Investigation of the Genotoxic, Antigenotoxic and Antioxidant Profile of Different Extracts from Equisetum arvense L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Soxhlet Extraction
2.4. Determination of Total Phenolic Content, Total Flavonoid Content and Antioxidant Activity
2.4.1. Preparation of Samples
2.4.2. Total Phenolic Content (TPC)
2.4.3. Total Flavonoid Content (TFC)
2.4.4. ABTS Radical Cation Decolorization Assay
2.4.5. Radical Scavenging Activity by DPPH• Assay
2.4.6. Ferric Reducing Antioxidant Power (FRAP) Assay
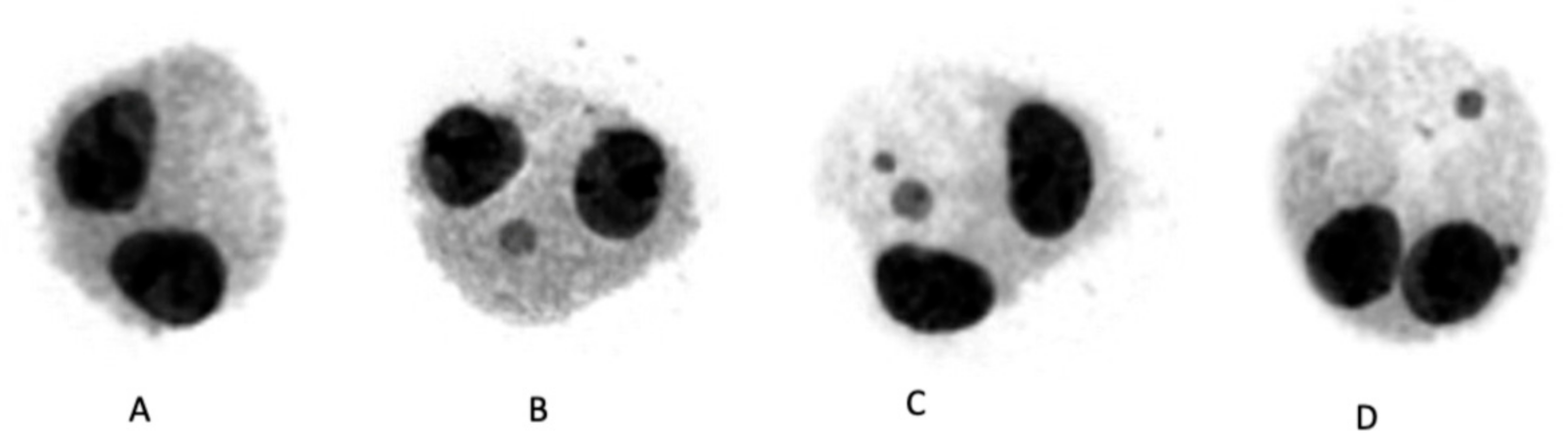
2.5. CBMN Assay
2.5.1. Ethics Statement
2.5.2. CBMN Assay Application
2.6. Statistical Analysis
2.7. E. arvense L. Extract Characterization by ICP-MS/MS, GC-MS and UHPLC-MS
2.7.1. ICP-MS/MS Analysis
2.7.2. GC-MS Analysis
2.7.3. UHPLC-MS Analysis
3. Results
3.1. Extraction Yields (Soxhlet Extraction)
3.2. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity
3.2.1. Total Phenolic and Flavonoid Content
3.2.2. Antioxidant Activity
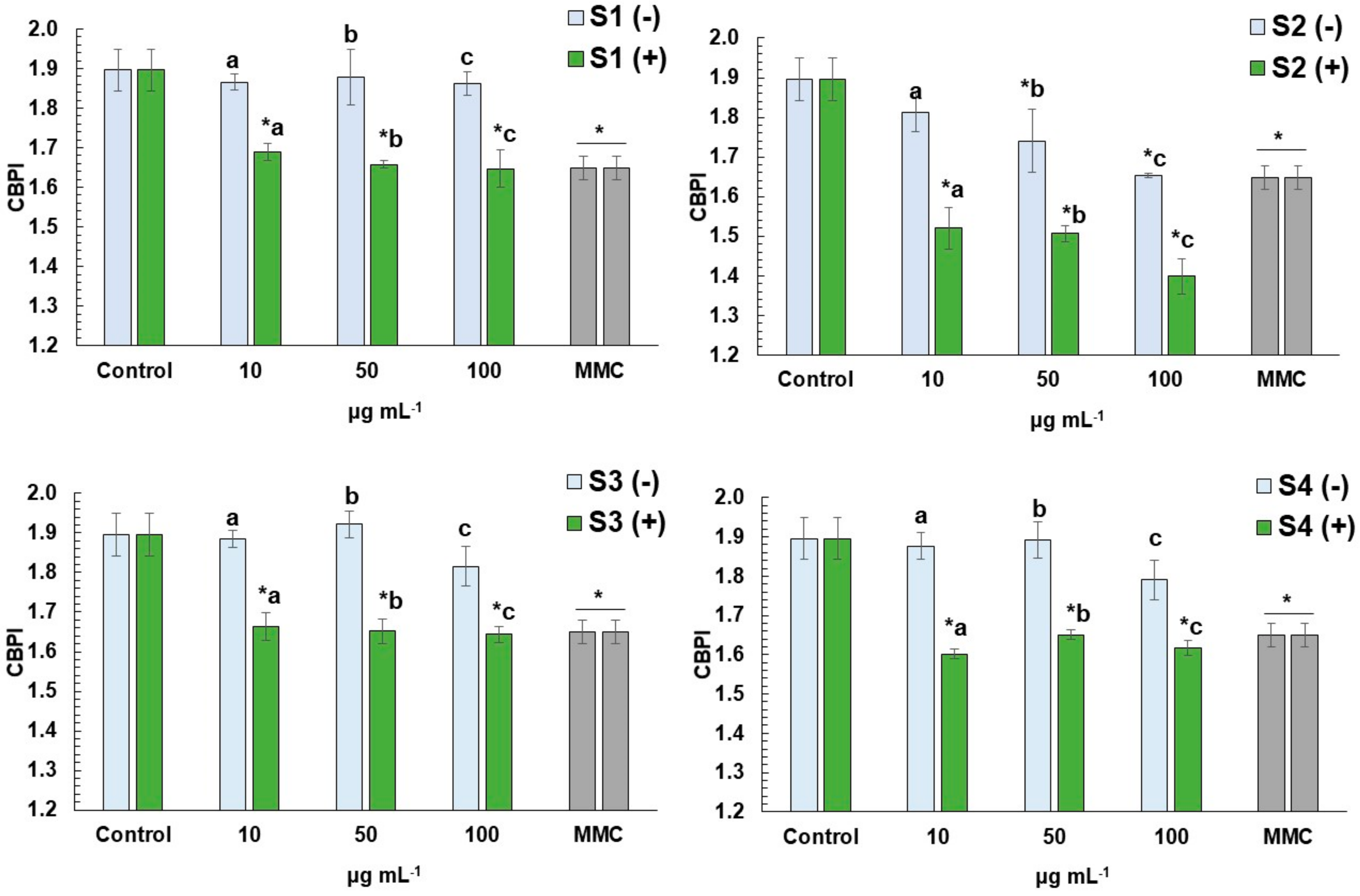
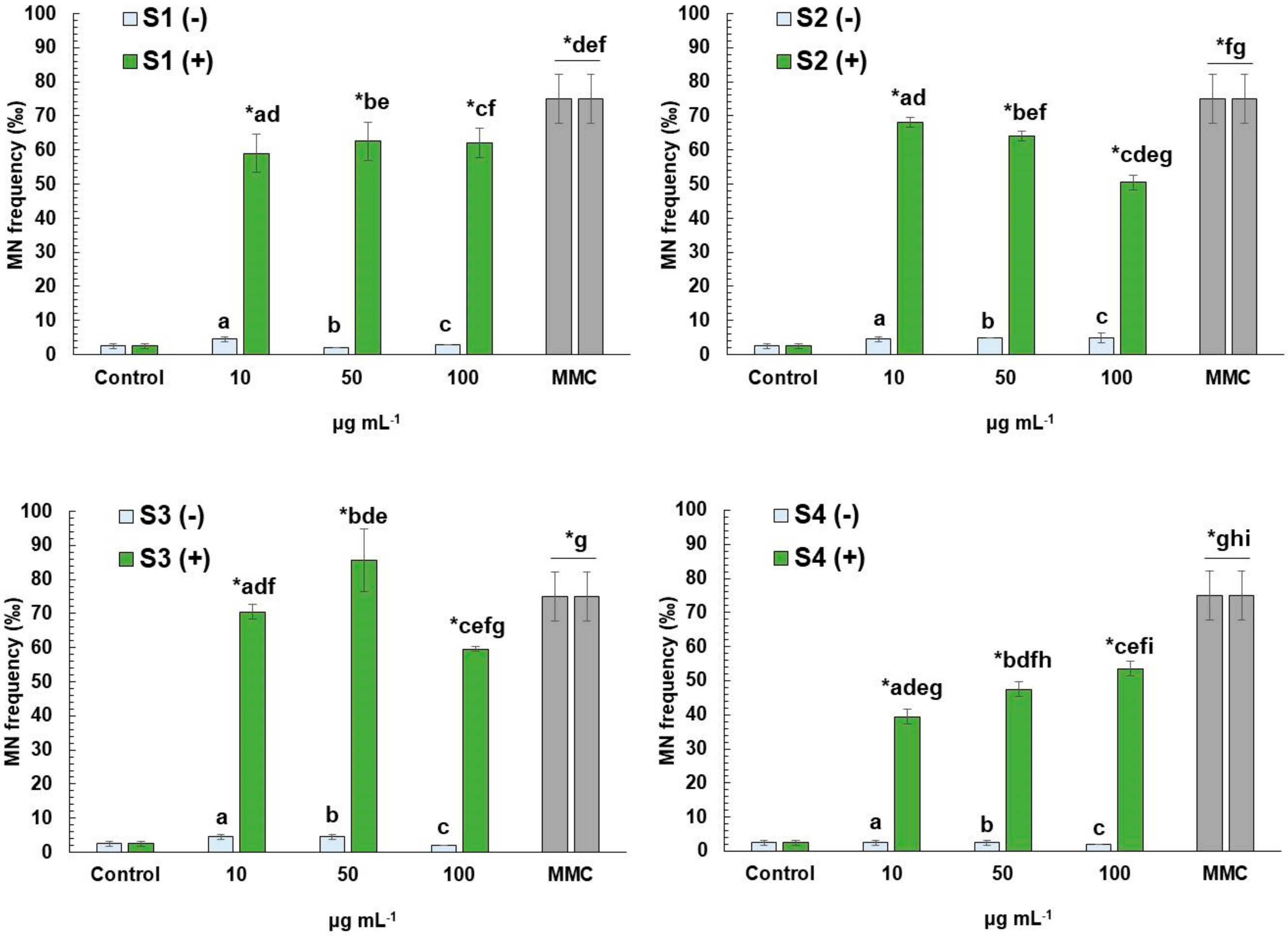
3.3. CBMN Assay in Human Lymphocytes
3.4. E. arvense L. S4 Extract Characterization by ICP-MS/MS, GC-MS and UHPLC-MS
4. Discussion
4.1. Characterization of Extracts
4.2. Cyto-Genotoxic Potential of Extracts
4.3. Cytoprotective and Antigenotoxic Effects of E. arvense L. Extracts against Mitomycin C
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS); World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44059 (accessed on 27 May 2022).
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.G.; Blanco, M.M.; Do Monte, F.H.M.; Russi, M.; Lanziotti, V.M.N.B.; Leal, L.K.A.M.; Cunha, G.M. Sedative and anticonvulsant effects of hydroalco-holic extract of Equisetum arvense. Fitoterapia 2005, 76, 508–513. [Google Scholar] [CrossRef]
- Sandhu, N.S.; Kaur, S.; Chopra, D. Pharmacognostic Evaluation of Equisetum arvense Linn. Int. J. PharmTech Res. 2010, 2, 1460–1464. Available online: https://sphinxsai.com/s_v2_n2/PT_V.2No.2/phamtech_vol2no.2_pdf/PT=77%20_1460-1464_.pdf (accessed on 27 May 2022).
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marín, S. Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. Int. J. Food Microbiol. 2012, 153, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cetojevic-Simin, D.D.; Canadanovic-Brunet, J.M.; Bogdanovic, G.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T.; Stojiljkovic, B.T. Antioxidative and antiproliferative activities of different horsetail (Equisetum arvense L.) extracts. J. Med. Food. 2010, 13, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Bhattacharya, B. Natural Antimutagens: A Review. Res. J. Med. Plant 2011, 5, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E. Effect of Plant Flavonoids on Immune and Inflammatory Cell Function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Imbimbo, P.; D’Elia, L.; Liberti, D.; Olivieri, G.; Monti, D.M. Towards green extraction methods from microalgae learning from the classics. Appl. Microbiol. Biotechnol. 2020, 104, 9067–9077. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength, and limitation. Med. Aromat. Plants. 2015, 4, 196. [Google Scholar] [CrossRef]
- Vlastos, D.; Mademtzoglou, D.; Drosopoulou, E.; Efthimiou, I.; Chartomatsidou, T.; Pandelidou, C.; Astyrakaki, M.; Chalatsi, E.; Mavragani-Tsipidou, P. Evaluation of the genotoxic and antigenotoxic effects of Chios mastic water by the in vitro micronucleus test on human lymphocytes and the in vivo wing somatic test on Drosophila. PLoS ONE 2013, 8, e69494. [Google Scholar] [CrossRef]
- Vlastos, D.; Drosopoulou, E.; Efthimiou, I.; Gavriilidis, M.; Panagaki, D.; Mpatziou, K.; Kalamara, P.; Mademtzoglou, D.; Mavragani-Tsipidou, P. Genotoxic and antigenotoxic assessment of Chios mastic oil by the in vitro micronucleus test on human lymphocytes and the in vivo wing somatic test on Drosophila. PLoS ONE 2015, 10, e0130498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drosopoulou, E.; Vlastos, D.; Efthimiou, I.; Kyrizaki, P.; Tsamadou, S.; Anagnostopoulou, M.; Kofidou, D.; Gavriilidis, M.; Mademtzoglou, D.; Mavragani-Tsipidou, P. In vitro and in vivo evaluation of the genotoxic and antigenotoxic potential of the major Chios mastic water constituents. Sci. Rep. 2018, 8, 12200. [Google Scholar] [CrossRef] [PubMed]
- Lorge, E.; Thybaud, V.; Aardema, M.J.; Oliver, J.; Wakata, A.; Lorenzon, G.; Marzin, D. SFTG international collaborative study on in vitro micronucleus test I. General conditions and overall conclusions of the study. Mutat. Res. 2006, 607, 13–36. [Google Scholar] [CrossRef]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Method 925.10; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Gomide, R. Operações Com Sistemassólidosgranulares; Catalogação da CâmaraBrasileira de Publicação de Livros: São Paulo, Brazil, 1983; Volume 1, pp. 27–30. Available online: https://scholar.google.com/scholar_lookup?title=Operação%20Com%20Sistemas%20sólidos%20Granulares&author=R.%20Gomide&publication_year=1983 (accessed on 27 May 2022).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radicalcation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Surrallés, J.; Carbonell, E.; Marcos, R.; Degrassi, F.; Antoccia, A.; Tanzarella, C. A collaborative study on the improvement of the micronucleus test in cultured human lymphocytes. Mutagenesis 1992, 7, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Surrallés, J.; Xamena, N.; Creus, A.; Catalan, J.; Norppa, H.; Marcos, R. Induction of micronuclei by five pyrethroid insecticides in whole-blood and isolated human lymphocyte cultures. Mutat. Res. 1995, 341, 169–184. [Google Scholar] [CrossRef]
- Fenech, M. The advantages and disadvantages of the cytokinesis-block micronucleus method. Mutat. Res. 1997, 392, 11–18. [Google Scholar] [CrossRef]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN project: Detailed description of the scoring criteria for the cytokinesis block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- (ISO)17294-1; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 1: General Guidelines. International Organization for Standardization: Geneva, Switzerland, 2014. Available online: https://www.iso.org/standard/32957.html (accessed on 22 September 2020).
- (ISO)17294-1; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/62962.html (accessed on 22 September 2020).
- Yaldiz, G.; Koca Caliskan, U.; Aka, C. In vitro screening of natural drug potentials for mass production. Not. Bot. Horti Agrobot. Cluj Napoca. 2017, 45, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Gründemann, C.; Lengen, K.; Sauer, B.; Garcia-Käufer, M.; Zehl, M.; Huber, R. Equisetum arvense (common horsetail) modulates the function of inflammatory immunocompetent cells. BMC Complement. Altern. Med. 2014, 14, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 389–469. [Google Scholar]
- Guedes, A.R.; de Souza, A.R.C.; Barbi, R.C.T.; Escobar, E.L.N.; Zanoello, E.F.; Corazza, M.L. Extraction of Synadenium grantii Hook f. using conventional solvents and supercritical CO2 + ethanol. J. Supercrit. Fluids 2020, 160, 8–10. [Google Scholar] [CrossRef]
- Santzouk, G.; Santzouk, S.; Gerodimou, I.; Angeli, P.; Dormousoglou, M. Red Grape Skin and Seeds: Extraction and Characterization of Flavonoids (Anthocyanins) and Wine Production Waste Biomass Valorization. Bulg. Chem. Commun. 2019, 51, 108–110. Available online: http://www.bcc.bas.bg/bcc_volumes/Volume_51_Special_B_2019/BCC-51-B-IPROPBIO-012-Santzouk-108-110.pdf (accessed on 23 September 2019).
- Coelho, J.P.; Robalo, M.P.; Boyadzhieva, S.; Stateva, R.P. Microwave-Assisted Extraction of Phenolic Compounds from Spent Coffee Grounds. Process Optimization Applying Design of Experiments. Molecules 2021, 26, 7320. [Google Scholar] [CrossRef]
- Herbst, G.; Hamerski, F.; Errico, M.; Corazza, M.L. Pressurized liquid extraction of brewer’s spent grain: Kinetics and crude extracts characterization. J. Ind. Eng. Chem. 2021, 102, 370–383. [Google Scholar] [CrossRef]
- Guerras, L.S.; Sengupta, D.; Martín, M.; El-Halwagi, M.M. Multilayer Approach for Product Portfolio Optimization: Waste to Added-Value Products. ACS Sustain. Chem. Eng. 2021, 9, 18, 6410–6426. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.S.; Bithika, G.; Rimlee, N.; Roy, S.B.; Pallab, K.; Kumar, D.B. Pharmacognostical evaluation and phytochemical screening of the plant Equisetum arvense L. World J. Pharm. Pharm. Sci. 2016, 5, 1537–1543. [Google Scholar] [CrossRef]
- Uslu, M.E.; Erdoğan, İ.; Bayraktar, O.; Ateş, M. Optimization of Extraction Conditions for Active Components in Equisetum arvense Extract. Rom. Biotechnol. Lett. 2013, 18, 8115–8131. Available online: https://gcris.iyte.edu.tr/bitstream/11147/5274/1/5274.pdf (accessed on 22 September 2020).
- Nagai, T.; Myoda, T.; Nagashima, T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005, 91, 389–394. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Simin, N.; Cvefic, J.; Jovin, E.; Orcic, D.; Bozin, B. Phenolic compounds in field horsetail (Equisetum arvense L.) as natural antioxidants. Molecules 2008, 13, 1455–1464. [Google Scholar] [CrossRef] [Green Version]
- Duda-Chodak, A.; Tarko, T.; Rus, M. Antioxidant Activity and Total Polyphenol Content of Selected Herbal Medicinal Products Used in Poland. Herba Pol. 2011, 57, 48–57. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-539d9492-1336-4a0b-90d3-11cc27aad29c/c/1.pdf (accessed on 22 September 2020).
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C.F.R. In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Necip, A.; Isik, M. Bioactivities of Hypericum perforatum L. and Equisetum arvense L. fractions obtained with different solvents. Int. J. Life Sci. Biotechnol. 2019, 2, 221–230. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.J.; Jiang, F.; Yang, Y.; Wang, X.X.; Zhang, Z.; Li, Z.; Li, L. Caffeic acid improves cell viability and protects against DNA damage: Involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz. J. Med. Biol. Res. 2015, 48, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Zhao, H.Y.; Song, Y.L.; Zhang, Q.W.; Wang, Y.T. Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquidchromatography-triple quadrupole mass spectrometry with novel shell-type column. J. Sep. Sci. 2011, 34, 2576–2585. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, Q.W.; Li, S.L.; Wang, Y.; Ye, W.C.; Zhao, J.; Wang, Y.T. Simultaneous quantification of major flavonoids in “Bawanghua”, the edible flower of Hylocereusundatus using pressurised liquid extraction and high performance liquid chromatography. Food Chem. 2012, 135, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Kirsch-Volders, M.; Decordier, I.; Elhajouji, A.; Plas, G.; Aardema, M.J.; Fenech, M. In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis 2011, 26, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Calliste, C.A.; Allais, D.P.; Simon, S.; Marfak, A.; Delage, C.; Duroux, J.L. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem. 2003, 80, 399–407. [Google Scholar] [CrossRef]
- Alexandru, V.; Petrusca, D.N.; Gille, E. Investigation of Pro-Apoptotic Activity of Equisetum arvense L. Water Extract on Human Leukemia U 937 Cells. Rom. Biotechnol. Lett. 2007, 12, 3139–3147. Available online: https://e-repository.org/rbl/vol.12/iss.2/3.pdf (accessed on 22 September 2020).
- Bhat, A.A.; Ahamad, B.; Rehman, M.U.; Ahmad, P. Impact of ethanolic extract of Equisetum arvense (EA1) on pancreatic carcinoma AsPC-1 cells. Saudi J. Biol. Sci. 2020, 5, 1260–1264. [Google Scholar] [CrossRef]
- Harutyunyan, K.; Balayan, K.; Tadevosyan, G.; Hayrapetyan, M.; Musayelyan, R.; Grigoryan, R. Genotoxic potential of selected medicinal plant extracts in human whole blood cultures. J. Herbmed. Pharmacol. 2019, 8, 160–162. [Google Scholar] [CrossRef]
- Al Mohammed, H.I.; Paray, B.A.; Rather, I.A. Anticancer Activity of EA1 Extracted from Equisetum arvense. Pak. J. Pharm. Sci. 2017, 30, 1947–1950. Available online: https://faculty.ksu.edu.sa/sites/default/files/5-sup-706_0.pdf (accessed on 22 September 2020).
- Téllez, M.G.; Rodríguez, H.B.; Olivares, G.Q.; Sortibrán, A.N.; Cetto, A.A.; Rodríguez-Arnaiz, R. A phytotherapeutic extract of Equisetum myriochaetum is not genotoxic either in the in vivo wing somatic test of Drosophila or in the in vitro human micronucleus test. J. Ethnopharmacol. 2007, 111, 182–189. [Google Scholar] [CrossRef]
- Milovanović, V.; Radulović, N.; Todorović, Z.; Stanković, M.; Stojanović, G. Antioxidant, antimicrobial and genotoxicity screening of hydro-alcoholic extracts of five Serbian Equisetum species. Plant Foods Hum. Nutr. 2007, 62, 113–119. [Google Scholar] [CrossRef]
- Kour, J.; Ali, M.N.; Ganaie, H.A.; Tabassum, N. Amelioration of the cyclophosphamide induced genotoxic damage in mice by the ethanolic extract of Equisetum arvense. Toxicol. Rep. 2017, 4, 226–233. [Google Scholar] [CrossRef]
- Joksić, G.; Stanković, M.; Novak, A. Antibacterial medicinal plants Equisetiherba and Ononidis radix modulate micronucleus formation in human lymphocytes in vitro. J. Environ. Pathol. Toxicol. Oncol. 2003, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sortibrán, A.C.; Téllez, M.O.; Andrade-Cetto, A.; Kischinevzky, C.S.; Rodríguez-Arnaiz, R. Antimutagenic activity of two medicinal phytoextracts in somatic cells of Drosophila melanogaster. Pharm. Biol. 2011, 49, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, E.; Fülöp, I.; Farczádi, L.; Croitoru, M.D. Polyphenolic determination from medicinal plants used in veterinary medicine by an UHPLC-LC-MS/MS method. Farmacia 2020, 68, 1129–1135. [Google Scholar] [CrossRef]
- Barcelos, G.R.; Grotto, D.; Angeli, J.P.; Serpeloni, J.M.; Rocha, B.A.; Bastos, J.K.; Barbosa, F., Jr. Evaluation of antigenotoxic effects of plant flavonoids quercetin and rutin on HepG2 cells. Phytother. Res. 2011, 25, 1381–1388. [Google Scholar] [CrossRef]
- Abraham, S.K.; Eckhardt, A.; Oli, R.G.; Stopper, H. Analysis of in vitro chemoprevention of genotoxic damage by phytochemicals, as single agents or as combinations. Mutat. Res. 2012, 744, 117–124. [Google Scholar] [CrossRef]
- Li, P.; Xu, G.; Li, S.P.; Wang, Y.T.; Fan, T.P.; Zhao, Q.S.; Zhang, Q.W. Optimizing ultraperformance liquid chromatographic analysis of 10 diterpenoid compounds in Salvia miltiorrhiza using central composite design. J. Agric. Food Chem. 2008, 56, 1164–1171. [Google Scholar] [CrossRef]
- Jovtchev, G.; Gateva, S.; Stankov, A. Lilium compounds kaempferol and jatropham can modulate cytotoxic and genotoxic effects of radiomimetic zeocin in plants and human lymphocytes in vitro. Environ. Toxicol. 2016, 31, 751–764. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Gurgul, A.; Youn, I.; Maldonado, A.; Wahid, F.; Che, C.T.; Khan, T. UHPLC-MS/MS-GNPS based phytochemical investigation of Equisetum arvense L. And evaluation of cytotoxicity against human melanoma and ovarian cancer cells. Saudi J. Biol. Sci. 2022, 29, 103271. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar]
- Nadathur, S.R.; Carney, J.R.; Gould, S.J.; Bakalinsky, A.T. Palmitic acid is the major fatty acid responsible for significant anti-N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) activity in yogurt. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 1996, 359, 179–189. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic acid protects against cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Doi, K.; Wei, M.; Kitano, M.; Uematsu, N.; Inoue, M.; Wanibuchi, H. Enhancement of preneoplastic lesion yield by Chios Mastic Gum in a rat liver medium-term carcinogenesis bioassay. Toxicol. Appl. Pharm. 2009, 234, 135–142. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef]
| Extraction | Polarity # | Time (min) | Yield (%) * |
|---|---|---|---|
| S1 | 6.6 | 360 | 15.0 ± 0.4 ac |
| S2 | 5.2 | 360 | 9.8 ± 0.5 bce |
| S3 | 10.2 | 360 | 24.9 ± 0.8 abd |
| S4 | 7.1 | 360 | 15.2 ± 0.4 de |
| Extract | TPC (mg GAE g−1) | TFC (mg CE g−1) |
|---|---|---|
| S1 | 196 ± 3 ab | 130 ± 4 ab |
| S2 | 257 ± 29 ac | 168 ± 14 acd |
| S3 | 63 ± 1 bc | 36 ± 1 bc |
| S4 | 220 ± 12 | 131 ± 9 d |
| Extract | AA (μmol TE g−1) | ||
|---|---|---|---|
| ABTS | DPPH | FRAP | |
| S1 | 1292 ± 27 ab | 1847 ± 158 a | 834 ± 34 a |
| S2 | 1788 ± 221 ac | 2217 ± 272 b | 979 ± 111 b |
| S3 | 243 ± 1 bcd | 374 ± 13 abc | 202 ± 4 abc |
| S4 | 1417 ± 141 d | 1581 ± 401 c | 858 ± 86 c |
| Element | Concentration (μg g−1) |
|---|---|
| Mg | 28,680.6 |
| Si | 7823.4 |
| K | 187,136.1 |
| Ca | 32,139.8 |
| P | 3988.8 |
| Mn | 257.7 |
| Cu | 237.1 |
| Zn | 1165.6 |
| GC/MS | Proposed Compound | Molecular Formula | MW |
| n-Hexadecanoic acid (Palmitic acid) | C16H32O2 | 256.4 | |
| Oleic Acid | C18H34O2 | 282.5 | |
| Hexadecanoic acid,14-methyl-, methyl ester | C18H36O2 | 284.5 | |
| Stigmasta-5,24(28)-dien-3ol (Isofucosterol) | C29H48O | 412.7 | |
| γ-Sitosterol (Fucosterol) | C29H50O | 414.7 | |
| Stigmastan-3,5-diene | C29H48 | 396.7 | |
| Campesterol | C28H48O | 400.7 | |
| UHPLC/MS | Proposed Compound | Molecular Formula | MW |
| Caffeic acid | C9H8O4 | 180.2 | |
| Quercetin-3,7-di-O-glucoside | C27H30O17 | 626.5 | |
| Kaempferol-3,7-di-O-glucoside | C27H30O16 | 610.5 | |
| Kaempferol-3-O-rutinoside-7-O-glucoside | C33H40O20 | 756.7 | |
| Kaempferol-3-O-sophoroside | C27H30O16 | 610.5 | |
| Kaempferol-3-O-glucoside | C21H20O11 | 448.4 | |
| Kaempferol | C15H10O6 | 286.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dormousoglou, M.; Efthimiou, I.; Antonopoulou, M.; Fetzer, D.L.; Hamerski, F.; Corazza, M.L.; Papadaki, M.; Santzouk, S.; Dailianis, S.; Vlastos, D. Investigation of the Genotoxic, Antigenotoxic and Antioxidant Profile of Different Extracts from Equisetum arvense L. Antioxidants 2022, 11, 1393. https://doi.org/10.3390/antiox11071393
Dormousoglou M, Efthimiou I, Antonopoulou M, Fetzer DL, Hamerski F, Corazza ML, Papadaki M, Santzouk S, Dailianis S, Vlastos D. Investigation of the Genotoxic, Antigenotoxic and Antioxidant Profile of Different Extracts from Equisetum arvense L. Antioxidants. 2022; 11(7):1393. https://doi.org/10.3390/antiox11071393
Chicago/Turabian StyleDormousoglou, Margarita, Ioanna Efthimiou, Maria Antonopoulou, Damian L. Fetzer, Fabiane Hamerski, Marcos L. Corazza, Maria Papadaki, Samir Santzouk, Stefanos Dailianis, and Dimitris Vlastos. 2022. "Investigation of the Genotoxic, Antigenotoxic and Antioxidant Profile of Different Extracts from Equisetum arvense L." Antioxidants 11, no. 7: 1393. https://doi.org/10.3390/antiox11071393








