432. [Google Scholar] [CrossRef]
- Zheng, W.; Doyle, T.J.; Kushi, L.H.; Sellers, T.A.; Hong, C.P.; Folsom, A.R. Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am. J. Epidemiol. 1996, 144, 175–182. [Google Scholar] [CrossRef] [PubMed]
- FʼGuyer, S.; Afaq, F.; Mukhtar, H. Photochemoprevention of skin cancer by botanical agents. Photodermatol. Photoimmunol. Photomed. 2003, 19, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.O.; Wang, Y.; Stebbins, W.G.; Gao, D.; Zhou, X.; Phelps, R. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis 2006, 27, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, B.Y.; Wu, N.L.; Tsui, W.H.; Lin, T.J.; Su, C.C. Anti-photoaging effects of soy isoflavone extract (aglycone and acetylglucoside form) from soybean cake. Int. J. Mol. Sci. 2010, 11, 4782–4795. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Khan, N.; Syed, D.N.; Mukhtar, H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem. Photobiol. 2010, 86, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Koshihara, Y.; Neichi, T.; Murota, S.; Lao, A.; Fujimoto, Y.; Tatsuno, T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochimica et Biophysica Acta 1984, 792, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Gilaberte, Y.; Philips, N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem. Photobiol. Sci. 2010, 9, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Gilaberte, Y.; Philips, N.; Juarranz, A. Fernblock, a nutriceutical with photoprotective properties and potential preventive agent for skin photoaging and photoinduced skin cancers. Int. J. Mol. Sci. 2011, 12, 8466–8475. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, A.; Garcia-Lopez, M.A.; Fernandez-Penas, P.; Alonso-Lebrero, J.L.; Benedicto, I.; Lopez-Cabrera, M.A. Polypodium leucotomos extract inhibits solar-simulated radiation-induced TNF-α and iNOS expression, transcriptional activation and apoptosis. Exp. Dermatol. 2007, 16, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lebrero, J.L.; Domínguez-Jiménez, C.; Tejedor, R.; Brieva, A.; Pivel, J.P. Photoprotective properties of a hydrophilic extract of the fern Polypodium leucotomos on human skin cells. J. Photochem. Photobiol. 2003, 70, 31–37. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Tejedor, R.; de la Fuente, H.; Garcia-Lopez, M.A.; Alonso-Lebrero, J.L.; Pivel, J.P.; Gonzalez, S.; Gonzalez-Amaro, R.; Sanchez–Madrid, F. Polypodium leucotomos induces pretection of UV-induced apoptosis in human skin cells. The Society of Investigative Dermatology. J. Investig. Dermatol. 2005, 125, 334–342. [Google Scholar] [PubMed]
- Middelkamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Garcia-Caballero, T.; Rius-Diaz, F.; Fitzpatrick, T.B. Orally administered Polypodium leucotomos extract decreases psoralen-UVA-induced phototoxicity, pigmentation, and damage of human skin. J. Am. Acad. Dermatol. 2004, 50, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Capote, R.; Alonso-Lebrero, J.L.; Garcia, F.; Brieva, A.; Pivel, J.P.; Gonzalez, S. Polypodium leucotomos extract inhibits trans-urocanic acid photoisomerization photodecomposition. J. Photochem. Photobiol. 2006, 82, 173–179. [Google Scholar] [CrossRef]
- Gonzalez, S.; Pathak, M.A.; Cuevas, J.; Villarrubia, V.G.; Fitzpatrick, T.B. Topical or oral administration with an extract of Polypodium leucotomos prevents acute sunburn and psoralen-induced phototoxic reactions as well as depletion of Langerhans cells in human skin. Photodermatol. Photoimmunol. Photomed. 1997, 13, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Middel kamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Goukassian, D.; Rius-Diaz, F.; Mihm, M.C. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J. Am. Acad. Dermatol. 2004, 51, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial regulation of matrix metalloproteinases for skin health. Enzym. Res. 2011, 2011. [Google Scholar] [CrossRef]
- Frieling, U.M.; Schaumberg, D.A.; Kupper, T.S.; Muntwyler, J.; Hennekens, C.H. A randomized, 12-year primary-prevention trial of β carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch. Dermatol. 2000, 136, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.; Rhodes, L.E. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Qureshi, A.A.; Han, J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012, 72, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Peng, Q.; Li, T.; Nolan, B.; Bernard, J.J.; Wagner, G.C.; Lin, Y.; Shih, W.J.; Conny, A.H.; Lou, Y.P. Oral caffeine during voluntary exercise markedly inhibits skin carcinogenesis and decreases inflammatory cytokines in UVB-treated mice. Nutr. Cancer 2013, 65, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; Hendrix, S.O.; McNeeley, S.G.; Johnson, K.C.; Rosenberg, C.A.; Mossavar-Rahmani, Y.; Vitolins., M.; Kruger, M. Daily coffee consumption and prevalence of nonmelanoma skin cancer in Caucasian women. Eur. J. Cancer Prev. 2007, 16, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Ming, M.; He, Y.Y. Caffeine promotes ultraviolet B-induced apoptosis in human keratinocytes without complete DNA repair. J. Biol. Chem. 2011, 286, 22825–22832. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Fahey, J.W.; Healy, Z.R.; Wehage, S.L.; Benedict, A.L.; Min, C. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 17500–17505. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.E.; Melton, T.F.; Olson, E.R.; Zhang, J.; Saboda, K; Bowden, G.T. Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: Implications for chemoprevention of UVB-induced skin cancer. Cancer Res. 2009, 69, 7103–7110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.R.; Liu, W.L.; Luo, D. Protective effect of baicalin against multiple ultraviolet B exposure-mediated injuries in C57BL/6 mouse skin. Arch. Pharm. Res. 2011, 34, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. French maritime pine bark (Pinus maritima Lam.) extract (Flavangenol) prevents chronic UVB radiation-induced skin damage and carcinogenesis in melanin-possessing hairless mice. Photochem. Photobiol. 2010, 86, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.J.; Martin, J.R.; Wulff, B.C.; Stoner, G.D.; Tober, K.L.; Oberyszyn, T.M.; Kusevitt, D.F.; van Buskirk, A.M. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev. Res. 2009, 2, 665–672. [Google Scholar] [CrossRef]
- Da Silva, V.V.; Ropke, C.D.; Miranda, D.V.; de Almeida, R.L.; Sawada, T.C.; Rivelli, D.P. Photoprotective effect of Pothomorphe umbellata on UVB radiation-induced biomarkers involved in carcinogenesis of hairless mouse epidermis. Cutan. Ocul. Toxicol. 2009, 28, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Winghofer, B.; Müller, K.; Schulte-Mönting, J.; Mannel, M.; Schöpf, E.; Simon, J.C. Effect of oral administration of Hypericum perforatum extract (St. John’s Wort) on skin erythema and pigmentation induced by UVB, UVA, visible light and solar simulated radiation. Phytother. Res. 2003, 17, 141–146. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248-268. https://doi.org/10.3390/antiox4020248
Bosch R, Philips N, Suárez-Pérez JA, Juarranz A, Devmurari A, Chalensouk-Khaosaat J, González S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants. 2015; 4(2):248-268. https://doi.org/10.3390/antiox4020248
Chicago/Turabian StyleBosch, Ricardo, Neena Philips, Jorge A. Suárez-Pérez, Angeles Juarranz, Avani Devmurari, Jovinna Chalensouk-Khaosaat, and Salvador González. 2015. "Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals" Antioxidants 4, no. 2: 248-268. https://doi.org/10.3390/antiox4020248
APA StyleBosch, R., Philips, N., Suárez-Pérez, J. A., Juarranz, A., Devmurari, A., Chalensouk-Khaosaat, J., & González, S. (2015). Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants, 4(2), 248-268. https://doi.org/10.3390/antiox4020248






