Oxidative Stress Related to Plasmalemmal and Mitochondrial Phosphate Transporters in Vascular Calcification
Abstract
:1. Introduction
2. Plasmalemmal Phosphate Transporters
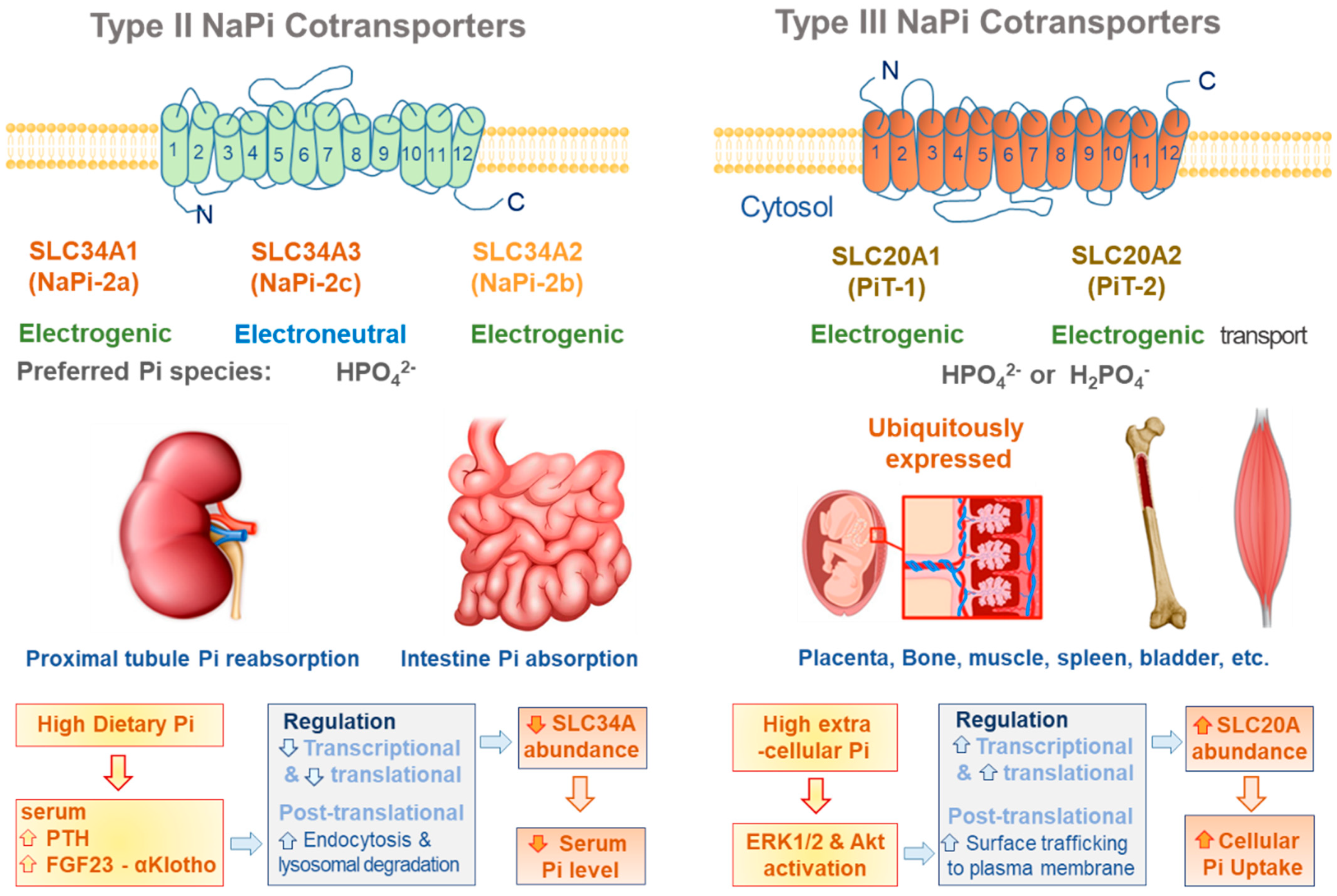
2.1. SLC34A Family
2.2. SLC20A Family
2.3. Cytosolic Phosphate Exporter
2.4. Oxidative Stress Related to Plasmalemmal Pi Transporters
3. Mitochondrial Phosphate Transporters
3.1. PiC
3.2. DIC
3.3. UCP2
3.4. Mg-ATP/Pi Carrier
3.5. Oxidative Stress Due to Mitochondrial Pi Transport
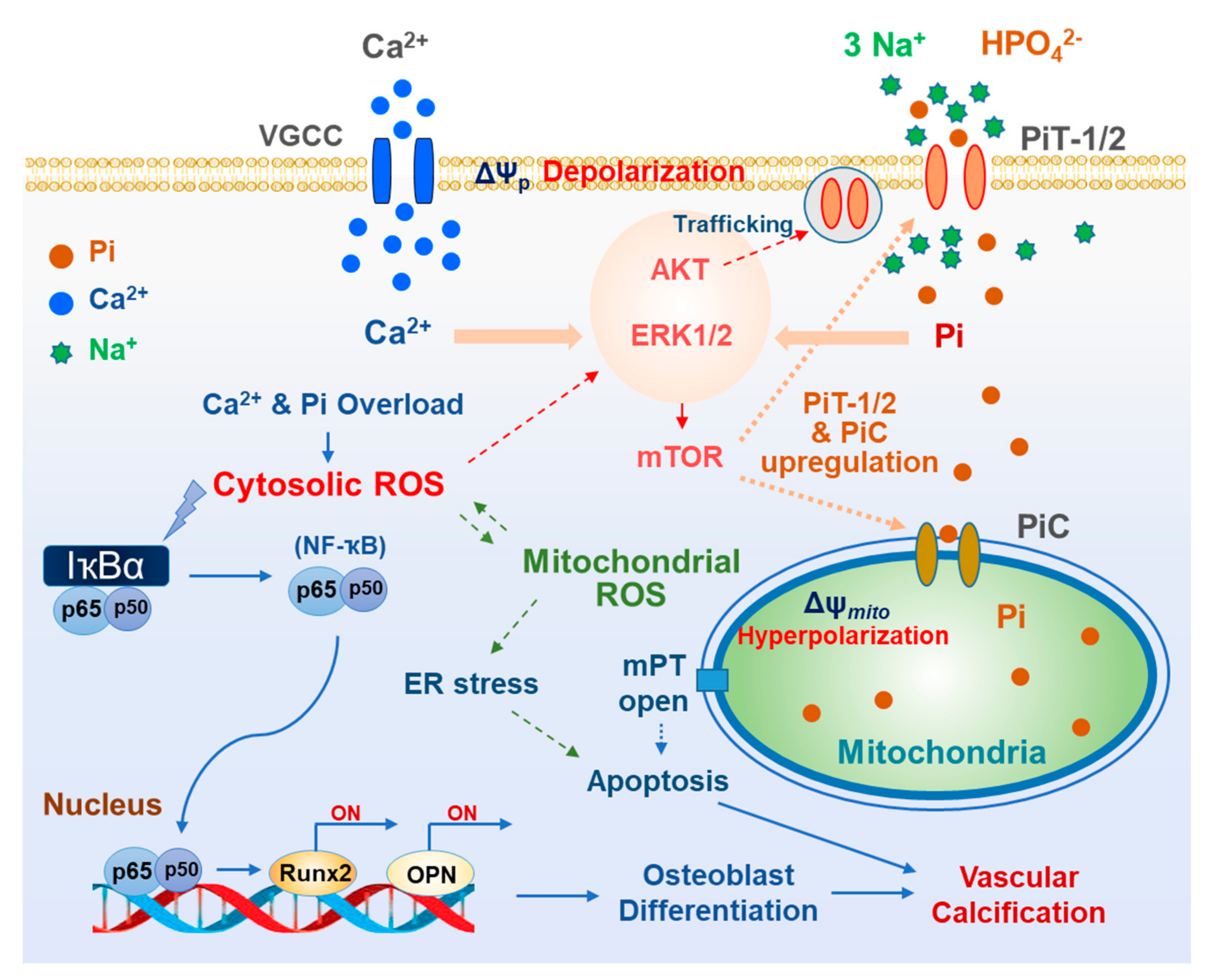
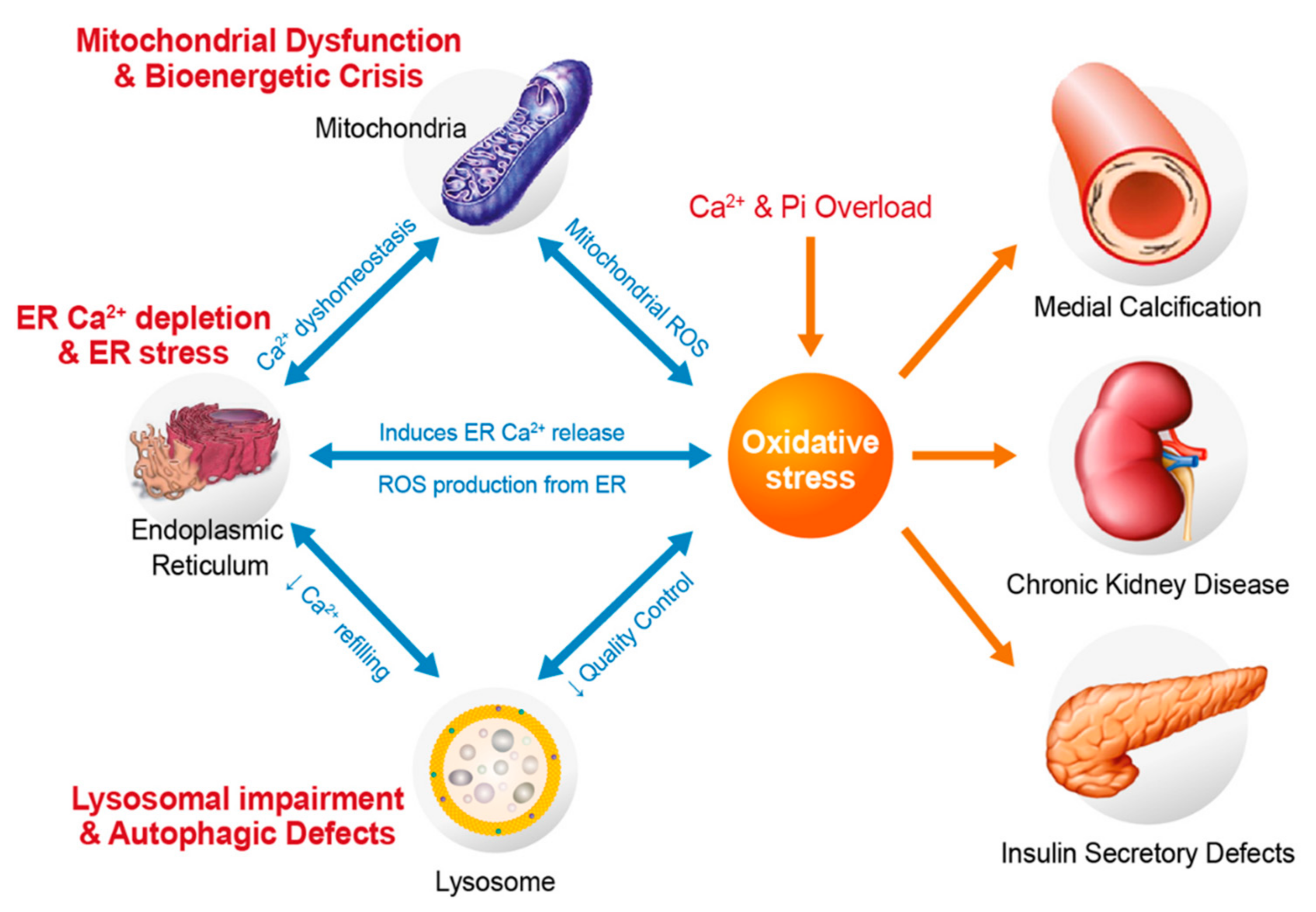
4. Oxidative Stress Related to Cytosolic and Mitochondrial Ca2+ and Pi Overloads
5. Oxidative Stress and Vascular Medial Calcification
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamamoto, Y.; Ishikawa, Y.; Shimpo, M.; Matsumura, M. Mönckeberg’s sclerosis. J. Gen. Fam. Med. 2021, 22, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular calcification-new insights into its mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.Y.; Shanahan, C.M. Medial arterial calcification: An overlooked player in peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1475–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, X.; Das, R.; Xu, S.; Cline, G.W.; Wiederkehr, A.; Wollheim, C.B.; Park, K.S. Mitochondrial phosphate transport during nutrient stimulation of ins-1e insulinoma cells. Mol. Cell. Endocrinol. 2013, 381, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Biber, J.; Hernando, N.; Forster, I. Phosphate transporters and their function. Annu. Rev. Physiol. 2013, 75, 535–550. [Google Scholar] [CrossRef]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transporters of the slc20 and slc34 families. Mol. Asp. Med. 2013, 34, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.A.; Hernando, N.; Forster, I.C.; Biber, J. The slc34 family of sodium-dependent phosphate transporters. Pflugers Arch. 2014, 466, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transport kinetics and structure-function relationships of slc34 and slc20 proteins. Curr. Top. Membr. 2012, 70, 313–356. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, K.; Haito-Sugino, S.; Kuwahara, S.; Ohi, A.; Nomura, K.; Ito, M.; Kuwahata, M.; Kido, S.; Tatsumi, S.; Kaneko, I.; et al. Sodium-dependent phosphate cotransporters: Lessons from gene knockout and mutation studies. J. Pharm. Sci. 2011, 100, 3719–3730. [Google Scholar] [CrossRef]
- Breusegem, S.Y.; Takahashi, H.; Giral-Arnal, H.; Wang, X.; Jiang, T.; Verlander, J.W.; Wilson, P.; Miyazaki-Anzai, S.; Sutherland, E.; Caldas, Y.; et al. Differential regulation of the renal sodium-phosphate cotransporters napi-iia, napi-iic, and pit-2 in dietary potassium deficiency. Am. J. Physiol. Renal Physiol. 2009, 297, F350–F361. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, C.; Meinild, A.K.; Murer, H.; Forster, I.C. Voltage- and substrate-dependent interactions between sites in putative re-entrant domains of a Na(+)-coupled phosphate cotransporter. Pflugers Arch. 2011, 461, 645–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschèn, I.; Setiawan, I.; Bröer, S.; Murer, H.; Lang, F. Effect of napi-mediated phosphate transport on intracellular ph. Pflugers Arch. 2001, 441, 802–806. [Google Scholar] [CrossRef]
- Segawa, H.; Aranami, F.; Kaneko, I.; Tomoe, Y.; Miyamoto, K. The roles of na/pi-ii transporters in phosphate metabolism. Bone 2009, 45 (Suppl. S1), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Onitsuka, A.; Furutani, J.; Kaneko, I.; Aranami, F.; Matsumoto, N.; Tomoe, Y.; Kuwahata, M.; Ito, M.; Matsumoto, M.; et al. Npt2a and npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development. Am. J. Physiol. Renal Physiol. 2009, 297, F671–F678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yang, H.Y.; Giachelli, C.M. Role of the sodium-dependent phosphate cotransporter, pit-1, in vascular smooth muscle cell calcification. Circ. Res. 2006, 98, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Villa-Bellosta, R.; Bogaert, Y.E.; Levi, M.; Sorribas, V. Characterization of phosphate transport in rat vascular smooth muscle cells: Implications for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Alesutan, I.; Leibrock, C.B.; Quintanilla-Martinez, L.; Kuhn, V.; Feger, M.; Mia, S.; Ahmed, M.S.; Rosenblatt, K.P.; Kuro, O.M.; et al. Spironolactone ameliorates pit1-dependent vascular osteoinduction in klotho-hypomorphic mice. J. Clin. Investig. 2013, 123, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, S.; Suzuki, A.; Asano, S.; Nishiwaki-Yasuda, K.; Shibata, M.; Nagao, S.; Yamamoto, N.; Matsuyama, M.; Sato, Y.; Yan, K.; et al. Phosphate overload induces podocyte injury via type iii na-dependent phosphate transporter. Am. J. Physiol. Renal Physiol. 2011, 300, F848–F856. [Google Scholar] [CrossRef] [Green Version]
- Werner, A.; Dehmelt, L.; Nalbant, P. Na+-dependent phosphate cotransporters: The napi protein families. J. Exp. Biol. 1998, 201, 3135–3142. [Google Scholar] [CrossRef]
- Bergwitz, C.; Roslin, N.M.; Tieder, M.; Loredo-Osti, J.C.; Bastepe, M.; Abu-Zahra, H.; Frappier, D.; Burkett, K.; Carpenter, T.O.; Anderson, D.; et al. Slc34a3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter napi-iic in maintaining phosphate homeostasis. Am. J. Hum. Genet. 2006, 78, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Parra, E.; Tuñón, J.; Egido, J.; Ortiz, A. Phosphate: A stealthier killer than previously thought? Cardiovasc. Pathol. 2012, 21, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Ohi, A.; Hanabusa, E.; Ueda, O.; Segawa, H.; Horiba, N.; Kaneko, I.; Kuwahara, S.; Mukai, T.; Sasaki, S.; Tominaga, R.; et al. Inorganic phosphate homeostasis in sodium-dependent phosphate cotransporter npt2b⁺/⁻ mice. Am. J. Physiol. Renal Physiol. 2011, 301, F1105–F1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.G.; Edwards, R.H.; Miller, A.D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc. Natl. Acad. Sci. USA 1994, 91, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, B.; Johann, S.V.; Klinger, H.P.; Blair, D.G.; Rubinson, H.; Dunn, K.J.; Sass, P.; Vitek, S.M.; Robins, T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990, 1, 119–127. [Google Scholar]
- Kavanaugh, M.P.; Miller, D.G.; Zhang, W.; Law, W.; Kozak, S.L.; Kabat, D.; Miller, A.D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA 1994, 91, 7071–7075. [Google Scholar] [CrossRef] [Green Version]
- Olah, Z.; Lehel, C.; Anderson, W.B.; Eiden, M.V.; Wilson, C.A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J. Biol. Chem. 1994, 269, 25426–25431. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Quan, X.; Xu, S.; Das, R.; Cha, S.K.; Kong, I.D.; Shong, M.; Wollheim, C.B.; Park, K.S. Intracellular alkalinization by phosphate uptake via type iii sodium-phosphate cotransporter participates in high-phosphate-induced mitochondrial oxidative stress and defective insulin secretion. FASEB J. 2016, 30, 3979–3988. [Google Scholar] [CrossRef] [Green Version]
- Beck, L.; Leroy, C.; Beck-Cormier, S.; Forand, A.; Salaün, C.; Paris, N.; Bernier, A.; Ureña-Torres, P.; Prié, D.; Ollero, M.; et al. The phosphate transporter pit1 (slc20a1) revealed as a new essential gene for mouse liver development. PLoS ONE 2010, 5, e9148. [Google Scholar] [CrossRef] [Green Version]
- Jensen, N.; Schrøder, H.D.; Hejbøl, E.K.; Füchtbauer, E.M.; de Oliveira, J.R.; Pedersen, L. Loss of function of slc20a2 associated with familial idiopathic basal ganglia calcification in humans causes brain calcifications in mice. J. Mol. Neurosci. 2013, 51, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Shen, Y.; Si, N.; Fan, S.; Zhang, Y.; Xu, W.; Shi, L.; Zhang, X. Slc20a2-deficient mice exhibit multisystem abnormalities and impaired spatial learning memory and sensorimotor gating but normal motor coordination abilities. Front. Genet. 2021, 12, 639935. [Google Scholar] [CrossRef]
- Crouthamel, M.H.; Lau, W.L.; Leaf, E.M.; Chavkin, N.W.; Wallingford, M.C.; Peterson, D.F.; Li, X.; Liu, Y.; Chin, M.T.; Levi, M.; et al. Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: Redundant roles for pit-1 and pit-2. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2625–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.T.; Nguyen, T.T.; Ly, D.D.; Xia, J.-B.; Qi, X.-F.; Lee, I.-K.; Cha, S.-K.; Park, K.-S. Oxidative stress by Ca2+ overload is critical for phosphate-induced vascular calcification. Am. J. Physiol. -Heart Circ. Physiol. 2020, 319, H1302–H1312. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Leaf, E.M.; Chia, J.J.; Cox, T.C.; Speer, M.Y.; Giachelli, C.M. Pit-2, a type iii sodium-dependent phosphate transporter, protects against vascular calcification in mice with chronic kidney disease fed a high-phosphate diet. Kidney Int. 2018, 94, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Li, Q.L.; Pan, Q.; Zhang, H.Y.; Fu, X.Y.; Yao, F.; Wang, J.N.; Yang, A.K. Xenotropic and polytropic retrovirus receptor 1 (xpr1) promotes progression of tongue squamous cell carcinoma (tscc) via activation of nf-κb signaling. J. Exp. Clin. Cancer Res. 2019, 38, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secco, D.; Wang, C.; Arpat, B.A.; Wang, Z.; Poirier, Y.; Tyerman, S.D.; Wu, P.; Shou, H.; Whelan, J. The emerging importance of the spx domain-containing proteins in phosphate homeostasis. New Phytol. 2012, 193, 842–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legati, A.; Giovannini, D.; Nicolas, G.; López-Sánchez, U.; Quintáns, B.; Oliveira, J.R.; Sears, R.L.; Ramos, E.M.; Spiteri, E.; Sobrido, M.J.; et al. Mutations in xpr1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 2015, 47, 579–581. [Google Scholar] [CrossRef]
- Ansermet, C.; Moor, M.B.; Centeno, G.; Auberson, M.; Hu, D.Z.; Baron, R.; Nikolaeva, S.; Haenzi, B.; Katanaeva, N.; Gautschi, I.; et al. Renal fanconi syndrome and hypophosphatemic rickets in the absence of xenotropic and polytropic retroviral receptor in the nephron. J. Am. Soc. Nephrol. 2017, 28, 1073–1078. [Google Scholar] [CrossRef] [Green Version]
- Krämer, R. Structural and functional aspects of the phosphate carrier from mitochondria. Kidney Int. 1996, 49, 947–952. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, L.; Palmieri, F.; Runswick, M.J.; Walker, J.E. Identification by bacterial expression and functional reconstitution of the yeast genomic sequence encoding the mitochondrial dicarboxylate carrier protein. FEBS Lett. 1996, 399, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Fiermonte, G.; De Leonardis, F.; Todisco, S.; Palmieri, L.; Lasorsa, F.M.; Palmieri, F. Identification of the mitochondrial atp-mg/pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J. Biol. Chem. 2004, 279, 30722–30730. [Google Scholar] [CrossRef] [Green Version]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. Ucp2 transports c4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, F. The mitochondrial transporter family slc25: Identification, properties and physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Runswick, M.J.; Powell, S.J.; Nyren, P.; Walker, J.E. Sequence of the bovine mitochondrial phosphate carrier protein: Structural relationship to adp/atp translocase and the brown fat mitochondria uncoupling protein. EMBO J. 1987, 6, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Dolce, V.; Fiermonte, G.; Palmieri, F. Tissue-specific expression of the two isoforms of the mitochondrial phosphate carrier in bovine tissues. FEBS Lett. 1996, 399, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, F.; Bisaccia, F.; Capobianco, L.; Dolce, V.; Fiermonte, G.; Iacobazzi, V.; Zara, V. Transmembrane topology, genes, and biogenesis of the mitochondrial phosphate and oxoglutarate carriers. J. Bioenerg. Biomembr. 1993, 25, 493–501. [Google Scholar] [CrossRef]
- Dolce, V.; Iacobazzi, V.; Palmieri, F.; Walker, J.E. The sequences of human and bovine genes of the phosphate carrier from mitochondria contain evidence of alternatively spliced forms. J. Biol. Chem. 1994, 269, 10451–10460. [Google Scholar] [CrossRef]
- Boulet, A.; Vest, K.E.; Maynard, M.K.; Gammon, M.G.; Russell, A.C.; Mathews, A.T.; Cole, S.E.; Zhu, X.; Phillips, C.B.; Kwong, J.Q.; et al. The mammalian phosphate carrier slc25a3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 2018, 293, 1887–1896. [Google Scholar] [CrossRef] [Green Version]
- Fonyó, A.; Ligeti, E. The role of intramitochondrial pi in stimulation of respiration by calcium and strontium. FEBS Lett. 1978, 93, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Aguilar, M.; Douglas, D.L.; Gibson, A.K.; Domeier, T.L.; Molkentin, J.D.; Baines, C.P. Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J. Mol. Cell. Cardiol. 2014, 72, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Kwong, J.; Davis, J.; Baines, C.; Sargent, M.; Karch, J.; Wang, X.; Huang, T.; Molkentin, J. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014, 21, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- Seifert, E.L.; Ligeti, E.; Mayr, J.A.; Sondheimer, N.; Hajnóczky, G. The mitochondrial phosphate carrier: Role in oxidative metabolism, calcium handling and mitochondrial disease. Biochem. Biophys. Res. Commun. 2015, 464, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, F.; Scarcia, P.; Monné, M. Diseases caused by mutations in mitochondrial carrier genes slc25: A review. Biomolecules 2020, 10, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, J.A.; Merkel, O.; Kohlwein, S.D.; Gebhardt, B.R.; Böhles, H.; Fötschl, U.; Koch, J.; Jaksch, M.; Lochmüller, H.; Horváth, R.; et al. Mitochondrial phosphate-carrier deficiency: A novel disorder of oxidative phosphorylation. Am. J. Hum. Genet. 2007, 80, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Mayr, J.A.; Zimmermann, F.A.; Horváth, R.; Schneider, H.-C.; Schoser, B.; Holinski-Feder, E.; Czermin, B.; Freisinger, P.; Sperl, W. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul. Disord. 2011, 21, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc. Res. 2009, 83, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.W.; Varanyuwatana, P.; Halestrap, A.P. The mitochondrial phosphate carrier interacts with cyclophilin d and may play a key role in the permeability transition. J. Biol. Chem. 2008, 283, 26312–26323. [Google Scholar] [CrossRef] [Green Version]
- Varanyuwatana, P.; Halestrap, A.P. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion 2012, 12, 120–125. [Google Scholar] [CrossRef]
- Basso, E.; Petronilli, V.; Forte, M.A.; Bernardi, P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin a and by cyclophilin d ablation. J. Biol. Chem. 2008, 283, 26307–26311. [Google Scholar] [CrossRef] [Green Version]
- Poncet, D.; Pauleau, A.L.; Szabadkai, G.; Vozza, A.; Scholz, S.R.; Le Bras, M.; Brière, J.J.; Jalil, A.; Le Moigne, R.; Brenner, C.; et al. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vmia. J. Cell Biol. 2006, 174, 985–996. [Google Scholar] [CrossRef] [Green Version]
- Poncet, D.; Larochette, N.; Pauleau, A.L.; Boya, P.; Jalil, A.A.; Cartron, P.F.; Vallette, F.; Schnebelen, C.; Bartle, L.M.; Skaletskaya, A.; et al. An anti-apoptotic viral protein that recruits bax to mitochondria. J. Biol. Chem. 2004, 279, 22605–22614. [Google Scholar] [CrossRef] [Green Version]
- Pauleau, A.L.; Galluzzi, L.; Scholz, S.R.; Larochette, N.; Kepp, O.; Kroemer, G. Unexpected role of the phosphate carrier in mitochondrial fragmentation. Cell Death Differ. 2008, 15, 616–618. [Google Scholar] [CrossRef] [Green Version]
- Nishi, Y.; Fujimoto, S.; Sasaki, M.; Mukai, E.; Sato, H.; Sato, Y.; Tahara, Y.; Nakamura, Y.; Inagaki, N. Role of mitochondrial phosphate carrier in metabolism-secretion coupling in rat insulinoma cell line ins-1. Biochem. J. 2011, 435, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, F. Mitochondrial carrier proteins. FEBS Lett. 1994, 346, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Lewis, R.Y.; Combatsiaris, T.P.; Lin, Y.; Shapiro, L.; Charron, M.J.; Scherer, P.E. Predominant expression of the mitochondrial dicarboxylate carrier in white adipose tissue. Biochem. J. 1999, 344 Pt 2, 313–320. [Google Scholar] [CrossRef]
- Fiermonte, G.; Dolce, V.; Arrigoni, R.; Runswick, M.J.; Walker, J.E.; Palmieri, F. Organization and sequence of the gene for the human mitochondrial dicarboxylate carrier: Evolution of the carrier family. Biochem. J. 1999, 344 Pt 3, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Coty, W.A.; Pedersen, P.L. Phosphate transport in rat liver mitochondria. Kinetics and energy requirements. J. Biol. Chem. 1974, 249, 2593–2598. [Google Scholar] [CrossRef]
- Hlouschek, J.; Ritter, V.; Wirsdörfer, F.; Klein, D.; Jendrossek, V.; Matschke, J. Targeting slc25a10 alleviates improved antioxidant capacity and associated radioresistance of cancer cells induced by chronic-cycling hypoxia. Cancer Lett. 2018, 439, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.N.; Chappell, J.B. The transport of inorganic phosphate by the mitochondrial dicarboxylate carrier. Biochem. J. 1973, 134, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lash, L.H. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharmacol. Exp. Ther. 1998, 285, 608–618. [Google Scholar]
- Kamga, C.K.; Zhang, S.X.; Wang, Y. Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. Am. J. Physiol. Cell Physiol. 2010, 299, C497–C505. [Google Scholar] [CrossRef] [Green Version]
- Huypens, P.; Pillai, R.; Sheinin, T.; Schaefer, S.; Huang, M.; Odegaard, M.L.; Ronnebaum, S.M.; Wettig, S.D.; Joseph, J.W. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia 2011, 54, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punzi, G.; Porcelli, V.; Ruggiu, M.; Hossain, M.F.; Menga, A.; Scarcia, P.; Castegna, A.; Gorgoglione, R.; Pierri, C.L.; Laera, L. Slc25a10 biallelic mutations in intractable epileptic encephalopathy with complex i deficiency. Hum. Mol. Genet. 2018, 27, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Baffy, G.; Perret, P.; Krauss, S.; Peroni, O.; Grujic, D.; Hagen, T.; Vidal-Puig, A.J.; Boss, O.; Kim, Y.-B.; et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, β cell dysfunction, and type 2 diabetes. Cell 2001, 105, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.Y.; Ma, S.; Tse, G.; Wong, W.T.; Huang, Y. Uncoupling protein 2 in cardiovascular health and disease. Front. Physiol. 2018, 9, 1060. [Google Scholar] [CrossRef] [Green Version]
- Fleury, C.; Neverova, M.; Collins, S.; Raimbault, S.; Champigny, O.; Levi-Meyrueis, C.; Bouillaud, F.; Seldin, M.F.; Surwit, R.S.; Ricquier, D.; et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997, 15, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Langin, D.; Larrouy, D.; Barbe, P.; Millet, L.; Viguerie-Bascands, N.; Andreelli, F.; Laville, M.; Vidal, H. Uncoupling protein-2 (ucp2) and uncoupling protein-3 (ucp3) expression in adipose tissue and skeletal muscle in humans. Int. J. Obes. Relat. Metab. Disord. 1999, 23 (Suppl. S6), S64–S67. [Google Scholar] [CrossRef] [Green Version]
- Rupprecht, A.; Bräuer, A.U.; Smorodchenko, A.; Goyn, J.; Hilse, K.E.; Shabalina, I.G.; Infante-Duarte, C.; Pohl, E.E. Quantification of uncoupling protein 2 reveals its main expression in immune cells and selective up-regulation during t-cell proliferation. PLoS ONE 2012, 7, e41406. [Google Scholar] [CrossRef]
- Pecqueur, C.; Alves-Guerra, M.C.; Gelly, C.; Levi-Meyrueis, C.; Couplan, E.; Collins, S.; Ricquier, D.; Bouillaud, F.; Miroux, B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J. Biol. Chem. 2001, 276, 8705–8712. [Google Scholar] [CrossRef] [Green Version]
- Berardi, M.J.; Chou, J.J. Fatty acid flippase activity of ucp2 is essential for its proton transport in mitochondria. Cell. Metab. 2014, 20, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, S.; Xie, P.; Hou, T.; Yu, T.; Fu, X. Overexpressed ucp2 regulates mitochondrial flashes and reverses lipopolysaccharide-induced cardiomyocytes injury. Am. J. Transl. Res. 2018, 10, 1347–1356. [Google Scholar] [PubMed]
- Jezek, P.; Holendova, B.; Garlid, K.D.; Jaburek, M. Mitochondrial uncoupling proteins: Subtle regulators of cellular redox signaling. Antioxid. Redox Signal. 2018, 29, 667–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Ma, Y.; Wang, R.; Zhang, J.; Jing, L.; Li, P.A. Deletion of mitochondrial uncoupling protein 2 exacerbates mitochondrial damage in mice subjected to cerebral ischemia and reperfusion injury under both normo- and hyperglycemic conditions. Int. J. Biol. Sci. 2020, 16, 2788–2802. [Google Scholar] [CrossRef] [PubMed]
- del Arco, A.; Satrústegui, J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J. Biol. Chem. 2004, 279, 24701–24713. [Google Scholar] [CrossRef] [Green Version]
- Satrústegui, J.; Pardo, B.; Arco, A.d. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol. Rev. 2007, 87, 29–67. [Google Scholar] [CrossRef] [Green Version]
- Aprille, J.R.; Austin, J. Regulation of the mitochondrial adenine nucleotide pool size. Arch. Biochem. Biophys. 1981, 212, 689–699. [Google Scholar] [CrossRef]
- Ryu, J.; Ko, J.M.; Shin, C.-H. A 9-year-old korean girl with fontaine progeroid syndrome: A case report with further phenotypical delineation and description of clinical course during long-term follow-up. BMC Med. Genet. 2019, 20, 188. [Google Scholar] [CrossRef]
- Writzl, K.; Maver, A.; Kovacic, L.; Martinez-Valero, P.; Contreras, L.; Satrustegui, J.; Castori, M.; Faivre, L.; Lapunzina, P.; van Kuilenburg, A.B.P.; et al. De novo mutations in slc25a24 cause a disorder characterized by early aging, bone dysplasia, characteristic face, and early demise. Am. J. Hum. Genet. 2017, 101, 844–855. [Google Scholar] [CrossRef] [Green Version]
- Ehmke, N.; Graul-Neumann, L.; Smorag, L.; Koenig, R.; Segebrecht, L.; Magoulas, P.; Scaglia, F.; Kilic, E.; Hennig, A.F.; Adolphs, N.; et al. De novo mutations in slc25a24 cause a craniosynostosis syndrome with hypertrichosis, progeroid appearance, and mitochondrial dysfunction. Am. J. Hum. Genet. 2017, 101, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Cavero, S.; Traba, J.; Del Arco, A.; Satrústegui, J. The calcium-dependent atp-mg/pi mitochondrial carrier is a target of glucose-induced calcium signalling in saccharomyces cerevisiae. Biochem. J. 2005, 392, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harborne, S.P.D.; King, M.S.; Crichton, P.G.; Kunji, E.R.S. Calcium regulation of the human mitochondrial atp-mg/pi carrier slc25a24 uses a locking pin mechanism. Sci. Rep. 2017, 7, 45383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traba, J.; Satrústegui, J.; del Arco, A. Characterization of scamc-3-like/slc25a41, a novel calcium-independent mitochondrial atp-mg/pi carrier. Biochem. J. 2009, 418, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, N.E.; Chandramoorthy, H.C.; Shanmughapriya, S.; Zhang, X.Q.; Vallem, S.; Doonan, P.J.; Malliankaraman, K.; Guo, S.; Rajan, S.; Elrod, J.W.; et al. Slc25a23 augments mitochondrial Ca2⁺ uptake, interacts with mcu, and induces oxidative stress-mediated cell death. Mol. Biol. Cell 2014, 25, 936–947. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Quan, X.; Hwang, K.H.; Xu, S.; Das, R.; Choi, S.K.; Wiederkehr, A.; Wollheim, C.B.; Cha, S.K.; Park, K.S. Mitochondrial oxidative stress mediates high-phosphate-induced secretory defects and apoptosis in insulin-secreting cells. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E933–E941. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; French, S.; Evans, F.J.; Joubert, F.; Balaban, R.S. Metabolic network control of oxidative phosphorylation: Multiple roles of inorganic phosphate. J. Biol. Chem. 2003, 278, 39155–39165. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ros generation and uncoupling (review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Hernansanz-Agustín, P.; Ramos, E.; Navarro, E.; Parada, E.; Sánchez-López, N.; Peláez-Aguado, L.; Cabrera-García, J.D.; Tello, D.; Buendia, I.; Marina, A.; et al. Mitochondrial complex i deactivation is related to superoxide production in acute hypoxia. Redox Biol. 2017, 12, 1040–1051. [Google Scholar] [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ros signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, M.D.; Goncalves, R.L.; Orr, A.L.; Vargas, L.; Gerencser, A.A.; Borch Jensen, M.; Wang, Y.T.; Melov, S.; Turk, C.N.; Matzen, J.T.; et al. Suppressors of superoxide-h(2)o(2) production at site i(q) of mitochondrial complex i protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 2016, 24, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selivanov, V.A.; Zeak, J.A.; Roca, J.; Cascante, M.; Trucco, M.; Votyakova, T.V. The role of external and matrix ph in mitochondrial reactive oxygen species generation. J. Biol. Chem. 2008, 283, 29292–29300. [Google Scholar] [CrossRef] [Green Version]
- Al-Aly, Z. Phosphate, oxidative stress, and nuclear factor-κb activation in vascular calcification. Kidney Int. 2011, 79, 1044–1047. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Zheng, L.; Xu, H.; Tang, D.; Lin, L.; Zhang, J.; Li, C.; Wang, W.; Yuan, Q.; Tao, L.; et al. Oxidative stress contributes to vascular calcification in patients with chronic kidney disease. J. Mol. Cell. Cardiol. 2020, 138, 256–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor runx2 by akt signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodenbeck, S.D.; Zarse, C.A.; McKenney-Drake, M.L.; Bruning, R.S.; Sturek, M.; Chen, N.X.; Moe, S.M. Intracellular calcium increases in vascular smooth muscle cells with progression of chronic kidney disease in a rat model. Nephrol. Dial. Transplant. 2016, 32, 450–458. [Google Scholar] [CrossRef]
- Gordeeva, A.V.; Zvyagilskaya, R.A.; Labas, Y.A. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry 2003, 68, 1077–1080. [Google Scholar] [CrossRef]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef] [Green Version]
- Stefani, D.D.; Rizzuto, R.; Pozzan, T. Enjoy the trip: Calcium in mitochondria back and forth. Annu. Rev. Biochem. 2016, 85, 161–192. [Google Scholar] [CrossRef]
- Giorgio, V.; Burchell, V.; Schiavone, M.; Bassot, C.; Minervini, G.; Petronilli, V.; Argenton, F.; Forte, M.; Tosatto, S.; Lippe, G.; et al. Ca(2+) binding to f-atp synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017, 18, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of atp production by mitochondrial Ca(2+). Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, M.J.; Brown, L.J. Calcium activation of mitochondrial glycerol phosphate dehydrogenase restudied. Arch. Biochem. Biophys. 1996, 326, 79–84. [Google Scholar] [CrossRef]
- Beis, I.; Newsholme, E.A. Effects of calcium ions on adenine nucleotide translocase from cardiac muscle. J. Mol. Cell. Cardiol. 1976, 8, 863–876. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Bolaños, J.P. A-ketoglutarate dehydrogenase complex moonlighting: Ros signalling added to the list: An editorial highlight for ‘reductions in the mitochondrial enzyme α-ketoglutarate dehydrogenase complex in neurodegenerative disease—beneficial or detrimental?’. J. Neurochem. 2016, 139, 689–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mráček, T.; Holzerová, E.; Drahota, Z.; Kovářová, N.; Vrbacký, M.; Ješina, P.; Houštěk, J. Ros generation and multiple forms of mammalian mitochondrial glycerol-3-phosphate dehydrogenase. Biochim. Biophys. Acta 2014, 1837, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Kowaltowski, A.J.; Castilho, R.F.; Grijalba, M.T.; Bechara, E.J.; Vercesi, A.E. Effect of inorganic phosphate concentration on the nature of inner mitochondrial membrane alterations mediated by Ca2+ ions. A proposed model for phosphate-stimulated lipid peroxidation. J. Biol. Chem. 1996, 271, 2929–2934. [Google Scholar] [CrossRef] [Green Version]
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial ca2+ transport: Mechanisms, molecular structures, and role in cells. Biochemistry 2019, 84, 593–607. [Google Scholar] [CrossRef]
- Ligeti, L.; Barlow, C.; Chance, B.; Kovach, A.G.B.; Oconnor, M. P-31 nmr-spectroscopy of brain and heart. Adv. Exp. Med. Biol. 1983, 159, 281–292. [Google Scholar]
- Duvvuri, B.; Lood, C. Mitochondrial calcification. Immunometabolism 2021, 3, e210008. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.; Nicholls, D.G. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003, 278, 19062–19070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, A.-C.; Liu, T.; Winslow, R.L.; O’Rourke, B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: Two components of ca2+ uptake and role of phosphate buffering. J. Gen. Physiol. 2012, 139, 465–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, M.; Kinoshita, S.; Ozono, K.; Michigami, T. Inorganic phosphate activates the akt/mtorc1 pathway and shortens the life span of an α-klotho-deficient model. J. Am. Soc. Nephrol. 2016, 27, 2810–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.; Lee, H.J.; Jung, Y.M.; Jung, Y.J. Mtor-mediated antioxidant activation in solid tumor radioresistance. J. Oncol. 2019, 2019, 5956867. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.M.; Xu, M.J.; Cai, Y.; Zhao, G.; Guan, Y.; Kong, W.; Tang, C.; Wang, X. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int. 2011, 79, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Agharazii, M.; St-Louis, R.; Gautier-Bastien, A.; Ung, R.V.; Mokas, S.; Larivière, R.; Richard, D.E. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am. J. Hypertens. 2015, 28, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Liberman, M.; Bassi, E.; Martinatti, M.K.; Lario, F.C.; Wosniak, J., Jr.; Pomerantzeff, P.M.; Laurindo, F.R. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Vitamin d, reactive oxygen species and calcium signalling in ageing and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150434. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Yamashita, M.; Horimai, C.; Hayashi, M. Smooth muscle-selective nuclear factor-κb inhibition reduces phosphate-induced arterial medial calcification in mice with chronic kidney disease. J. Am. Heart Assoc. 2017, 6, e007248. [Google Scholar] [CrossRef] [Green Version]
- Speer, M.Y.; Yang, H.-Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.-L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 2009, 104, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Chavkin, N.W.; Chia, J.J.; Crouthamel, M.H.; Giachelli, C.M. Phosphate uptake-independent signaling functions of the type iii sodium-dependent phosphate transporter, pit-1, in vascular smooth muscle cells. Exp. Cell Res. 2015, 333, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, T.; Chan, Y.Y.; Kawanami, A.; Balmes, G.; Landreth, G.E.; Murakami, S. Extracellular signal-regulated kinase 1 (erk1) and erk2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol. Cell. Biol. 2009, 29, 5843–5857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, C.; Xiao, G.; Jiang, D.; Yang, Q.; Hatch, N.E.; Roca, H.; Franceschi, R.T. Identification and functional characterization of erk/mapk phosphorylation sites in the runx2 transcription factor. J. Biol. Chem. 2009, 284, 32533–32543. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Jiang, D.; Thomas, P.; Benson, M.D.; Guan, K.; Karsenty, G.; Franceschi, R.T. Mapk pathways activate and phosphorylate the osteoblast-specific transcription factor, cbfa1. J. Biol. Chem. 2000, 275, 4453–4459. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.; Yang, J.; Liu, L.; Yang, X.; Yao, Z.; Ma, C.; Zhu, H.; Su, J.; Zhao, Q.; Feng, K.; et al. Erk1/2 inhibition reduces vascular calcification by activating mir-126-3p-dkk1/lrp6 pathway. Theranostics 2021, 11, 1129–1146. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, Y.; Yang, X.; Sun, L.; Liu, M.; Wang, Q.; Ma, X.; Zhang, W.; Li, X.; Hu, W.; et al. Inhibition of erk1/2 and activation of lxr synergistically reduce atherosclerotic lesions in apoe-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 948–959. [Google Scholar] [CrossRef] [Green Version]
- Alkagiet, S.; Tziomalos, K. Vascular calcification: The role of micrornas. Biomol. Concepts 2017, 8, 119–123. [Google Scholar] [CrossRef]
- Jansen, F.; Stumpf, T.; Proebsting, S.; Franklin, B.S.; Wenzel, D.; Pfeifer, P.; Flender, A.; Schmitz, T.; Yang, X.; Fleischmann, B.K.; et al. Intercellular transfer of mir-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting lrp6. J. Mol. Cell. Cardiol. 2017, 104, 43–52. [Google Scholar] [CrossRef]
- Deng, L.; Huang, L.; Sun, Y.; Heath, J.M.; Wu, H.; Chen, Y. Inhibition of foxo1/3 promotes vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Bai, Y.; Zhang, J.; Zhang, S.; Xu, J. Effects of extracellular acid stimulation on rat vascular smooth muscle cell in gas6/axl or pi3k/akt signaling pathway. Clin. Exp. Hypertens. 2016, 38, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, A.; Sinha, S.; Hyde, G.D.; Borland, S.J.; Taylor, R.F.; Pond, E.; Eyre, H.J.; Inkson, C.A.; Gilmore, A.; Ashton, N.; et al. Fti-277 inhibits smooth muscle cell calcification by up-regulating pi3k/akt signaling and inhibiting apoptosis. PLoS ONE 2018, 13, e0196232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Chiu, J.F.; Gagne, J.; Fukagawa, N.K. Age-related differences in insulin-like growth factor-1 receptor signaling regulates akt/foxo3a and erk/fos pathways in vascular smooth muscle cells. J. Cell. Physiol. 2008, 217, 377–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, W.; Saaoud, F.; Wang, Y.; Wang, Q.; Hodge, J.; Hui, Y.; Yin, S.; Lessner, S.M.; Kong, X.; et al. Mir155 modulates vascular calcification by regulating akt-foxo3a signalling and apoptosis in vascular smooth muscle cells. J. Cell. Mol. Med. 2021, 25, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, T.G.; Park, S.; Yun, H.R.; Nguyen, N.N.Y.; Jo, Y.H.; Jang, M.; Kim, J.; Kim, J.; Kang, I.; et al. Mitochondrial ros-derived pten oxidation activates pi3k pathway for mtor-induced myogenic autophagy. Cell Death Differ. 2018, 25, 1921–1937. [Google Scholar] [CrossRef]
- Furmanik, M.; van Gorp, R.; Whitehead, M.; Ahmad, S.; Bordoloi, J.; Kapustin, A.; Schurgers, L.J.; Shanahan, C.M. Endoplasmic reticulum stress mediates vascular smooth muscle cell calcification via increased release of grp78 (glucose-regulated protein, 78 kda)-loaded extracellular vesicles. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 898–914. [Google Scholar] [CrossRef]
- Panda, D.K.; Bai, X.; Sabbagh, Y.; Zhang, Y.; Zaun, H.-C.; Karellis, A.; Koromilas, A.E.; Lipman, M.L.; Karaplis, A.C. Defective interplay between mtorc1 activity and endoplasmic reticulum stress-unfolded protein response in uremic vascular calcification. Am. J. Physiol. -Renal Physiol. 2018, 314, F1046–F1061. [Google Scholar] [CrossRef]
- Ly, L.D.; Xu, S.; Choi, S.K.; Ha, C.M.; Thoudam, T.; Cha, S.K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.K.; Park, K.S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef]
- Duan, X.; Zhou, Y.; Teng, X.; Tang, C.; Qi, Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem. Biophys. Res. Commun. 2009, 387, 694–699. [Google Scholar] [CrossRef]
- Masuda, M.; Miyazaki-Anzai, S.; Levi, M.; Ting, T.C.; Miyazaki, M. Perk-eif2α-atf4-chop signaling contributes to tnfα-induced vascular calcification. J. Am. Heart Assoc. 2013, 2, e000238. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki-Anzai, S.; Masuda, M.; Demos-Davies, K.M.; Keenan, A.L.; Saunders, S.J.; Masuda, R.; Jablonski, K.; Cavasin, M.A.; Kendrick, J.; Chonchol, M.; et al. Endoplasmic reticulum stress effector ccaat/enhancer-binding protein homologous protein (chop) regulates chronic kidney disease-induced vascular calcification. J. Am. Heart Assoc. 2014, 3, e000949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman, M.; Johnson, R.C.; Handy, D.E.; Loscalzo, J.; Leopold, J.A. Bone morphogenetic protein-2 activates nadph oxidase to increase endoplasmic reticulum stress and human coronary artery smooth muscle cell calcification. Biochem. Biophys. Res. Commun. 2011, 413, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.T.; Nguyen, T.T.; Park, K.-S. Oxidative Stress Related to Plasmalemmal and Mitochondrial Phosphate Transporters in Vascular Calcification. Antioxidants 2022, 11, 494. https://doi.org/10.3390/antiox11030494
Nguyen NT, Nguyen TT, Park K-S. Oxidative Stress Related to Plasmalemmal and Mitochondrial Phosphate Transporters in Vascular Calcification. Antioxidants. 2022; 11(3):494. https://doi.org/10.3390/antiox11030494
Chicago/Turabian StyleNguyen, Nhung Thi, Tuyet Thi Nguyen, and Kyu-Sang Park. 2022. "Oxidative Stress Related to Plasmalemmal and Mitochondrial Phosphate Transporters in Vascular Calcification" Antioxidants 11, no. 3: 494. https://doi.org/10.3390/antiox11030494





