A Comprehensive Review of C. capsularis and C. olitorius: A Source of Nutrition, Essential Phytoconstituents and Pharmacological Activities
Abstract
:1. Introduction
2. Use in Local and Traditional Food and Medicine
3. Nutritional Composition of Jute
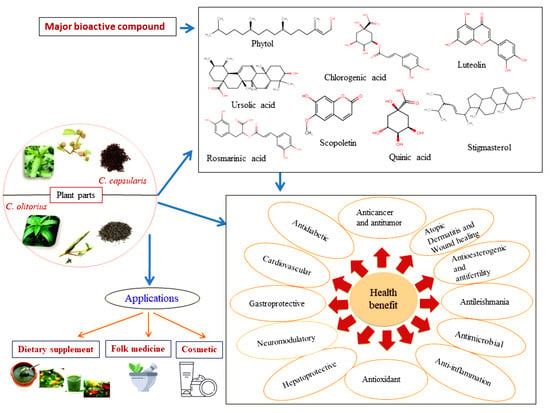
4. Bioactive Compounds in Jute
4.1. Polyhenols
4.2. Flavanoids
4.3. Cardiac Glycosides and Their Aglycones
4.4. Triterpenoids
4.5. Organic Acid/Essential Oils
4.6. Fatty Acid
4.7. Lipid Composition
4.8. Phytosterols and Hydrocarbons
4.9. Polysaccharides and Other Sugars
4.10. Volatile Compounds
5. Biological Activity
5.1. Antitumor and Anticancer Promoting Activity
5.2. Gastroprotective Effect
5.3. Antinociceptive, Anti-Inflammatory, and Antipyretic
5.4. Antioxidant Activity
5.5. Cardiovascular Activity
5.6. Antidiabetic and Bioadsorbent Properties
5.7. Hepatobiliary, Renal, and Haematological Activity
5.8. Anticonvulsant Activity
5.9. Neuromodulatory Activity
5.10. Antioesterogenic and Antifertility Activity
5.11. Dermatitis and Wound Healing Activities
5.12. Antimicrobial Activity
5.13. Antimalarial Activity
5.14. Antinutrients and Insecticidal Effect
5.15. Toxicology
| Biological Activities | Species | Plant Parts | Extraction Solvent/Fed Diet | Main Findings | References |
|---|---|---|---|---|---|
| Antioxidant activity | C. olitorius | Leaf stem | 96% alcohol | Both leaves and stem displayed DPPH radical scavenging (95.1% and 97.1%), respectively, at 400 µg/mL. | [70] |
| ,, | Leaf | Distilled water | Mucilaginous polysaccharides (PSc) showed more fantastic antioxidant activities than soluble fraction (SF) extract. Antioxidant activities were about 90% against DPPH•, 78% against lipid peroxidation, and 69% against β-carotene at 1.5 mg/mL. | [41] | |
| Cardiovascular activity | C. olitorius | Seed | Methanol | Compounds such as Corchoroside A, Corchoroside B, Strophanthidin trioside, Coroloside, and Olitoriside are widely used for heart failure treatment. | [87] |
| Antitumor and anticancer promoting activity | C. capsularis C. olitorius | Leaf | Methanol | Phytol and mono galactosyldiacylglycerol at concentrations of 15 g/mL and 30 g/mL completely inhibited the induction of Epsterin-Barr virus (EBV) early antigen in Raji cells, and viable cells decreased about 20% in the inducer-treated Raji cells. | [17] |
| C. olitorius | Leaf | Ethanol | ECO treatment had a dose-dependent effect on HepG2 cell proliferation and 12.5 µg/mL effectively triggered apoptosis by increasing caspase-9 activity mitochondria as well as caspase-mediated pathways. | [14] | |
| C. olitorius | Whole plant | Methanol | Cytotoxicity against HeLa, HL460 lung cancer cell line, and PC3 prostate cancer cell line, indicating antitumor potential and Galactolipid 1 antitumor promoting activity. | [13] | |
| C. olitorius | Leaf | Dichloromethane (DCM) and aqueous | Two extract-induced apoptotic in Colo-320 and Colo-741 cells lines at 50 µg/mL concentration, and extract-induced apoptosis was more effective in metastatic Colo-741, colon adenocarcinoma cell lines. | [12] | |
| C. olitorius | Stem | Dichloromethane | Extracts have growth-inhibiting effects on humans MCF-7 and MDA-MB-231 adenocarcinoma. Stigmasterol demonstrated cytostatic activity against Hep-2 and McCoy cells. | [45] | |
| C. olitorius | Leaf | 67% methanol and chloroform | Polyphenol-enriched extracts (PEEs) of C. olitorius leaves significantly reduced the viability of tumor Caco-2 cancer cells without any detrimental effects on the healthy CCD841 line. | [49] | |
| C. olitorius | Leaf | Aqueous | Significant antiproliferative effects on SUIT-2, A-375 and AGS cells at a concentration as low as 2.54 mg/mL. Moreover, extracts strongly inhibited angiogenesis and the growth of A-375 and AGS tumors. | [94] | |
| Antiulcer activity | C.olitorius | Leaf | Aqueous | Oral aqueous extract dose-dependently inhibited gastric ulcers. The extract (400 mg/kg) had the highest cure rate (94.08%) and (33.75%) for acetic acid and ethanol/aspirin-induced ulcers, respectively. | [97] |
| Antidiabetic and bioadsorbent properties | C. olitorius | Leaf | Methanol | The dose-dependent inhibition of α-amylase and α-glucosidase (12.5–50.0 µg/mL), as well as ACE (10.0–50.0 µg/mL), was observed. | [65] |
| C. olitorius | Leaf | Phytol and terpenes have hepatoprotective and antiadipogenic properties and may help manage insulin resistance and metabolic disorders associated with diabetes and obesity. | [84] | ||
| C. olitorius | Leaf | Methanol | The extract reduced blood glucose on a dose-dependent basis. | [105] | |
| C. olitorius | Leaf | Ethanol | Extract significantly reduced (p > 0.05) the activities of catalase, SOD, and liver function enzymes. Oral administration of extract had an anti-hyperglycemic effect in streptozotocin-induced diabetics, suggesting it could be used to treat diabetes mellitus. | [165] | |
| C. olitorius | Seed | Ethanol | Significantly reduced blood sugar levels in normoglycaemic, OGTT, and diabetic rats, as well as a suppressed postprandial increase in glucose-loaded rats, and a decreased blood glucose level in diabetic rats. | [32] | |
| Antiobesity effect | C. olitorius | Leaf | Ethanol | It reduces oxidative stress and increases β-oxidation in the liver, which helps prevent diet-induced obesity. | [16] |
| Stem Leaf | Dichloromethane | Oleanolic acid possesses glucose-lowering properties. | [45] | ||
| Antifertility activity | C. olitorius | Seed | Methanol | Seed extract inhibited male reproductive capacity in sexually mature mice, since it interferes with gonadal steroidogenesis. | [144] |
| Antinociceptive, anti-inflammatory, and antipyretic | C. capsularis | Leaf | Chloroform | Reduce the number of abdominal constrictions that was confirming its traditional use of inflammatory and pain-related diseases. It is also linked to curing chronic urinary bladder inflammation. | [29] |
| Anti-inflammatory and antipyretic | C. olitorius | Root | Distilled water | Extract significantly lowered the elevated temperature after the brewer’s injection. Compared with controls, approximately 50 mg/kg of active ingredient had the least granuloma weight. | [166] |
| C. olitorius | Aerial part | Aqueous ethanol | Isoquercetin-rich extracts reduced carrageenan-induced rat paw edema in inflammatory exudates and demonstrated activity against metastatic melanoma, leukemia, and osteosarcoma cell lines. | [57] | |
| C. olitorius | Leaf | chloroform: methanol (2:1, v/v) | α-linolenic acid and linoleic acid ability to anti-inflammatory properties and wound healing promotion. | [72] | |
| Proliferative activity | C. olitorius | Leaf | Chloroform and methanol | Polysaccharides rich in uronic acid showed proliferative activity toward the murine splenocyte. | [20] |
| Anticonvulsant activity | C. olitorius | Seed | Methanol | Seed extract significantly increased the level of catecholamines in mice brain after a 6-week treatment. | [165] |
| Gastroprotective activity | C. olitorius | Leaf | Ethanol | Extract administration significantly inhibited gastric wall mucus depletion, and a group treated with 400 mg/kg produced a significant amount of gastric mucus. | [100] |
| Hepatobiliary, renal and haematological activity | C. olitorius | Leaf | Leaf powder | Reduces hepatic cholesterol while increasing neutral fecal bile acid and neutral sterol excretion. | [131] |
| C. olitorius | Seed | Powder | A significant increase was seen in AST, ALT, as well as total lipid of liver, while serum AST and ALT dropped. | [48] | |
| C. olitorius Leaf | Leaf | Water-soluble extract | Reduced the levels of serum biomarkers for liver injury and reduced the overexpression of inflammatory cytokines and lipid metabolism-related proteins in the liver. | [50] | |
| C. olitorius | Leaf | Leaf powder | Increased δ-ALAD, hepatic catalase, and SOD activities were observed in association with a decrease in serum AST and AST activity. | [130] | |
| Wound healing | C. olitorius | Leaf | Methanol and aqueous | An excision wound model showed significant wound healing activity for both powder and aqueous extract. | [148] |
| Neuromodulatory activity | C. olitorius | Leaf | Ethanol | Extracts significantly correlated with improved cognitive function and reduced neurodegeneration induced by LPS. | [138] |
| Phytoalexin activity | C. olitorius | Fresh young leaf | 95% ethanol | Isolated stress metabolites and volatile compounds had good activity against the microorganisms. Antifungal substances are released or fungioxic compounds are synthesized in response to infection or injury. | [84] |
| Antileishmanial activity | C. capsularis | Leaf | Chloroform | Showed potent antileishmanial activity against L. donovani promastigotes with an IC50 value of 79 μg/mL and exhibited very specific apoptotic features by targeting LdTryR. | [80,167] |
| Antibacterial activity | C. capsularis C. olitorius | Leaf | Lipophilic extracts | Both of the species of Corchorus showed antibacterial activity against (Gram+ and Gram−) bacteria. Fractions of C. capsularis leaves extract were found to be more effective against E. coli. | [151] |
| C. olitorius | Leaf | Petroleum ether, methanol, and ethyl acetate + water | Antibacterial or antifungal activity was observed in all extracts, and petroleum ether was demonstrated with zone diameters of 14 to 20 mm. | [166] | |
| C. olitorius | Leaf | Ethanol | Simultaneous administration of antibiotics to patients who eat C. olitorius regularly needs to be reappraised because of possible synergism and antagonism. | [150] | |
| C. olitorius | Seed | Petroleum ether, chloroform, methanol | Isolated cardenolide glycosides at 150 g/mL were the most effective against the bacteria tested, with a 20–25 mm zone of inhibition. | [168] | |
| C. olitorius | Leaf | 95% ethanol | Volatile components are effective against Gram-positive and Gram-negative bacteria. | [84] | |
| Antimalarial activity | C. capsularis | Leaf | Acetone, ethyl acetate, ethanol | Different extracts demonstrate mosquitocidal activity against Anopheles stephensi, a common malaria vector, and Aedes aegypti, a dengue vector. | [153] |
| Antimicrobial activity Antibacterial activity | C. olitorius | Leaf stem | 96% alcohol | Leaf and stem oil components showed significant antibacterial activity with MIC values of 0.40–0.8 and 1.6- > 3.2 mg/mL, respectively. | [70] |
| C. capsularis | Leaf | Methanol | The highest activities against Gram-positive, Gram-negative bacteria and fungi, with a 0.9 to 1.5 mm zone of inhibition. | [96] | |
| C. olitorius | Leaf | Methanol and aqueous | Both extracts had antimicrobial activities, but methanolic extracts displayed more comprehensive inhibition and activity indices. | [149] | |
| Antifungal activity | C. olitorius | Leaf | Petroleum ether, methanol, and ethyl acetate + water | All extracts displayed varying antifungal activity, and ethyl acetate + water extract showed prominent activity against Geotrichum candidum. | [166] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-azino-bis(3 ethylbenzothiazoline-6-sulphonic acid) |
| AFB1 | Aflatoxin B |
| δ-ALAD | Hepatic delta-aminolevulinic acid dehydratase |
| CPT1A | Carnitine palmitoyl transferase 1A |
| COX-2 | Cyclooxigenase-2 |
| DAG | Diacylglycerol |
| DCM | dichloromethane; |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| EC50 | Half maximal effective concentration |
| EGFR | Epidermal growth factor receptor |
| FFA | Free fatty acids |
| FRAP | Ferric reducing ability of plasma |
| GABA | γ-amino butyric acid |
| G-6-PD | Glucose-6-phosphate dehydrogenase |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| HE | Hydrophilic extract |
| HFD | High-fat diet |
| HPLC | High-performance liquid chromatography |
| HDL-C | High-density lipoprotein cholesterol |
| HepG2 | Human hepatocellular carcinoma |
| IC50 | Half maximal inhibitory concentration |
| IgE | Immunoglobulin E |
| ICAD | Inhibitor of caspase-activated DNase |
| LDLR | Low-density lipoprotein receptor-deficient |
| LE | Lipophilic extract |
| LLC | Lipoprotein-low cholesterol |
| LPS | Lipopolysaccharide |
| iNOS | Inducible nitric oxide synthase |
| LC-MS | Liquid chromatography-mass spectrometry |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MECO | Methanolic extract of C. olitorius |
| NMR | Nuclear Magnetic Resonance |
| PARP | Poly ADP-ribose polymerase |
| PEEs | Polyphenol-enriched extracts |
| PGE2 | Prostaglandin E2 |
| PSc | Polysaccharides |
| PTS | Phytosterol |
| PPARα | Peroxisome proliferator activated receptor alpha |
| QE/g | Quercetin equivalent per gram |
| ROS | Reactive oxygen species |
| SGOT | Serum glutamic oxaloacetic transaminase |
| SGPT | Serum glutamic pyruvic transaminase |
| SOD | Superoxide dismutase |
| STAT | Signal transducer and activator of transcription |
| TAG | Triacylglycerol |
| TFC | Total flavonoids content |
| T2DM | Type 2 diabetes mellitus |
References
- Beyene, B. Review on Application and Management of Medicinal Plants for the Livelihood of the Local Community. J. Resour. Dev. Manag. 2016, 22, 33–39. [Google Scholar]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akomas, S.; Ijioma, S.; Emelike, C. In-vivo and In-vitro spasmolytic Effect of Ficus sur Forssk Ethanol Leaf Extract on the Gastrointestinal Tract. Br. Biotechnol. J. 2014, 4, 1182–1190. [Google Scholar] [CrossRef]
- Loumerem, M.; Alercia, A. Descriptors for jute (Corchorus olitorius L.). Genet. Resour. Crop Evol. 2016, 63, 1103–1111. [Google Scholar] [CrossRef]
- Kumari, N.; Choudhary, S.B.; Sharma, H.K.; Singh, B.K.; Kumar, A.A. Health-promoting properties of Corchorus leaves: A review. J. Herb. Med. 2019, 15, 100240. [Google Scholar] [CrossRef]
- Choudhary, S.B.; Sharma, H.K.; Karmakar, P.G.; Anil Kumar, A.; Saha, A.R.; Hazra, P.; Mahapatra, B.S. Nutritional profile of cultivated and wild jute (Corchorus) species. Aust. J. Crop Sci. 2013, 7, 1973–1982. [Google Scholar]
- Choudhary, S.B.; Sharma, H.K.; Anil Kumar, A.; Maruthi, R.T.; Karmakar, P.G. The genus Corchorus L. (Malvaceae) in India: Species distribution and ethnobotany. Genet. Resour. Crop Evol. 2017, 64, 1675–1686. [Google Scholar] [CrossRef]
- Islam, M.M. Biochemistry, Medicinal and Food values of Jute (Corchorus capsularis L. and C. olitorius L.) leaf: A Review. Int. J. Enhanc. Res. Sci. Technol. Eng. 2013, 2, 35–44. [Google Scholar]
- Steyn, N.P.; Olivier, J.; Winter, P.; Burger, S.; Nesamvuni, C. A survey of wild, green, leafy vegetables and their potential in combating micronutrient deficiencies in rural populations. S. Afr. J. Sci. 2001, 97, 276–278. [Google Scholar]
- Dansi, A.; Adjatin, A.; Adoukonou-Sagbadja, H.; Faladé, V.; Yedomonhan, H.; Odou, D.; Dossou, B. Traditional leafy vegetables and their use in the Benin Republic. Genet. Resour. Crop Evol. 2008, 55, 1239–1256. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Somchit, M.N.; Zaiton, H.; Mat Jais, A.M.; Sulaiman, M.R.; Farah, W.O.; Nazaratulmawarina, R.; Fatimah, C.A. The in vitro antibacterial activity of Corchorus olitorius extracts. Int. J. Pharmacol. 2006, 2, 213–215. [Google Scholar] [CrossRef] [Green Version]
- Soykut, G.; Becer, E.; Calis, I.; Yucecan, S.; Vatansever, S. Apoptotic effects of Corchorus olitorius L. leaf extracts in colon adenocarcinoma cell lines. Prog. Nutr. 2018, 20, 689–698. [Google Scholar] [CrossRef]
- Taiwo, B.J.; Taiwo, G.O.; Olubiyi, O.O.; Fatokun, A.A. Polyphenolic compounds with anti-tumour potential from Corchorus olitorius (L.) Tiliaceae, a Nigerian leaf veget. Bioorg. Med. Chem. Lett. 2016, 26, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Huang, S.Y.; Wu, M.Y.; Chen, Y.C.; Tsang, S.F.; Chyuan, J.H.; Hsu, H.Y. Induction of apoptosis by ethanolic extract of Corchorus olitorius leaf in human hepatocellular carcinoma (HepG2) cells via a mitochondria-dependent pathway. Molecules 2012, 17, 9348–9360. [Google Scholar] [CrossRef] [Green Version]
- Ademiluyi, A.O.; Oboh, G.; Aragbaiye, F.P.; Oyeleye, S.I.; Ogunsuyi, O.B. Antioxidant properties and in vitro α-amylase and α-glucosidase inhibitory properties of phenolics constituents from different varieties of Corchorus spp. J. Taibah Univ. Med. Sci. 2015, 10, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yamasaki, M.; Katsube, T.; Sun, X.; Yamasaki, Y.; Shiwaku, K. Antiobesity effect of polyphenolic compounds from molokheiya (Corchorus olitorius L.) leaves in LDL receptor-deficient mice. Eur. J. Nutr. 2011, 50, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, T.F.; Ang, R.W.; Kazaki, K.O.; Asan, A.F.M.F.H.; Li, M.I.A.; Ondo, A.K. Antitumor Promoters in Leaves of Jute (Corchorus capsularis and Corchorus olitorius). Food Sci. Technol. Res. 2002, 8, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Oyedele, D.J.; Asonugho, C.; Awotoye, O.O. Heavy metals in soil and accumulation by edible vegetables after phosphate fertilizer application. Electron. J. Environ. Agric. Food Chem. 2006, 5, 1446–1453. [Google Scholar]
- Velempini, P.; Riddoch, I.; Batisani, N. Seed treatments for enhancing germination of wild okra (Corchorus olitorius). Exp. Agric. 2003, 39, 441–447. [Google Scholar] [CrossRef]
- Ohtani, K.; Okai, K.; Yamashita, U.; Yuasa, I.; Misaki, A. Characterization of an Acidic Polysaccharide Isolated from the Leaves of Corchorus olitorius (Moroheiya). Biosci. Biotechnol. Biochem. 1995, 59, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Hosen, S.M.I.; Das, D.; Kobi, R.; Chowdhury, D.U.S.; Alam, M.J.; Rudra, B.; Bakar, M.A.; Islam, S.; Rahman, Z.; Al-Forkan, M. Study of arsenic accumulation in rice and evaluation of protective effects of Chorchorus olitorius leaves against arsenic contaminated rice induced toxicities in Wistar albino rats. BMC Pharmacol. Toxicol. 2016, 17, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orieke, D.; Ohaeri, O.C.; Ijeh, I.I.; Ijioma, S.N. Gastrointestinal and uterine smooth muscles relaxant and anti-inflammatory effects of Corchorus olitorius leaf extract in laboratory animal models. J. Ethnopharmacol. 2020, 247, 112224. [Google Scholar] [CrossRef] [PubMed]
- Nduche, M.U.; Omosun, G.; Okwulehie, I.C. Ethnobotanical Survey of Plants Used as Remedy for Fertility Conditions in Ebonyi State of Nigeria. Sch. Acad. J. Biosiences. 2015, 3, 214–221. [Google Scholar]
- Akaneme, F.I. Identification and preliminary phytochemical analysis of herbs that can arrest threatened miscarriage in Orba and Nsukka towns of Enugu State. African J. Biotechnol. 2008, 7, 006–011. [Google Scholar] [CrossRef]
- Sadat, A.; Chakraborty, K. Jute-A biological elixir with multifaceted applications: An overview. Int. J. Res. Pharm. Sci. 2015, 6, 323–332. [Google Scholar]
- Whitlock, B.A.; Karol, K.G.; Alverson, W.S. Chloroplast DNA sequences confirm the placement of the enigmatic Oceanopapaver within Corchorus (Grewioideae: Malvaceae S.L., formerly Tiliaceae). Int. J. Plant Sci. 2003, 164, 35–41. [Google Scholar] [CrossRef]
- Ahmed, F. Nutraceutical Potential of Molokhia (Corchorus olitorius L.): A Versatile Green Leafy Vegetable. Pharmacogn. Res. 2021, 10, 24–30. [Google Scholar] [CrossRef]
- Castro, M.P.T.; Obtinalla, C.O.; Lucente, M.E. Evaluation of Jute (Corchorus olitorius) Leaves as a Sushi Wrap. Adv. Nutr. Food Sci. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Sulaiman, M.R.; Gopalan, H.K.; Abdul Ghani, Z.D.F.; Raden Nur, R.N.S.; Mat Jais, A.M.; Abdullah, F.C. Antinociceptive and anti-inflammatory properties of Corchorus capsularis leaves chloroform extract in experimental animalmodels. Yakugaku Zasshi. 2007, 127, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, Z.A.; Kumar, G.H.; Nor, R.N.S.R.M.; Sulaiman, M.R.; Fatimah, C.A.; Jais, A.M.M.; Somchit, M.N.; Ismail, M.S. Antinociceptive, anti-inflammatory and antipyretic properties of an aqueous extract of Corchorus capsularis leaves in experimental animal models. Pharm. Biol. 2009, 47, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Zeghichi, S.; Kallithraka, S.; Simopoulos, A.P. Nutritional composition of molokhia (Corchorus olitorius) and stamnagathi (Cichorium spinosum). World Rev. Nutr. Diet. 2003, 91, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Osaronowen, M.; Udo, E.; Oricha, S. Anti-diabetic Activity of Ethanolic Seed Extract of Corchorus olitorius. IJSBAR 2013, 12, 8–22. [Google Scholar]
- Adebo, H.O.; Ahoton, L.E.; Quenum, F.J.B.; Adoukonou-Sagbadja, H.; Bello, D.O.; Chrysostome, C.A.A.M. Ethnobotanical Knowledge of Jute (Corchorus olitorius L.) in Benin. European J. Med. Plants. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water extractable phytochemicals from some tropical spices. Pharm. Biol. 2012, 50, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-snafi, A.E. The Constituents and Pharmacology of Corchorus aestuans-A Review. Pharm. Chem. J. 2016, 3, 208–214. [Google Scholar]
- Yoshikawa, M.; Shimada, H.; Saka, M.; Yoshizumi, S. Medicinal Foodstuffs. V. 1 Moroheiya. (1): Absolute Stereostructures of Corchoionosides A, B, and C, Histamine Release Inhibitors from the Leaves of Vietnamese Corchorus olitorius L. (Tiliaceae). Chem. Pharm. Bull. 1997, 45, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonibare, M.A.; Moody, J.O.; Adesanya, E.O.; Momordica, L.; Seem, P.B.; Ocimum, L. Use of medicinal plants for the treatment of measles in Nigeria. J. Ethnopharmacol. 2009, 122, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Hadid, A.F.A.; El-shinawy, M.; El-beltagy, A.S.; Medany, M. Studies on the production of off-season Jew’s Mallow “Melokhia” in Egypt. Egypt. J. Hort. 1994, 21, 187–193. [Google Scholar]
- Idirs, S.; Yisa, J.; Ndamitso, M.M. Nutritional composition of Corchorus olitorius leaves. J. Anim. Prod. Adv. 2009, 5, 2. [Google Scholar] [CrossRef]
- Biswas, A.; Dey, S.; Li, D.; Liu, Y.; Zhang, J.; Huang, S.; Pan, G.; Deng, Y. Comparison of Phytochemical Profile, Mineral Content, and in Vitro Antioxidant Activities of Corchorus capsularis and Corchorus olitorius Leaf Extracts from Different Populations. J. Food Qual. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ben Yakoub, A.R.; Abdehedi, O.; Jridi, M.; Elfalleh, W.; Bkhairia, I.; Nasri, M.; Ferchichi, A. Bioactive polysaccharides and their soluble fraction from Tossa jute (Corchorus olitorius L.) leaves. Food Biosci. 2020, 37, 100741. [Google Scholar] [CrossRef]
- Gupta, S.; Prakash, J. Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum. Nutr. 2009, 64, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, T. Extraction, Identification and Estimation of Caffeine and Catechin from Corchorus capsularis Leaves Extract. Ph.D. Thesis, East West University, Dhaka, Bangladesh, 2014. [Google Scholar]
- Samuel, F.O.; Ayoola, P.B.; Ejoh, S.I. Nutrient, antinutrient and sensory evaluation of Corchorus olitorius fruit. Ife J. Agric. 2020, 32, 13–20. [Google Scholar]
- Ragasa, C.Y.; Vivar, J.L.A.; Tan, M.C.S.; Shen, C.C. Chemical constituents of Corchorus olitorius L. Int. J. Pharmacognf. Phytochem. Res. 2016, 8, 2085–2089. [Google Scholar]
- Musa, A.; Ogbadoyi, E.O. Effect of Cooking and Sun Drying On Micronutrients, Antinutrients an Toxic Substances in Corchorus olitorius (Jute Mallow). J. Nutr. Food Sci. 2012, 2, 1000140. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Nakayama, M.; Koshioka, M.; Ippoushi, K.; Yamaguchi, Y.; Kohata, K.; Yamauchi, Y.; Ito, H.; Higashio, H. Phenolic antioxidants from the leaves of Corchorus olitorius L. J. Agric. Food Chem. 1999, 47, 3963–3966. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Bano, S.; Javed, K.; Asad Mueed, M. A comprehensive review on the chemistry and pharmacology of Corchorus species - A source of cardiac glycosides, triterpenoids, ionones, flavonoids, coumarins, steroids and some other compounds. J. Sci. Ind. Res. (India) 2006, 65, 283–298. [Google Scholar]
- Guzzetti, L.; Panzeri, D.; Ulaszewska, M.; Sacco, G.; Forcella, M.; Fusi, P.; Tommasi, N.; Fiorini, A.; Campone, L.; Labra, M. Assessment of Dietary Bioactive Phenolic Compounds and Agricultural Sustainability of an African Leafy Vegetable Corchorus olitorius L. Front. Nutr. 2021, 8, 667812. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, H.H.L.; Kim, Y.; Lee, H.B.; Lee, E.; Park, J.H.; Park, H.Y. Corchorus olitorius L. ameliorates alcoholic liver disease by regulating gut-liver axis. J. Funct. Foods 2021, 85, 104648. [Google Scholar] [CrossRef]
- Meyer, A.S.; Donovan, J.L.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Fruit Hydroxycinnamic Acids Inhibit Human Low-Density Lipoprotein Oxidation in Vitro. J. Agric. Food Chem. 1998, 46, 1783–1787. [Google Scholar] [CrossRef]
- Price, K.R.; Rhodes, M.J.C. Analysis of the major flavonol glycosides present in four varieties of onion (Allium cepa) and changes in composition resulting from autolysis. J. Sci. Food Agric. 1997, 74, 331–339. [Google Scholar] [CrossRef]
- Ben Yakoub, A.R.; Abdehedi, O.; Jridi, M.; Elfalleh, W.; Nasri, M.; Ferchichi, A. Flavonoids, phenols, antioxidant, and antimicrobial activities in various extracts from Tossa jute leave (Corchorus olitorus L.). Ind. Crops Prod. 2018, 118, 206–213. [Google Scholar] [CrossRef]
- Ali, M.M.; Ahmed, K.S.; Hossain, H.; Roy, B.; Rokeya, B.; Rahman, M.T.; Jahan, I.A.; Rahman, M.M. Total antioxidant capacity and profiling of polyphenolic compounds in jute leaves by hplc-dad. Food Res. 2021, 5, 343–348. [Google Scholar] [CrossRef]
- Chhikara, N.; Devi, H.R.; Jaglan, S.; Sharma, P.; Gupta, P.; Panghal, A. Bioactive compounds, food applications and health benefits of Parkia speciosa (stinky beans): A review. Agric. Food Secur. 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Handoussa, H.; Hanafi, R.; Eddiasty, I.; El-Gendy, M.; El Khatib, A.; Linscheid, M.; Mahran, L.; Ayoub, N. Anti-inflammatory and cytotoxic activities of dietary phenolics isolated from Corchorus olitorius and Vitis vinifera. J. Funct. Foods. 2013, 5, 1204–1216. [Google Scholar] [CrossRef]
- Ramadevi, D. Phytochemical examination of Corchorus olitorius leaves. Int. J. Curr. Pharm. Rev. Res. 2013, 4, 42–46. [Google Scholar]
- Mukherjee, K.K.; Mitra, S.K.; Ganguly, S.N. A new coumarin from the seeds of jute (Corchorus olitorius L.). Nat. Prod. Sci. 1998, 4, 51–52. [Google Scholar]
- Nakamura, T.; Goda, Y.; Sakai, S.; Kondo, K.; Akiyama, H.; Toyoda, M. Cardenolide glycosides from seeds of Corchorus olitorius. Phytochemistry 1998, 49, 2097–2101. [Google Scholar] [CrossRef]
- Mahato, S.B.; Sahu, N.P.; Roy, S.K.; Pramanik, B.N. Cardiac Glycosides from Corchorus olitorius. J. Chem. Soc. 1998, 2065–2068. [Google Scholar] [CrossRef]
- Manzoor-i-Khuda, M.; Habermehl, G. Chemical Constituents of Corchorus capsularis and C.olitorius (Jute Plant), Part IV* Isolation of Corosolic Acid, Ursolic Acid and Oxo-corosin and Correlation of Corosin with Tormentic Acid. Z. Fur Nat. Sect. B J. Chem. Sci. 1979, 34, 1320–1325. [Google Scholar] [CrossRef]
- Chen, S.; Wu, B.; Fang, J.; Liu, Y.; Zhang, H.; Fang, L. Analysis of flavonoids from lotus (Nelumbo nucifera) leaves using high performance liquid chromatography/photodiode array detector tandem electrospray ionization mass spectrometry and an extraction method optimized by orthogonal design. J. Chromatogr. A. 2012, 1227, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Quader, M.A.; Ahmed, M.D.; Hasan, C.M.; Waterman, P.G. Capsugenin-30-O-β-glucopyranoside: A new glucoside from the leaves of Corchorous capsularis. J. Nat. Prod. 1987, 50, 479–481. [Google Scholar] [CrossRef]
- Hassan, A.; Mekhael, M.; Hanna, A.; Simon, A.; Tóth, G.; Duddeck, H. Phytochemical investigation of Corchorus olitorius and Corchorus capsularis (Family Tiliaceae) that grow in Egypt. Egypt. Pharm. J. 2019, 18, 123–134. [Google Scholar] [CrossRef]
- Hasan, C.M.; Islam, A.; Ahmed, M.; Ahmed, M.D.; Waterman, P.G. Capsugenin, a dammarane triterpene from Corchorus capsularis. Phytochemistry 1984, 23, 2583–2587. [Google Scholar] [CrossRef]
- Quader, M.A.; Gray, A.I.; Layud, C.; Massiot, G.; Hasan, C.M.; Ahmed, M.D. Capsugenin-25,30-O-β-diglucopyranoside: A new glycoside from the leaves of Corchorus capsularis. J. Nat. Prod. 1990, 53, 527–530. [Google Scholar] [CrossRef]
- Driss, D.; Kaoubaa, M.; Ben Mansour, R.; Kallel, F.; eddine Abdelmalek, B.; Ellouz Chaabouni, S. Antioxidant, Antimutagenic and Cytotoxic Properties of Essential Oil from Corchorus olitorius L. Flowers and Leaf. Free Radic. Antioxid. 2016, 6, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Murakami, T.; Shimada, H.; Yoshizumi, S. Medicinal Foodstuffs. XIV. On the Bioactive Constituents of Moroheiya. (2): New Fatty Acids, Corchorifatty Acids A, B, C, D, E, and F, from the Leaves of Corchorus olitorius L. (Tiliaceae): Structures and Inhibitory Effect on NO Production. Chem. Pharm. Bull. 1998, 46, 1008–1014. [Google Scholar] [CrossRef] [Green Version]
- Al-Yousef, H.M.; Amina, M.; Ahamad, S.R. Comparative study on the chemical composition of Corchorus olitorius leaf and stem dry oils. Biomed. Res. 2017, 28, 4581–4587. [Google Scholar]
- Mahmoud, A.S.; Thao, N.; Mario, A. Corchorus olitorius Linn: A Rich Source of Ω3-Fatty Acids. Pharm. Anal. Acta 2016, 7, 6–8. [Google Scholar] [CrossRef] [Green Version]
- Siriamornpun, S.; Li, D.; Yang, L.; Kanchanamayoon, W.; Suttajit, M. Thai jute seed oil: A potential polyunsaturated fatty acid source. Songklanakarin J. Sci. Technol. 2006, 28, 11–15. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.P.; Meara, M.L. The component fatty acids and glycerides of coconut oils. J. Sci. Food Agric. 1952, 6, 237–240. [Google Scholar] [CrossRef]
- Hasan, H.T.; Kadhim, E.J. Phytochemical investigation of leaves and seeds of Corchorus olitorius l. Cultivated in iraq. Asian J. Pharm. Clin. Res. 2018, 11, 408–417. [Google Scholar] [CrossRef]
- Oikawa, T. NII-Electronic Library Service. Chem. Pharm. Bull. 2002, 2091. [Google Scholar]
- Yamamoto, K.; Asakawa, H.; Tokunaga, K.; Watanabe, H.; Matsuo, N.; Tokimitsu, I.; Yagi, N. Long-term ingestion of dietary diacylglycerol lowers serum triacylglycerol in type II diabetic patients with hypertriglyceridemia. J. Nutr. 2001, 131, 3204–3207. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, H.; Watanabe, H.; Onizawa, K.; Nagao, T.; Gotoh, N.; Yasukawa, T.; Tsushima, R.; Shimasaki, H.; Itakura, H. Double-blind controlled study on the effects of dietary diacylglycerol on postprandial serum and chylomicron triacylglycerol responses in healthy humans. J. Am. Coll. Nutr. 2000, 19, 789–796. [Google Scholar] [CrossRef]
- Negm, S.; El Shabrawy, O.; Arbid, M.; Radwan, A.S. Toxicological study of the different organs of Corchorus olitorius L. plant with special reference to their cardiac glycosides content. Z. Ern&Hrungswiss. 1980, 19, 28–32. [Google Scholar]
- Pramanik, P.K.; Chakraborti, S.; Bagchi, A.; Chakraborti, T. Bioassay-based Corchorus capsularis L. leaf-derived β-sitosterol exerts antileishmanial effects against Leishmania donovani by targeting trypanothione reductase. Sci. Rep. 2020, 10, 20440. [Google Scholar] [CrossRef]
- Ramadevi, D.; Lakshmi Sirisha, K.; Dhorababu, D.; Sampath Kumar, G.V. Phytochemical Examination of Corchorus capsularis Roots. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 137–140. [Google Scholar]
- Furumoto, T.; Wang, R.; Okazaki, K.; Hasan, A.; Ali, M.; Kondo, A.; Fukui, H. Antitumor Promoters in Leaves of Jute (Corchorus capsularis and Corchorus olitorius). Food Sci. Technol. Res. 2002, 8, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanaka, Y.; Hosoi, S.; Hidaka, T.; Usui, S. Identification and Determination of Unknown Chemicals in Crops by Dual-Column GC System Equipped with MS and ECD. J Food Hyg Soc Japan. 2002, 43, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeid, A.H.S.A. Stress metabolites from Corchorus olitorius L. leaves in response to certain stress agents. Food Chem. 2002, 76, 187–195. [Google Scholar] [CrossRef]
- Ogawa, M.; Hayasi, K.; Tomimori, S.; Konishi, N.; Nakayama, O. Contents of strophanthidin glycosides and digitoxigenin glycosides in”moroheiya” (Corchorus olitorius L.) and its products. J. Jpn. Soc. Food Sci. Technol. (Japan) 2002, 49, 282–287. [Google Scholar] [CrossRef]
- Mazumder, U.K.; Gupta, M.; Pal, D.; Bhattacharya, S. Chemical and toxicological evaluation of methanol extract of Cuscuta reflexa Roxb. stem and Corchorus olitorius Linn. seed on hematological parameters and hepatorenal functions in mice. Acta Pol. Pharm.-Drug Res. 2003, 60, 317–323. [Google Scholar]
- Matsufuji, H.; Sakai, S.; Chino, M.; Goda, Y.; Toyoda, M.; Takeda, M. Relationship between cardiac glycoside contents and color of Corchorus olitorius seeds. J. Health Sci. 2001, 47, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A.E. The contents and pharmacological importance of Corchorus capsularis-A review. IOSR J. 2016, 6, 58–63. [Google Scholar]
- Goda, Y.; Sakai, S.; Nakamura, T.; Akiyama, H.; Toyoda, M. Identification and Analyses of Main Cardiac Glycosides in Corchorus olitorius Seeds and Their Acute Oral Toxicity in mice. F. Hyg. Saf. Sci. 1377, 68–70. [Google Scholar] [CrossRef]
- Handoussa, H.; Hanafi, R.S.; El-Khatib, A.H.; Linscheid, M.W.; Mahran, L.G.; Ayoub, N.A. Computer-assisted HPLC method development using DryLab for determination of major phenolic components in Corchorus olitorius and Vitis vinifera by using HPLC-PDA-ESI-TOF- MSn. Res. Rev. J. Bot. Sci. 2017, 6, 9–16. [Google Scholar]
- Darcansoy İşeri, Ö.; Yurtcu, E.; Sahin, F.I.; Haberal, M. Corchorus olitorius (jute) extract induced cytotoxicity and genotoxicity on human multiple myeloma cells (ARH-77). Pharm. Biol. 2013, 51, 766–770. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Pieters, R.; Abdel-Aziem, S.; Van der Walt, A.; Bezuidenhout, C.; Giesy, J.; Abdel-Wahhab, M. Aqueous extract of Corchorus olitorius decreases cytotoxicity of aflatoxin B1 and fumonisin B1 in H4IIE-luc cells. Hepatoma Res. 2015, 1, 75. [Google Scholar] [CrossRef] [Green Version]
- Tosoc, J.P.S.; Nuñeza, O.M.; Sudha, T.; Darwish, N.H.E.; Mousa, S.A. Anticancer effects of the Corchorus olitorius aqueous extract and its bioactive compounds on human cancer cell lines. Molecules 2021, 26, 6033. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J.; et al. The cytotoxicity of kahweol in HT-29 human colorectal cancer cells is mediated by apoptosis and suppression of heat shock protein 70 expression. Biomol. Ther. 2015, 23, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rume, J.M. Phytochemical, Antimicrobial and Biological Investigations of Methanolic Extract of Leavrs of Corchorus Capsularis. Master Thesis, East West University, Dhaka, Bangladesh, 2010. [Google Scholar]
- Malfertheiner, P.; Chan, F.K.; McColl, K.E. Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar]
- Mezui, C.; Amang, A.P.; Nkenfou, C.; Sando, Z.; Betou, D.; Moulioum, H.T.P.V. Anti-Ulcer and Antioxidant Activities of the Leaf Aqueous Extract of Corchorus olitorius (Tiliaceae) in Rats. Int. J. Phytopharm. 2016, 7, 17–28. [Google Scholar]
- Devaraj, V.C.; Asad, M.; Prasad, S. Effect of leaves and fruits of Moringa oleifera on gastric and duodenal ulcers. Pharm. Biol. 2007, 45, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Al-Batran, R.; Al-Bayaty, F.; Ameen Abdulla, M.; Jamil Al-Obaidi, M.M.; Hajrezaei, M.; Hassandarvish, P.; Fouad, M.; Golbabapour, S.; Talaee, S. Gastroprotective effects of Corchorus olitorius leaf extract against ethanol-induced gastric mucosal hemorrhagic lesions in rats. J. Gastroenterol. Hepatol. 2013, 28, 1321–1329. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, Z.A.; Safarul, M.; Valsala, R.; Sulaiman, M.R.; Fatimah, C.A.; Somchit, M.N.; Mat Jais, A.M. The influences of temperature and naloxone on the antinociceptive activity of Corchorus olitorius L. in mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 2005, 372, 55–62. [Google Scholar] [CrossRef]
- Oyewole, A.L.; Owoyele, B.V.; Alimi, M.L.; Sanni, S.A.; Oyeleke, S.A. Anti-inflammatory and antipyretic properties of Corchorus olitorius aqueous root extract in Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 363–368. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Wang, Y.W.; Chen, S.L.; Zhuang, S.R.; Wang, C.K. Anti-inflammatory effects of phenolic crude extracts from five fractions of Corchorus olitorius L. Food Chem. 2013, 138, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Ijioma, N.; Madubuike, K.; Nwankudu, O.; Nwosu, C.; Emelike, C. Uterine Relaxation Potential of Ethanol Leaf Extract of Moringa oleifera Lam. VIA the Muscarinic Receptor Pathway. Br. J. Pharm. Res. 2014, 4, 2455–2462. [Google Scholar] [CrossRef]
- Parvin, S.; Marzan, M.; Rahman, S.; Das, A.K.; Haque, S.; Rahmatullah, M. Preliminary phytochemical screening, antihyperglycemic, analgesic and toxicity studies on methanolic extract of aerial parts of Corchorus olitorius L. J. Appl. Pharm. Sci. 2015, 5, 068–071. [Google Scholar] [CrossRef] [Green Version]
- Borokini, T.I.; Lawal, I.O. Traditional medicine practices among the Yoruba people of Nigeria: A historical perspective. J. Med. Plants Stud. 2014, 2, 20–33. [Google Scholar]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Nisha, P.; Mini, S. In vitro antioxidant and antiglycation properties of methanol extract and its different solvent fractions of Musa paradisiaca L. (Cv.Nendran) inflorescence. Int. J. Food Prop. 2014, 17, 399–409. [Google Scholar] [CrossRef]
- Mibei, E.K.; Ojijo, N.K.O.; Karanja, S.M.; Kinyua, J.K. Phytochemical and antioxidant analysis of methanolic extracts of four african indegenous leafy vegetables. Ann. Food Sci. Technol. 2012, 13, 37–42. [Google Scholar]
- Sadat, A.; Hore, M.; Chakraborty, K.; Roy, S. Phytochemical Analysis and Antioxidant Activity of Methanolic Extract of Leaves of Corchorus olitorius. Int. J. Curr. Pharm. Res. 2017, 9, 59–63. [Google Scholar] [CrossRef]
- Oboh, G.; Raddatz, H.; Henle, T. Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of Jute (Corchorus olitorius) leaf. Int. J. Food Sci. Nutr. 2009, 60, 124–134. [Google Scholar] [CrossRef]
- Kaboré, S.; Touré, A.; Kouamé, B.K.; Bamba, A.E.; Kabran, A.F.; Kadio, B.D.; Konan, D.J.; Attioua, K.B.; Coulibaly, W.K.; Kablan, A.L.; et al. Phytochemical screening and antioxidant activity of leaves of Amaranthus hybridus L., Corchorus olitorius L and Hibiscus sabdariffa L. grown in northen of Côte d’Ivoire. GSC Biol. Pharm. Sci. 2021, 16, 182–189. [Google Scholar] [CrossRef]
- Morsy, N.E.; Rayan, A.M.; Youssef, K.M. Physico Chemical Properties, Antioxidant Activity, Phytochemicals and Sensory Evaluation of Rice-Based Extrudates Containing Dried Corchorus olitorius L. Leaves. J. Food Process. Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Hussien, N.M.; Labib, S.E.; Hefnawy, H.T.M. Phytochemical studies and antioxidant activity of leaves extracts of Corchorus olitorius L. (Molokhia). Zagazig J. Agric. Res. 2017, 44, 2231–2239. [Google Scholar] [CrossRef]
- Chigurupati, S.; Aladhadh, H.S.; Alhowail, A.; Selvarajan, K.K.; Bhatia, S. Phytochemical composition, antioxidant and antidiabetic potential of methanolic extract from Corchorus olitorius Linn. Grown in Saudi Arabia. Med. Plants 2020, 12, 71–76. [Google Scholar] [CrossRef]
- Airaodion, A.I.; Ogbuagu, E.O.; Ewa, O.; Ogbuagu, U.; Awosanya, O.O.; Adekale, O.A. Increase in Tannin Content of Some Selected Nigerian Vegetables during Blanching and Juicing. Asian J. Biochem. Genet. Mol. Biol. 2019, 2, 1–10. [Google Scholar] [CrossRef]
- Mesli, F.; Ghalem, M.; Daoud, I.; Ghalem, S. Potential inhibitors of angiotensin converting enzyme 2 receptor of COVID-19 by Corchorus olitorius Linn using docking, molecular dynamics, conceptual DFT investigation and pharmacophore mapping. J. Biomol. Struct. Dyn. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, P.; Anil Kumar, P.; Saraswat, M.; Petrash, J.M.; Reddy, G.B. Inhibition of aldose reductase by tannoid principles of Emblica officinalis: Implications for the prevention of sugar cataract. Mol. Vis. 2004, 10, 148–154. [Google Scholar]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Oboh, G.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Inhibitory effects of aqueous extracts of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. Journal of Food and Nutrition Research. J. Food Nutr. Res. 2010, 49, 14–20. [Google Scholar]
- Kwon, Y.I.; Apostolidis, E.; Kim, Y.C.; Shetty, K. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Food. 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Olusanya, A.R.; Ifeoluwa, B.S. Antidiabetic and safety properties of ethanolic leaf extract of Corchorus olitorius in alloxan-induced diabetic rats. Diabetes Food Plan. 2018, 11, 57. [Google Scholar]
- Ozioma, O.K.; Solomon, O.A.; Ocholi, E.D.; Somadina, C.R. Platelet Aggregation Effect of Corchorus olitorus Extract as an Index of Managing Blood Clotting Disorders. IOSR J. Dent. Med. Sci. 2014, 13, 58–60. [Google Scholar] [CrossRef]
- Gomaa, A.A.; El-Sers, D.A.; Al-Zokeim, N.I.; Gomaa, M.A. Amelioration of experimental metabolic syndrome induced in rats by orlistat and Corchorus olitorius leaf extract; role of adipo/cytokines. J. Pharm. Pharmacol. 2019, 71, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Ai, W.; Zhang, F.; Yang, K.; Wang, L.; Zhu, X.; Gao, P.; Shu, G.; Jiang, Q.; et al. Phytol increases adipocyte number and glucose tolerance through activation of PI3K/Akt signaling pathway in mice fed high-fat and high-fructose diet. Biochem. Biophys. Res. Commun. 2017, 489, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Mercan, N.; Toros, P.; Söyler, G.; Hanoglu, A.; Kükner, A. Effects of Corchorus olitorius and protacatechuic acid on diabetic rat testis tissue. Int. J. Morphol. 2020, 38, 1330–1335. [Google Scholar] [CrossRef]
- Lee, H.B.; Oh, M.J.; Do, M.H.; Kim, Y.S.; Park, H.Y. Molokhia leaf extract prevents gut inflammation and obesity. J. Ethnopharmacol. 2020, 257, 112866. [Google Scholar] [CrossRef]
- Abdallah, H.M.I.; Abdel Jaleel, G.A.; Mohammed, H.S.; Mahmoud, S.S.; Yassin, N.A.; Gamal El Din, A.A.; Shaffie, N.M.; Bassyouni, F.A. Phytochemical screening, gas chromatography-mass spectrometry analysis, and antidiabetic effects of Corchorus olitorius leaves in rats. Open Access Maced. J. Med. Sci. 2020, 8, 385–394. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, A.K. Plant Secondary Metabolites With Hepatoprotective Efficacy. In Nutraceuticals and Natural Product Pharmaceuticals; Academic Press: London, UK, 2019; ISBN 9780128164501. [Google Scholar]
- Saliu, J.A.; Ademiluyi, A.O.; Boligon, A.A.; Oboh, G.; Schetinger, M.R.C.; Rocha, J.B.T. Dietary supplementation of jute leaf (Corchorus olitorius) modulates hepatic delta-aminolevulinic acid dehydratase (δ-ALAD) activity and oxidative status in high-fat fed/low streptozotocin-induced diabetic rats. J. Food Biochem. 2019, 43, e12949. [Google Scholar] [CrossRef]
- Innami, S.; Tabata, K.; Shimizu, J.; Kusunoki, K.; Ishida, H.; Matsuguma, M.; Wada, M.; Sugiyama, N.; Kondo, M. Dried green leaf powders of Jew’s mellow (Corchorus), persimmon (Diosphyros kaki) and sweet potato (Ipomoea batatas poir) lower hepatic cholesterol concentration and increase fecal bile acid excretion in rats fed a cholesterol-free diet. Plant Foods Hum. Nutr. 1998, 52, 55–66. [Google Scholar] [CrossRef]
- Innami, S.; Nakamura, K.; Tabata, K.; Wada, M.; Takita, T. Water-soluble viscous substance of Jew’s mellow leaves lowers serum and liver cholesterol concentrations and increases fecal steroid excretion in rats fed a high cholesterol diet. J. Nutr. Sci. Vitaminol. 1995, 41, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Mazumder, U.K.; Pal, D.; Bhattacharya, S.; Chakrabarty, S. Studies on brain biogenic amines in methanolic extract of Cuscuta reflexa Roxb. and Corchorus olitorius linn. seed treated mice. Acta Pol. Pharm. Drug Res. 2003, 60, 207–210. [Google Scholar]
- Oyedeji, K.O.; Bolarinwa, A.F.; Akinbode, A.A. Effect of Corchorus olitorius extract on reproductive functions in male albino rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 427–431. [Google Scholar]
- Haridy, L.A.M.; Shakerali, S.; Alghamdi, R.K. Protective Role of Corchorus olitorius L. Leaves Extract against Experimentally-induced Hepatotoxicity. 2020. Available online: https://eijppr.com/IHPNOqf (accessed on 30 May 2022).
- Das, A.K.; Sahu, R.; Dua, T.K.; Bag, S.; Gangopadhyay, M.; Sinha, M.K.; Dewanjee, S. Arsenic-induced myocardial injury: Protective role of Corchorus olitorius leaves. Food Chem. Toxicol. 2010, 48, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Wagdy, R.; Abdelkader, R.M.; El-Khatib, A.H.; Linscheid, M.W.; Hamdi, N.; Handoussa, H. Neuromodulatory Activity of Dietary Phenolics Derived from Corchorus olitorius L. J. Food Sci. 2019, 84, 1012–1022. [Google Scholar] [CrossRef]
- Gay, N.H.; Phopin, K.; Suwanjang, W.; Songtawee, N.; Ruankham, W. Neuroprotective Effects of Phenolic and Carboxylic Acids on Oxidative Stress-Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells. Neurochem. Res. 2018, 43, 619–639. [Google Scholar] [CrossRef]
- Gray, N.E.; Morré, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in Centella asiatica protect against amyloid-β toxicity. J. Alzheimer’s Dis. 2014, 40, 359–373. [Google Scholar] [CrossRef] [Green Version]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and its Neuroprotective Effects on Brain Ischemia and Neurodegenerative Diseases. Curr. Drug Targets. 2018, 19, 1710–1720. [Google Scholar] [CrossRef]
- Gupta, M.; Mazumder, U.K.; Pal, D.K.; Bhattacharya, S. Onset of puberty and ovarian steroidogenesis following adminstration of methanolic extract of Cuscuta reflexa Roxb. stem and Corchorus olitorius Linn. seed in mice. J. Ethnopharmacol. 2003, 89, 55–59. [Google Scholar] [CrossRef]
- Yadav, S.B.; Tripathi, V.; Singh, R.K.; Pandey, H.P. Antioxidant activity of Cuscuta reflexa stems. Indian J. Pharm. Sci. 2000, 62, 477–478. [Google Scholar]
- Pal, D.; Gupta, M.; Mazumder, U. Effects of methanol extracts of Cuscuta reflexa Roxb. stem and Corchorus olitorius Linn. seed on male reproductive system of mice. Orient. Pharm. Exp. Med. 2009, 9, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, S.; Hiramoto, K.; Fujikawa, T.; Kondo, H.; Konishi, N.; Sudo, S.; Iwashima, M.; Ooi, K. Skin Hydrating Effects of Corchorus olitorius; Extract in a Mouse Model of Atopic Dermatitis. J. Cosmet. Dermatological Sci. Appl. 2014, 4, 42341. [Google Scholar] [CrossRef] [Green Version]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the Ω-6 and Ω-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Dai, Y.S.; Yang, Y.H.; Wang, L.C.; Lee, J.H.; Chiang, B.L. Linoleic acid metabolite levels and transepidermal water loss in children with atopic dermatitis. Ann. Allergy Asthma Immunol. 2008, 100, 66–73. [Google Scholar] [CrossRef]
- Vya, B.; Boye, A.; Quansah, N. Antioxidant and wound healing studies on the extracts of Corchorus olitorius leaf. Sci. Res. Rev. J. 2013, 1, 67–73. [Google Scholar]
- Adegoke, A.A.; Adebayo-Tayo, B.C. Phytochemical composition and antimicrobial effects of C. orchorous olitorius leaf extracts on four bacterial isolates. J. Med. Plants Res. 2009, 3, 155–159. [Google Scholar]
- Senu, A.J.; Olusola, E.M.; Afolashade, O.O.; Biliaminu; Ajoke, S. The in-vitro effect of Corchorus olitorius (Linn.) on the antibacterial activities of five antibiotics. Glob. Res. J. Microbiol. 2012, 2, 113–117. [Google Scholar]
- Abir, R.R.; Marjia, M.; Rakhi, N.N.; Saha, O.; Hossain, M.A.; Rahaman, M.M. In Vitro Comparative Analysis of Antibacterial Activity of Different Fractions of Corchorus capsularis and Corchorus olitorius Leaves Extracts. Bangladesh J. Microbiol. 2020, 36, 69–73. [Google Scholar] [CrossRef]
- Sathiamoorthy, B.; Gupta, P.; Kumar, M.; Chaturvedi, A.K.; Shukla, P.K.; Maurya, R. New antifungal flavonoid glycoside from Vitex negundo. Bioorganic Med. Chem. Lett. 2007, 17, 239–242. [Google Scholar] [CrossRef]
- Elangovan, A.; Dhanasekaran, S.; Anandan, A.; Krishnappa, K.; Gokulakrishnan, J.; Elumalai, K. Mosquitocidal Activities of Corchorus capsularis L (Malvaceae) against a Common Malarial Vector, Anopheles Stephensi (Liston) and a Dengue Vector Aedes Aegypti (L) (Diptera: Culicidae). Int. J. Recent Sci. Res. 2012, 3, 564–568. [Google Scholar]
- Chenniappan, K.; Kadarkarai, M. Oviposition deterrent, ovicidal and gravid mortality effects of ethanolic extract of Andrographis paniculata Nees against the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Entomol. Res. 2008, 38, 119–125. [Google Scholar] [CrossRef]
- Sathiyamoorthy, P.; Lugasi-Evgi, H.; Schlesinger, P.; Kedar, I.; Gopas, J.; Pollack, Y.; Golan-Goldhirsh, A. Screening for cytotoxic and antimalarial activities in desert plants of the Negev and Bedouin market plant products. Pharm. Biol. 1999, 37, 188–195. [Google Scholar] [CrossRef]
- Mohammed, R.M.O. Phytochemical Investigation of Antimicrobial and Antioxidant Activity Leaves Extracts of Corchorus olitorius. OALib 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Roy, N. Role of Chorchorus capsularis phytochemicals on the feeding dynamics of Diacrisia casignetum Kollar (Lepidoptera: Arctiidae). J. Entomol. Zool. Stud. 2014, 2, 227–236. [Google Scholar]
- Schoonhoven, L.; Van Loon, J.J.A.; Dicke, M. Insect Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2005; Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkozje))/reference/referencespapers.aspx?referenceid=1939403 (accessed on 30 May 2022).
- Talukder, D.; Muslima Khanam, L.A. Toxicity of four plant based products against three stored product pests. J. Bio-Sci. 2009, 17, 149–153. [Google Scholar] [CrossRef]
- Mahfuz, I.; Khanam, L.A.M. Toxicity of Some Indigenous Plant Extracts Against Tribolium. J. Bio-Sci. 2007, 15, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Manning, P.J.; Sutherland, W.H.; Walker, R.J.; Williams, S.M.; De Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of High-Dose Vitamin E on Insulin. Emerg. Treat. Technol. 2004, 27, 2166–2171. [Google Scholar]
- Adedosu, O.T.; Akanni, O.E.; Afolabi, O.K.; Adedeji, A.L. Effects of Corchorus olitorius Extract on Certain Antioxidants and Biochemical Indices in Sodium Arsenite Exposed Rats. Am. J. Phytomed. Clin. Ther. 2015, 245–256. [Google Scholar]
- Dewanjee, S.; Gangopadhyay, M.; Sahu, R.; Karmakar, S. Cadmium induced pathophysiology: Prophylactic role of edible jute (Corchorus olitorius) leaves with special emphasis on oxidative stress and mitochondrial involvement. Food Chem. Toxicol. 2013, 60, 188–198. [Google Scholar] [CrossRef]
- Dewanjee, S.; Sahu, R.; Karmakar, S.; Gangopadhyay, M. Toxic effects of lead exposure in Wistar rats: Involvement of oxidative stress and the beneficial role of edible jute (Corchorus olitorius) leaves. Food Chem. Toxicol. 2013, 55, 78–91. [Google Scholar] [CrossRef]
- Omeje, K.O.; Ezike, T.C.; Omeje, H.C.; Ezema, B.O. Effect of ethanol extract of Corchorus olitorus leaf on glucose level and antioxidant enzymes of Streptozotocin-induced hyperglycemic rat. Biokemistri 2016, 28, 121–127. [Google Scholar] [CrossRef]
- Ilhan, S.; Savarouglu, F.; CColak, F. Antibacterial and Antifungal Activity of Corchorus olitorius L. (Molokhia) Extracts. Int. J. Nat. Eng. Sci. 2007, 1, 59–61. [Google Scholar]
- Pramanik, P.K.; Paik, D.; Pramanik, A.; Chakraborti, T. Parasitology International White jute (Corchorus capsularis L.) leaf extract has potent leishmanicidal activity against Leishmania donovani. Parasitol. Int. 2019, 71, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.K.; Mandal, M.; Senthilkumar, G.P.; Padhiari, A. Antibacterial activity of Cuscuta reflexa stem and Corchorus olitorius seed. Fitoterapia 2006, 77, 589–591. [Google Scholar] [CrossRef] [PubMed]




| Species | Location | Part | Uses |
|---|---|---|---|
| C. capsularis | Bangladesh and India | Leaves along with petiole and tender shoots | Usually, sauteed lightly and eaten along with grains of rice or rice gruel. |
| Malaysia | Leaves | known as “kancing baju”. | |
| Vietnam | Leaves | It is made into a soup with shrimp. | |
| Nigeria | Leaves | Used to prepare a stew called “ewedu”. Use jute leaves for treating iron and folic acid deficiency, as well as treatment of anemia. | |
| C. olitorius | Northern Sudan, Tunisian, and Mali | Leaves | Used to make into a common medicinal mucilaginous (slimy) soup or stew. |
| Egypt, Jordan, and Syria | Dried leaf, dried immature fruit | Ingredient as a soup-based dish, eaten as boiled vegetable with lemon and olive oil. Sometimes eat with meat over rice or lentis. | |
| Philippines | Leaves | Commonly eaten with bamboo shoots as a leafy vegetable. | |
| Japan | Leaves, dried leaves | Food item, thickener in soups, and young dried leaves as coffee/tea substitute. | |
| China | Seeds, dried leaves | Seeds are used as a flavoring agent, and dried leaves are used to made herbal tea. | |
| Europe | Leaves are used as soup. | ||
| Middle East | Leaves | Green leafy vegetables and stews with rice. | |
| Kenya | Leaves | Leaves are eaten with “ugali”, a staple in most communities. | |
| Turkey and Cyprus | Young leaves | Generally cooked into a chicken stew. | |
| Thailand | Leaves | Eaten blanched, together with plain rice congee. | |
| Ghana, and Sierra Leone | Leaves | Complement of staple foods. |
| Ingredients and Unit | Leaves | Saluyot (Boiled/100 Grams Edible Portion) | Seeds of C. olitorius | |
|---|---|---|---|---|
| C. capsularis | C. olitorius | |||
| Moisture (%) | 80.4–84.1 | 86.30 | - | 0.006–0.011 |
| Ash | 2.4 | 8.11 | 4.5–5.6 | 1870–2642 |
| Calories (kl cal) | 73 | 37.6 | 43–58 | - |
| Protein | 3.6 | 26.80 | 4.5–5.6 | 970–1140 |
| Crude fat | 1.7–2.0 | 5.40 | - | 5270–5900 |
| Carbohydrates 7.6–12.4 | 7.6–12.4 | 85 | 7600–12,400 | 59,910–87,340 |
| Fiber | 1.7–2.0 | 37.60 | 1700–2000 | 970–1910 |
| Lipid | 0.6 | 8460 | - | - |
| Vit. A | - | - | 6390 | 2.16–2.84 |
| Thiamine | 15 | - | 15 | - |
| Riboflavin | 28 | - | 28 | - |
| Niacin | 1.1–1.2 | - | 1.5 | - |
| Ascorbic acid | 75.80 | 257.8 | 95 | 0.52–0.88 |
| Vit. D | - | - | - | 0.36–0.39 |
| β-carotene | 6.41–7.85 | 5.44 | - | - |
| α-tocopherol | - | 14.0 | - | - |
| Na | 12 | 72.3 | 12 | 2.2–7.0 |
| K | 444 | 4.4 | 444 | 30–1230 |
| Ca | 298 | - | 266–366 | 1240–2610 |
| Mg | 33.66–37.99 | 34.35–37.84 | 5.95 | 910–1480 |
| Fe | 12.53 | 9.93–13.44 | 11.6 | 1240–1620 |
| P | 97–122 | - | 97122 | 0.62–0.97 |
| Chemical Classification | Compound Isolated | Plant Parts | Major Findings | Extraction Solvent | Method | References |
|---|---|---|---|---|---|---|
| Cardiac glycosides | C. olitorius | Seed and leaf | Strophanthidin glycosides and digitoxigenin glycosides | Methanol | HPLC | [85] |
| Seed | Coroloside and deglycocoroloside | [48] | ||||
| Root | Corchoroside | Column chromatography | [58] | |||
| Leaf | Capsulasone, cochorol, and capsularol | |||||
| Seed | Caredenolide glycoside | Methanol | [86] | |||
| Leaf | Ionone glucosides named corchoionosides A, B, and C, (65′,9Jt)-roseoside Two flavonol glucosides isoquercitrin and astragalin Two coumarin glucosides cichoriine and scopolin | Methanol | [36] | |||
| Seed | Corchoroside A, corchoroside B, strophanthidin trioside, coroloside, deglycoroloside, oitoriside, glucoevatromonoside, deglucocoroloside, evatromonoside, and digitoxigenin triglycoside | Methanol | [87] | |||
| Seed, root, leaf, and stem | Raffinose, coroloside, glucoevatromonoside erysimoside andolitoriside, and gluco-olitoriside | Chloroform | GC-MS | [4,65,86] | ||
| C. capsularis | Seed | Strophanthidin glycoside, strophanthidin trioside, corchoroside A, corchoroside B, hydrogen cyanide, and polar glycosides A and B | Chloroform–alcohol (2:1) | Column chromatography | [58,88] | |
| Cardenolide glycosides | C. olitorius | Seed | Canarigenin 3-O-B-D-glucopyranosyl-(1-4)-O-B-D allomethylpyranose/altromethylpyranose, cannogenol 3-O-β- D-glucopyranosyl-(1→4)-O-β-D-boivinopyranoside, periplogenin 3-O-β-D-glucopyranosyl-(1→4)-O-β-D- digitoxopyranoside, and digitoxigenin 3-β-D-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl-(1→4)-O-β-D-digitoxopyranoside | Methanol | Medium pressure liquid chromatography and HPLC | [60,89] |
| Strophanthidin glycosides | C. olitorius | Seed | Erysimoside (strophanthidin 3-O-β-Dglucopyranosyl (1→4)-O-β-D-digitoxopyranoside), olitoriside (strophanthidin3-O-β-D-glucopyranosyl (1→4)-O-β-Dboivinopyranoside), corchoroside A (strophanthidin 3-O-β-D boivinopyranoside), and helveticoside (strophanthidin 3-O- β-D digitoxopyranoside), | Methanol | Medium pressure liquid chromatographyand HPLC | [61,89] |
| Digitoxigenin glycosides | C. olitorius | Seed | Glucoevatromonoside (digitoxigenin-3-O-β-D glucopyranosyl-(1→4)–O-β-D-digitoxopyranoside), coroloside (digitoxigenin-3-O-β-D-glucopyranosyl-(1→4)-O-β-D-boivinopyranoside), deglucocoroloside (digitoxigenin-3-O-β-D-boivinopyranoside), evatromonoside (digitoxigenin-3-O-β-D digitoxopyranoside), digitoxigenin 3-O-β-D glucopyranosyl-(1→6)-β-D-glucopyranosyl-(1→4)-O-β-D digitoxopyranoside, and corchorusoside (A, B, C, D, and E) | Methanol | Medium pressure liquid chromatography and HPLC | [61,89] |
| Triterpenes | C. capsularis C. olitorius | Root | Corosin (R1 = OH, R2 = R3= H), ursolic acid (R = H), corosolic acid, and oxo-corocin | Ethanol | Silicagel-colum chromatograph | [62] |
| C. olitorius | Stem and leaf | Oleanolic acid | Dichloromethane | NMR spectra | [45,69] | |
| C. olitorius | Root | Corosic acid | Refluxing with hydrochloric acid | [65] | ||
| C. capsularis | Leaf | Capsin (R1 = Glucose, R2 = H) and capsugenin 30-O-Glycopyranose (R1 = H, R2 = Glucose) | Methanol and sulphuric acid | TLC | [64,66] | |
| ,, | Root | Betulin | Petroleum ether, chloroform, and methanol | column chromatography | [81] | |
| Ursane tnterpenes | C. capsularrs C. olltorius | Root | Corosm (capsularone), ursohc acid, and corosohc acid | 95% ethanol alcohol | TLC | [66] |
| Ionones | C. olitorius | Leaf | Corchoionoside-A, corchoionoside-B, corchoionoside-C (6S,9R)-roseoside, and Betulabuside | Methanol under reflux | TLC | [36,69] |
| Phenolics | C. olitorius | Leaf | Quinic acid, gallic acid, protocatechuic acid, 4-O-caffeoylquinic acid, caffeic acid, 1,3-di-O caffeoyquinic acid, p-coumaric acid, trans-Ferulic acid, 3,4-di-O caffeoyquinic acid, rosmarinic acid, and 4,5-di-O-caffeoyquinic acid | Ethanol, ethanol/aqueous, and aqueous | LC–MS | [53,57] |
| ,, | Leaf and seed | Quinic acid, chlorogenic acid, and 1,5-dicaffeoyl quinic acid 3,5-dicaffeoyl quinic acid | Petroleum ether Fraction | GC-MS | [36,90] | |
| ,, | Dried leaf | chlorogenic acid, catechin, and astragalin | water-soluble extract | HPLC-ESI-MS | [54] | |
| ,, | Leaf | 5-caffeoylquinic acid (chlorogenic acid), 3,5-dicaffeoylquinic acid (isochlorogenic acid), quercetin 3-galactoside, quercetin 3-glucoside, quercetin 3-(6-malonylglucoside), and quercetin 3-(6-malonylgalactoside) | Methanol | NMR and FAB-MS | [47] | |
| ,, | Chlorogenic acids, dicaffeoylquinic acids, and feruloyl-quinic acids | 67% methanol and chloroform | UHPLCDAD-HRMS | [49] | ||
| ,, | Methyl-1,4,5-tri-O-caffeoyl quinate and trans-3-(4-Hydroxy-3 methoxyphenyl) acrylic anhydride | Methanol | NMR, IR, MS | [13] | ||
| ,, | Astragalin, isoquercetin, quercetin-3 galactoside, quercetin-3-(6 malonyl glucoside), and guercetin-3-(6 malonyl galactose) | Methanol | HPLC | [36,47] | ||
| ,, | Quinic acid | Ethanol | HPLC | [57] | ||
| C. capsularis | Bark and leaf | Cyanidin and cyanidin glucoside | - | [48] | ||
| Flavonoids | C. olitorius | Leaf and seed | Quercetin, isoquercetin, astragalin, catechins, luteolin, and 3,5-dicaffeoylquinic acid (3,5-DCQA) | Petroleum ether fraction | GC-MS | [36] |
| ,, | Leaf | Naringin, apegenin-7-O-glucoside, quercetin (quercetin-3-O rhamonoside), kaempferol, naringenin, luteolin, apigenin, cirsiliol, and cirsilineol | Ethanol, ethanol/aqueous, aqueous | LC-MS | [53] | |
| ,, | Leaf, aerial part | Astragalin (kaempferol 3-O-β-D glucopyranoside), tolifolin (kaempferol 3-O-β- D- galactopyranoside), and jugalanin (kaempferol 3-O-α-L-arabinopyranoside) | Methanol | NMR and FAB-MS | [47,65] | |
| Isoquercetin (quercetin 3-O-β-D-glucopyranoside) | Aqueous ethanol | HPLC | [57] | |||
| C. olitorius C. capsularis | Leaves | Caffeic acid, trans-ferulic acid, rutin hydrate, ellagic acid, and quercetin hydrate | 80% ethanol | HPLC-DAD | [54] | |
| Flavonoid glycosides | C. olitorius | Leaf | Isoquercetin, astragaline, tolifolin and juglanin, oleanolic acid glyceryl monopalmitate, β-sitosterol, and β- sitosterol-3-glucoside | - | [65] | |
| ,, | Aerial part | Quercetin3-O-galactoside (hyperoside), quercetin 3-β-glucoside (isoquercitrin), quercetin 3-(6 (malonylglucoside), and luteolin7-O-hexoside, | Hydroalcohol | HPLC | [90] | |
| Coumarin glucosides | C. olitorius | Defatted seed | (4-,7-dihydroxy coumarin) | Chloroform | [59] | |
| C. olitorius | Leaf | Scopolin and cichoriin | Methanol | NMR | [36] | |
| Sterols | C. olitorius | Leaf | β- Sitosterol-β-D-Sitosterol-glucoside, β-sitosterol 3-O-B-D-glucopyranoside | - | [65] | |
| ,, | Stem and root | β- Sitosterol | Dichloromethane | NMR spectra | [45] | |
| ,, | Stem | β-sitosteryl fatty acid esters, β-sitosterol, and stigmasterol | Dichloromethane | NMR spectra | [45] | |
| C. capsularis | Leaf | β-sitosterol | Chloroform | FTIR, HNMR, CNMR GC–MS | [80] | |
| ,, | Seed, leaf, root and stem | β-Sitosterol | Dichloromethan, ethanol | Column chromatograph and Silicagel-colum chromatograph | [45,58,62] | |
| ,, | Vegetative part | 3-O-glucopyranosyl-β-sitosterol | Petroleum ether and alcoholic Potassium Hydroxide | GC-MS | [65] | |
| Fatty acids | C. olitorius | Leaf Leaf | Corchorifatty acid-A, B, C, D, E, and F and undecanoic acid | Methanol | Silica-gel, HPLC | [69] |
| Glyceryl monopalmitate | [65] | |||||
| ,, | Leaf | α-linorenic acid and linoleic acid | Chloroform and methanol (2:1) | GLC | [72] | |
| ,, | Leaf | ω3-octadecatriene ω-3 fatty acids/ω3-octadecatriene (49% of the total fatty acids) | Chloroform and methanol (2:1) | GCQTOF | [71] | |
| C. olitorius | Fresh young leaf | Scopoletin, fraxinol, isopimpinellin, xanthotoxol, and peucedanol | 95% ethanol | GC-MS | [84] | |
| C. olitorius | Seed Stem | Stearic acid (49.48%)- seeds Palmitic acid (59.94%)- stems | Petroleum ether and chloroform | Gas–liquid chromatograph | [65] | |
| C. capsularis | Seed, vegetative part | Palmitic acid major in the seeds (81.68%) and the vegetative part (54.10%) | ||||
| Volatile component | C. olitorius | Leaf | In control and treated leaves, a total of 45 and 49 components were identified, with cis-3-hexen-1-ol, cis-4-hexen-1-ol, terpinolene, tetradecanal, sabinene, and phytol being the most abundant findings. | 95% ethanol | GC-MS | [84] |
| Polysaccharides and other sugar | C. olitorius | Leaf | Uronic acid (65%) and composed of rhamnose, glucose, galacturonic acid, and gluconic acid. | Chloroform/Methanol | Chromatology | [20] |
| C. capsularis C. olitorius | Seed | Free sugars, glucose, sucrose, fructose, raffinose, arabinose, and galactose | Methanol in ethyl acetate | Silicagel-colum chromatograph | [62] | |
| C. capsularis C. olitorius | Root | Glucose, fructose, arabinose, and raffinose | Methanol in ethyl acetate | Silicagel-colum chromatograph | [62] | |
| C. capsularis | Seed | Sucrose, raffinose, stachynose, and verbascose | ||||
| C. capsularis | Leaf and bark | Fructose and galactose | - | [48] | ||
| Essential oil | C. olitorius C. capsularis | Leaf | Cedrane-5-one and γ-terpinene are major components of each species, respectively | Methylene chloride | GC-MS | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, A.; Dey, S.; Huang, S.; Deng, Y.; Birhanie, Z.M.; Zhang, J.; Akhter, D.; Liu, L.; Li, D. A Comprehensive Review of C. capsularis and C. olitorius: A Source of Nutrition, Essential Phytoconstituents and Pharmacological Activities. Antioxidants 2022, 11, 1358. https://doi.org/10.3390/antiox11071358
Biswas A, Dey S, Huang S, Deng Y, Birhanie ZM, Zhang J, Akhter D, Liu L, Li D. A Comprehensive Review of C. capsularis and C. olitorius: A Source of Nutrition, Essential Phytoconstituents and Pharmacological Activities. Antioxidants. 2022; 11(7):1358. https://doi.org/10.3390/antiox11071358
Chicago/Turabian StyleBiswas, Ashok, Susmita Dey, Siqi Huang, Yong Deng, Ziggiju Mesenbet Birhanie, Jiangjiang Zhang, Delara Akhter, Liangliang Liu, and Defang Li. 2022. "A Comprehensive Review of C. capsularis and C. olitorius: A Source of Nutrition, Essential Phytoconstituents and Pharmacological Activities" Antioxidants 11, no. 7: 1358. https://doi.org/10.3390/antiox11071358