Tissue Oxidative Ecology along an Aridity Gradient in a Mammalian Subterranean Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling Sites and Sampling Sites’ Climate Data and Analysis
2.2. Animal Capture
2.3. Animal Housing
2.4. Age
2.5. Reagents
2.6. Euthanasia and Tissue Excision
2.7. Tissue Homogenization Procedure
2.8. TOS
2.9. MDA
2.10. TAC
2.11. SOD
2.12. OSI
2.13. Statistical Analyses
3. Results
3.1. Oxidative Damage
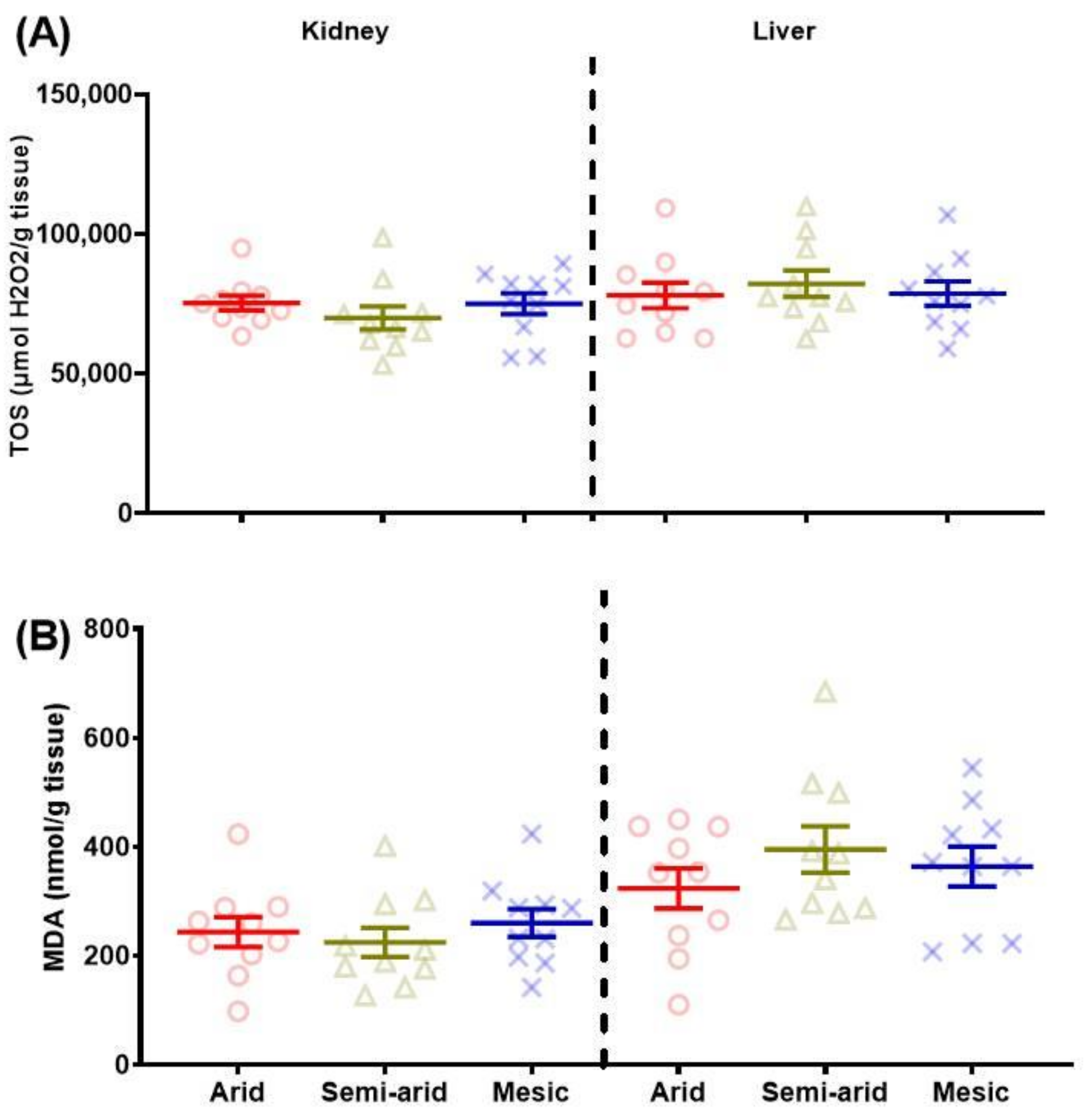
3.1.1. Total Oxidant Status (TOS)
3.1.2. Malondialdehyde (MDA)
3.2. Antioxidant Defense
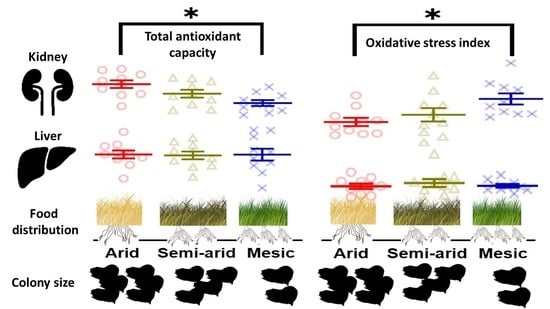
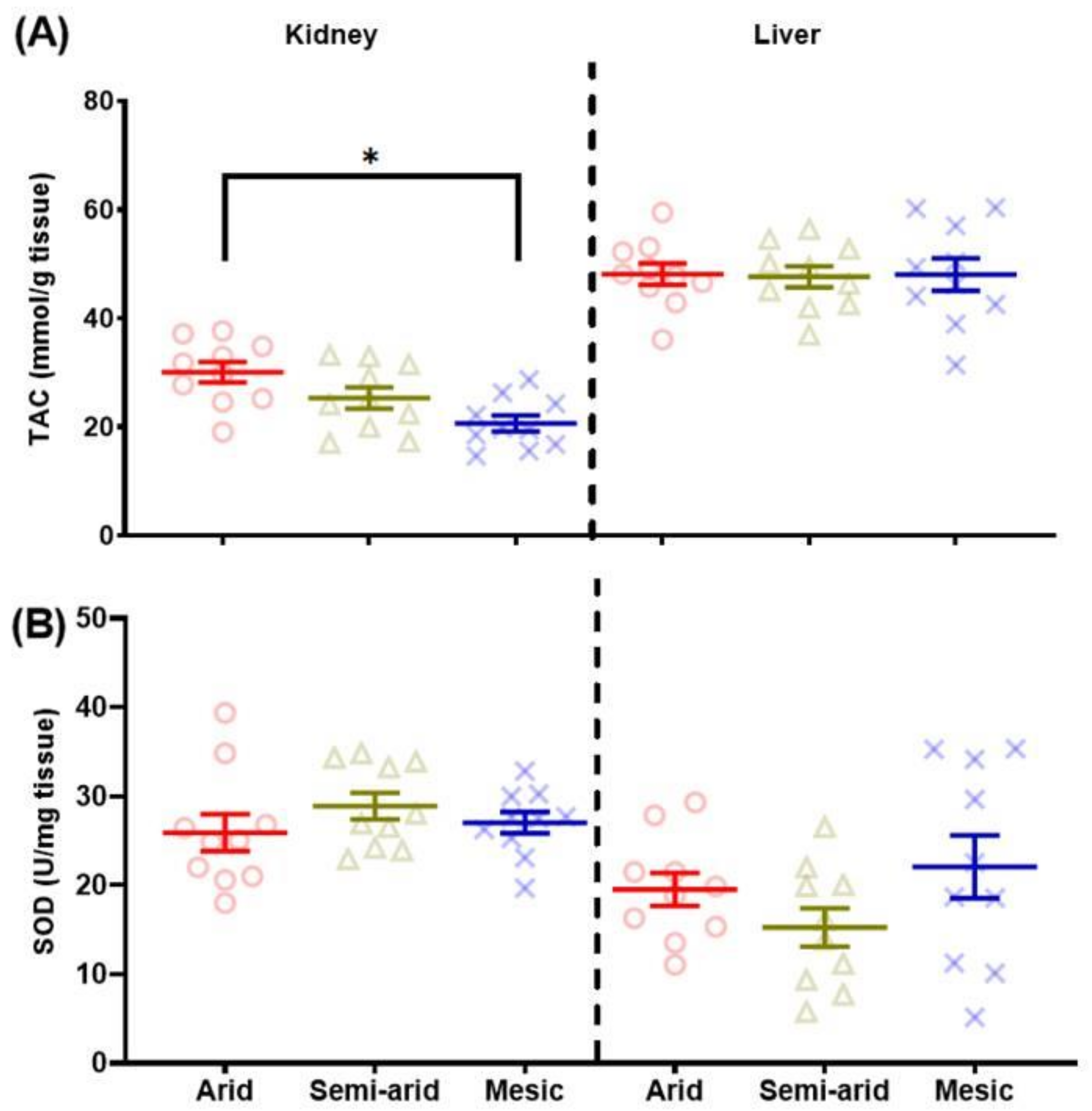
3.2.1. Total Antioxidant Capacity (TAC)
3.2.2. Superoxide Dismutase (SOD)
3.3. Oxidative Stress: OSI
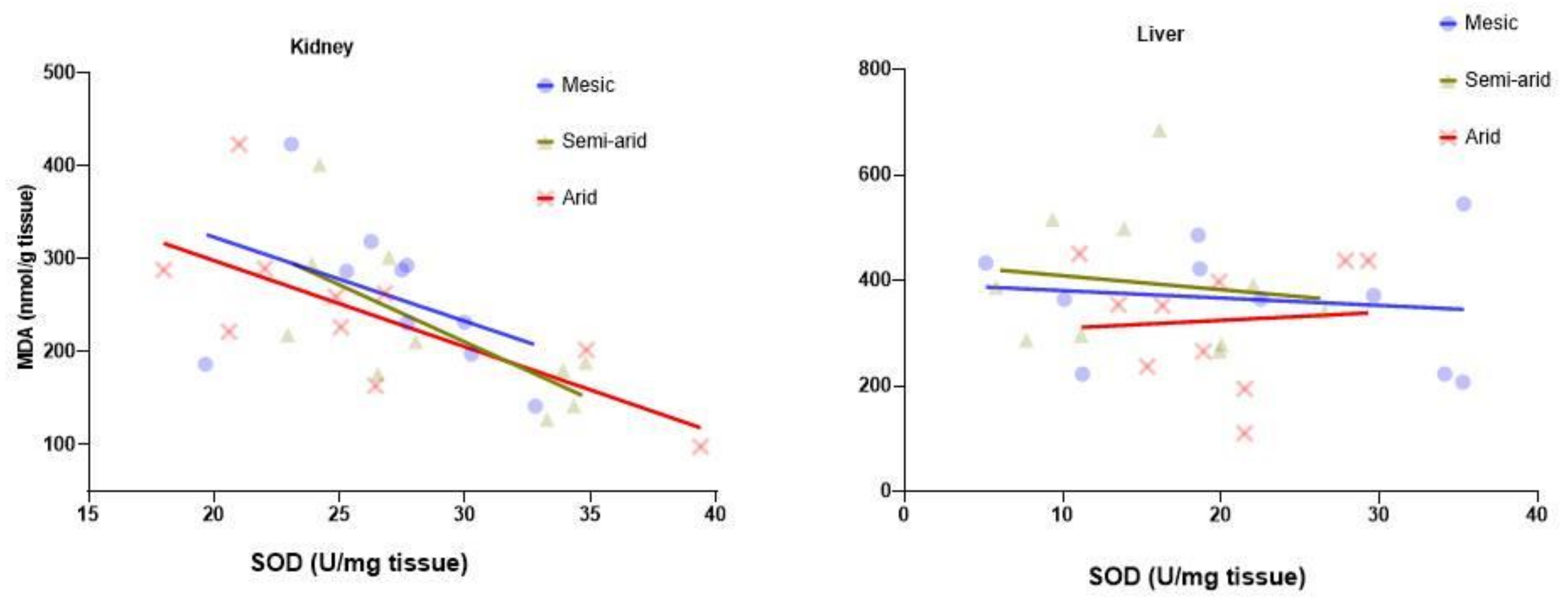
3.4. SOD-MDA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, J.A.; Jess, M.; Williams, S.E. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1665–1679. [Google Scholar] [CrossRef] [Green Version]
- Degen, A.A. Ecophysiology of Small Desert Mammals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Schwimmer, H.; Haim, A. Physiological adaptations of small mammals to desert ecosystems. Integr. Zool. 2009, 4, 357–366. [Google Scholar] [CrossRef]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Sahin, S. An aridity index defined by precipitation and specific humidity. J. Hydrol. 2012, 444, 199–208. [Google Scholar] [CrossRef]
- Park, C.-E.; Jeong, S.-J.; Joshi, M.; Osborn, T.J.; Ho, C.-H.; Piao, S.; Chen, D.; Liu, J.; Yang, H.; Park, H. Keeping global warming within 1.5 °C constrains emergence of aridification. Nat. Clim. Chang. 2018, 8, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Mosley, L.M. Drought impacts on the water quality of freshwater systems; review and integration. Earth-Sci. Rev. 2015, 140, 203–214. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Dai, A.; Wei, Y.; Kang, L. Drylands face potential threat under 2 °C global warming target. Nat. Clim. Chang. 2017, 7, 417–422. [Google Scholar] [CrossRef]
- Hu, W.; Ran, J.; Dong, L.; Du, Q.; Ji, M.; Yao, S.; Sun, Y.; Gong, C.; Hou, Q.; Gong, H. Aridity-driven shift in biodiversity–soil multifunctionality relationships. Nat. Commun. 2021, 12, 5350. [Google Scholar] [CrossRef]
- Shi, L.; Chen, B.; Wang, X.; Huang, M.; Qiao, C.; Wang, J.; Wang, Z. Antioxidant response to severe hypoxia in Brandt’s vole Lasiopodomys brandtii. Integr. Zool. 2022, 17, 581–595. [Google Scholar] [CrossRef]
- Perrino, E.; Signorile, G. Costa di Monopoli (Puglia): Check-list della flora vascolare. Inf. Bot. Ital. 2009, 41, 263–279. [Google Scholar]
- Galasso, G.; Domina, G.; Adorni, M.; Ardenghi, N.M.G.; Banfi, E.; Bedini, G.; Bertolli, A.; Brundu, G.; Calbi, M.; Cecchi, L. Notulae to the Italian alien vascular flora: 1. Ital. Bot. 2016, 1, 17–37. [Google Scholar] [CrossRef]
- Pokorny, L.; Riina, R.; Mairal, M.; Meseguer, A.S.; Culshaw, V.; Cendoya, J.; Serrano, M.; Carbajal, R.; Ortiz, S.; Heuertz, M. Living on the edge: Timing of Rand Flora disjunctions congruent with ongoing aridification in Africa. Front. Genet. 2015, 6, 154. [Google Scholar] [CrossRef] [Green Version]
- Fernández-González, F.; Loidi, J.; Moreno, J.C.; Del Arco, M.; Férnández-Cancio, A.; Galán, C.; García-Mozo, H.; Muñoz, J.; Pérez-Badia, R.; Sardinero, S. Impacts on plant biodiversity. In Impacts on Climatic Change in Spain; OCCE, Ministerio de Medio Ambiente: Madrid, Spain, 2005; pp. 183–248. [Google Scholar]
- Vleck, D. The energy cost of burrowing by the pocket gopher Thomomys bottae. Physiol. Zool. 1979, 52, 122–136. [Google Scholar] [CrossRef]
- Lovegrove, B. The cost of burrowing by the social mole rats (Bathyergidae) Cryptomys damarensis and Heterocephalus glaber: The role of soil moisture. Physiol. Zool. 1989, 62, 449–469. [Google Scholar] [CrossRef]
- Zelová, J.; Šumbera, R.; Okrouhlík, J.; Burda, H. Cost of digging is determined by intrinsic factors rather than by substrate quality in two subterranean rodent species. Physiol. Behav. 2010, 99, 54–58. [Google Scholar] [CrossRef]
- Van Jaarsveld, B.; Bennett, N.C.; Hart, D.W.; Oosthuizen, M.K. Locomotor activity and body temperature rhythms in the Mahali mole-rat (C. h. mahali): The effect of light and ambient temperature variations. J. Therm. Biol. 2019, 79, 24–32. [Google Scholar] [CrossRef]
- McAnulty, S.; McAnulty, L.; Pascoe, D.; Gropper, S.; Keith, R.; Morrow, J.; Gladden, L. Hyperthermia increases exercise-induced oxidative stress. Int. J. Sport. Med. 2005, 26, 188–192. [Google Scholar] [CrossRef]
- Belviranli, M.; Gökbel, H. Acute exercise induced oxidative stress and antioxidant changes. Eur. J. Gen. Med. 2006, 3, 126–131. [Google Scholar] [CrossRef]
- Spinks, A.C.; Bennett, N.C.; Jarvis, J.U. A comparison of the ecology of two populations of the common mole-rat, Cryptomys hottentotus hottentotus: The effect of aridity on food, foraging and body mass. Oecologia 2000, 125, 341–349. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Oosthuizen, M.; Mitchell, C.; Blount, J.D.; Bennett, N.C. Heat and dehydration induced oxidative damage and antioxidant defenses following incubator heat stress and a simulated heat wave in wild caught four-striped field mice Rhabdomys dilectus. PLoS ONE 2020, 15, e0242279. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. Int. J. Biometeorol. 2019, 63, 1569–1584. [Google Scholar] [CrossRef]
- França, M.; Panek, A.; Eleutherio, E. Oxidative stress and its effects during dehydration. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 621–631. [Google Scholar] [CrossRef]
- Altan, Ö.; Pabuçcuoğlu, A.; Altan, A.; Konyalioğlu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef]
- Georgescu, V.P.; de Souza Junior, T.P.; Behrens, C.; Barros, M.P.; Bueno, C.A.; Utter, A.C.; McAnulty, L.S.; McAnulty, S.R. Effect of exercise-induced dehydration on circulatory markers of oxidative damage and antioxidant capacity. Appl. Physiol. Nutr. Metab. 2017, 42, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Hillman, A.R.; Vince, R.V.; Taylor, L.; McNaughton, L.; Mitchell, N.; Siegler, J. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl. Physiol. Nutr. Metab. 2011, 36, 698–706. [Google Scholar] [CrossRef]
- King, M.A.; Clanton, T.L.; Laitano, O. Hyperthermia, dehydration, and osmotic stress: Unconventional sources of exercise-induced reactive oxygen species. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R105–R114. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [Green Version]
- Vollaard, N.B.; Shearman, J.P.; Cooper, C.E. Exercise-induced oxidative stress. Sport. Med. 2005, 35, 1045–1062. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox. Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Costantini, D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019, 222, jeb194688. [Google Scholar] [CrossRef] [Green Version]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants–A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radical in Biology and Medicine, 2nd ed.; Oxford Science Publications: Oxford, UK, 1989. [Google Scholar]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Coleman, J.C.; Downs, C.T. Variation in urine concentrating ability and water balance of the Black-tailed Tree Rat Thallomys nigricauda, along an aridity gradient. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 508–513. [Google Scholar] [CrossRef]
- Coleman, J.C.; Downs, C.T. Daily rhythms of body temperature and activity in free-living Black-tailed Tree Rats (Thallomys nigricauda) along an aridity gradient. Physiol. Behav. 2010, 99, 22–32. [Google Scholar] [CrossRef]
- Bozinovic, F.; Rojas, J.M.; Broitman, B.R.; Vásquez, R.A. Basal metabolism is correlated with habitat productivity among populations of degus (Octodon degus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 560–564. [Google Scholar] [CrossRef]
- Bozinovic, F.; Rojas, J.; Gallardo, P.; Palma, R.; Gianoli, E. Body mass and water economy in the South American olivaceous field mouse along a latitudinal gradient: Implications for climate change. J. Arid Environ. 2011, 75, 411–415. [Google Scholar] [CrossRef]
- van Jaarsveld, B.; Bennett, N.C.; Kemp, R.; Czenze, Z.J.; McKechnie, A.E. Heat tolerance in desert rodents is correlated with microclimate at inter-and intraspecific levels. J. Comp. Physiol. B 2021, 191, 575–588. [Google Scholar] [CrossRef]
- Conenna, I.; Santini, L.; Rocha, R.; Monadjem, A.; Cabeza, M.; Russo, D. Global patterns of functional trait variation along aridity gradients in bats. Glob. Ecol. Biogeogr. 2021, 30, 1014–1029. [Google Scholar] [CrossRef]
- Faulkes, C.G.; Bennett, N.C. Plasticity and constraints on social evolution in African mole-rats: Ultimate and proximate factors. Phil. Trans. R. Soc. B. 2013, 368, 20120347. [Google Scholar] [CrossRef] [Green Version]
- Faulkes, C.; Bennett, N.; Bruford, M.W.; O’brien, H.; Aguilar, G.; Jarvis, J. Ecological constraints drive social evolution in the African mole–rats. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1997, 264, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Faulkes, C.; Verheyen, E.; Verheyen, W.; Jarvis, J.; Bennett, N. Phylogeographical patterns of genetic divergence and speciation in African mole-rats (Family: Bathyergidae). Mol. Ecol. 2004, 13, 613–629. [Google Scholar] [CrossRef] [Green Version]
- McGowan, N.E.; Scantlebury, D.M.; Bennett, N.C.; Maule, A.G.; Marks, N.J. Thermoregulatory differences in African mole-rat species from disparate habitats: Responses and limitations. J. Therm. Biol. 2020, 88, 102495. [Google Scholar] [CrossRef]
- Bennett, N.C.; Faulkes, C.G. African Mole-Rats: Ecology and Eusociality; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Faulkes, C.G.; Bennett, N.C. Social evolution in African mole-rats–a comparative overview. In The Extraordinary Biology of the Naked Mole-Rat; Springer: Cham, Switzerland, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Jarvis, J.U.; O’Riain, M.J.; Bennett, N.C.; Sherman, P.W. Mammalian eusociality: A family affair. Trends Ecol. Evol. 1994, 9, 47–51. [Google Scholar] [CrossRef]
- Roper, T.; Bennett, N.; Conradt, L.; Molteno, A. Environmental conditions in burrows of two species of African mole-rat, Georhychus capensis and Cryptomys damarensis. J. Zool. 2001, 254, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Ivy, C.M.; Sprenger, R.J.; Bennett, N.C.; van Jaarsveld, B.; Hart, D.W.; Kirby, A.M.; Yaghoubi, D.; Storey, K.B.; Milsom, W.K.; Pamenter, M.E. The hypoxia tolerance of eight related African mole-rat species rivals that of naked mole-rats, despite divergent ventilatory and metabolic strategies in severe hypoxia. Acta Physiol. 2020, 228, e13436. [Google Scholar] [CrossRef]
- Logan, S.M.; Szereszewski, K.E.; Bennett, N.C.; Hart, D.W.; Van Jaarsveld, B.; Pamenter, M.E.; Storey, K.B. The brains of six African mole-rat species show divergent responses to hypoxia. J. Exp. Biol. 2020, 223, jeb215905. [Google Scholar] [CrossRef]
- Burda, H.; Šumbera, R.; Begall, S. Microclimate in burrows of subterranean rodents—Revisited. In Subterranean Rodents; Springer: Berlin/Heidelberg, Germany, 2007; pp. 21–33. [Google Scholar]
- Pamenter, M.E. Adaptations to a hypoxic lifestyle in naked mole-rats. J. Exp. Biol. 2022, 225, jeb196725. [Google Scholar] [CrossRef]
- Šumbera, R. Thermal biology of a strictly subterranean mammalian family, the African mole-rats (Bathyergidae, Rodentia)-a review. J. Therm. Biol. 2019, 79, 166–189. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.; Clarke, B.; Jarvis, J. A comparison of metabolic acclimation in two species of social mole-rats (Rodentia, Bathyergidae) in southern Africa. J. Arid Environ. 1992, 23, 189–198. [Google Scholar] [CrossRef]
- Wallace, K.M.; van Jaarsveld, B.; Bennett, N.C.; Hart, D.W. The joint effect of micro-and macro-climate on the thermoregulation and heat dissipation of two African mole-rat (Bathyergidae) sub-species, Cryptomys hottentotus mahali and C. h. pretoriae. J. Therm. Biol. 2021, 99, 103025. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.; Bennett, N.; Oosthuizen, M.; Waterman, J.; Hambly, C.; Scantlebury, D. Energetics and water flux in the subterranean rodent family Bathyergidae. Front. Ecol. Evol. 2022, 10, 867350. [Google Scholar] [CrossRef]
- Bennett, N.; Aguilar, G.; Jarvis, J.; Faulkes, C. Thermoregulation in three species of Afrotropical subterranean mole-rats (Rodentia: Bathyergidae) from Zambia and Angola and scaling within the genus Cryptomys. Oecologia 1994, 97, 222–227. [Google Scholar] [CrossRef]
- Bennett, N.; Jarvis, J. Coefficients of digestibility and nutritional values of geophytes and tubers eaten by southern African mole-rats (Rodentia: Bathyergidae). J. Zool. 1995, 236, 189–198. [Google Scholar] [CrossRef]
- Spinks, A.C.; Branch, T.A.; Croeser, S.; Bennett, N.C.; Jarvis, J.U. Foraging in wild and captive colonies of the common mole-rat Cryptomys hottentotus hottentotus (Rodentia: Bathyergidae). J. Zool. 1999, 249, 143–152. [Google Scholar] [CrossRef]
- Jarvis, J.U.; Bennett, N.C.; Spinks, A.C. Food availability and foraging by wild colonies of Damaraland mole-rats (Cryptomys damarensis): Implications for sociality. Oecologia 1998, 113, 290–298. [Google Scholar] [CrossRef]
- Lovegrove, B.; Painting, S. Variations in the foraging behaviour and burrow structures of the Damara molerat Cryptomys damarensis in the Kalahari Gemsbok National Park. Koedoe 1987, 30, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Okrouhlík, J.; Burda, H.; Kunc, P.; Knížková, I.; Šumbera, R. Surprisingly low risk of overheating during digging in two subterranean rodents. Physiol. Behav. 2015, 138, 236–241. [Google Scholar] [CrossRef]
- Hart, D.W.; van Jaarsveld, B.; Lasch, K.G.; Grenfell, K.L.; Oosthuizen, M.K.; Bennett, N.C. Ambient Temperature as a Strong Zeitgeber of Circadian Rhythms in Response to Temperature Sensitivity and Poor Heat Dissipation Abilities in Subterranean African Mole-Rats. J. Biol. Rhythm. 2021, 36, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Finn, K.T.; Janse van Vuuren, A.K.; Hart, D.W.; Süess, T.; Zöttl, M.; Bennett, N. Seasonal changes in locomotor activity patterns of wild social Natal mole-rats (Cryptomys hottentotus natalensis). Front. Ecol. Evol. 2022, 10, 819393. [Google Scholar] [CrossRef]
- McNab, B.K. The metabolism of fossorial rodents: A study of convergence. Ecology 1966, 47, 712–733. [Google Scholar] [CrossRef]
- Contreras, L.; McNab, B. Thermoregulation and energetics in subterranean mammals. Prog. Clin. Biol. Res. 1990, 335, 231–250. [Google Scholar]
- Lovegrove, B. Thermoregulation in the subterranean rodent Georychus capensis (Rodentia: Bathyergidae). Physiol. Zool. 1987, 60, 174–180. [Google Scholar] [CrossRef]
- Thirumalai, T.; Therasa, S.V.; Elumalai, E.; David, E. Intense and exhaustive exercise induce oxidative stress in skeletal muscle. Asian Pac. J. Trop. Dis. 2011, 1, 63–66. [Google Scholar] [CrossRef]
- Gündüz, F.; Şentürk, Ü.K. The effect of reactive oxidant generation in acute exercise-induced proteinuria in trained and untrained rats. Eur. J. Appl. Physiol. 2003, 90, 526–532. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lin, T.-J.; Lu, Y.-F.; Chen, C.-C.; Huang, C.-Y.; Lin, W.-T. Protective effects of L-arginine supplementation against exhaustive exercise-induced oxidative stress in young rat tissues. Chin. J. Physiol. 2009, 52, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.L.; Fu, R. Responses of glutathione system and antioxidant enzymes to exhaustive exercise and hydroperoxide. J. Appl. Physiol. 1992, 72, 549–554. [Google Scholar] [CrossRef]
- Liu, J.; Yeo, H.C.; Overvik-Douki, E.; Hagen, T.; Doniger, S.J.; Chu, D.W.; Brooks, G.A.; Ames, B.N. Chronically and acutely exercised rats: Biomarkers of oxidative stress and endogenous antioxidants. J. Appl. Physiol. 2000, 89, 21–28. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Mitochondria in exercise-induced oxidative stress. Neurosignals 2001, 10, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Nayanatara, A.; Nagaraja, H.; Anupama, B. The effect of repeated swimming stress on organ weights and lipid peroxidation in rats. Thai J. Pharm. Sci. 2005, 18, 3–9. [Google Scholar]
- Alessio, H.M.; Hagerman, A.E.; Romanello, M.; Carando, S.; Threlkeld, M.S.; Rogers, J.; Dimitrova, Y.; Muhammed, S.; Wiley, R.L. Consumption of green tea protects rats from exercise-induced oxidative stress in kidney and liver. Nutr. Res. 2002, 22, 1177–1188. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Vargas, N.T.; Hostler, D.; Parker, M.D.; Schlader, Z.J. Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. J. Appl. Physiol. 2020, 128, 715–728. [Google Scholar] [CrossRef]
- Pryor, R.R.; Pryor, J.L.; Vandermark, L.W.; Adams, E.L.; Brodeur, R.M.; Schlader, Z.J.; Armstrong, L.E.; Lee, E.C.; Maresh, C.M.; Casa, D.J. Acute kidney injury biomarker responses to short-term heat acclimation. Int. J. Environ. Res. Public Health 2020, 17, 1325. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.L.; Johnson, B.D.; Parker, M.D.; Hostler, D.; Pryor, R.R.; Schlader, Z. Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature 2021, 8, 108–159. [Google Scholar] [CrossRef]
- Bongers, C.C.; Alsady, M.; Nijenhuis, T.; Tulp, A.D.; Eijsvogels, T.M.; Deen, P.M.; Hopman, M.T. Impact of acute versus prolonged exercise and dehydration on kidney function and injury. Physiol. Rep. 2018, 6, e13734. [Google Scholar] [CrossRef]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Salo, D.C.; Donovan, C.M.; Davies, K.J. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic. Biol. Med. 1991, 11, 239–246. [Google Scholar] [CrossRef]
- King, M.A.; Leon, L.R.; Mustico, D.L.; Haines, J.M.; Clanton, T.L. Biomarkers of multiorgan injury in a preclinical model of exertional heat stroke. J. Appl. Physiol. 2015, 118, 1207–1220. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, S.; Ryan, A.; Gisolfi, C.; Moseley, P. Tissue-specific HSP70 response in animals undergoing heat stress. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 268, R28–R32. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Frøkiær, J.; Marples, D.; Kwon, T.-H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef] [PubMed]
- Segar, W.E. Chronic Hyperosmolality: A Condition Resulting From Absence of Thirst, Defective Osmoregulation, and Limited Ability to Concentrate Urine. Am. J. Dis. Child. 1966, 112, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Rodríguez, J.A.; Valenzuela-Soto, E.M. Enzymes involved in osmolyte synthesis: How does oxidative stress affect osmoregulation in renal cells? Life Sci. 2010, 87, 515–520. [Google Scholar] [CrossRef]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sport. Nutr. 2005, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Colantoni, A.; Delfanti, L.; Cossio, F.; Baciotti, B.; Salvati, L.; Perini, L.; Lord, R. Soil aridity under climate change and implications for agriculture in Italy. Appl. Math. Sci. 2015, 9, 2467–2475. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H. ERA5-Land: A state-of-the-art global reanalysis dataset for land applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- Romanenko, V. Computation of the autumn soil moisture using a universal relationship for a large area. Proc. Ukr. Hydrometeorol. Res. Inst. 1961, 3, 12–25. [Google Scholar]
- Hickman, G. A live-trap and trapping technique for fossorial mammals. S. Afr. J. Zool. 1979, 14, 9–12. [Google Scholar] [CrossRef]
- Bennett, N.C.; Faulkes, C.G.; Molteno, A.J. Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: Two components to a lifetime of socially induced infertility. Proc. Biol. Sci. 1996, 263, 1599–1603. [Google Scholar] [CrossRef]
- Bennett, N.C.; Ganswindt, A.; Ganswindt, S.B.; Jarvis, J.; Zöttl, M.; Faulkes, C. Evidence for contrasting roles for prolactin in eusocial naked mole-rats, Heterocephalus glaber and Damaraland mole-rats, Fukomys damarensis. Biol. Lett. 2018, 14, 20180150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, D.W.; Medger, K.; van Jaarsveld, B.; Bennett, N.C. Filling in the holes: The reproductive biology of the understudied Mahali mole-rat (Cryptomys hottentotus mahali). Can. J. Zool. 2021, 99, 801–811. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Finn, K.T.; van Vuuren, A.K.J.; Suess, T.; Hart, D.W.; Bennett, N.C. Defining the link between oxidative stress, behavioural reproductive suppression and heterothermy in the Natal mole-rat (Cryptomys hottentotus natalensis). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 261, 110753. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.J.; Hart, D.W.; Bennett, N.C. Plasma oxidative stress in reproduction of two eusocial African mole-rat species, the naked mole-rat and the Damaraland mole-rat. Front. Zool. 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.; Jarvis, J.; Wallace, D. The relative age structure and body masses of complete wild-captured colonies of two social mole-rats, the common mole-rat, Cryptomys hottentotus hottentotus and the Damaraland mole-rat, Cryptomys damarensis. J. Zool. 1990, 220, 469–485. [Google Scholar] [CrossRef]
- Golden, T.R.; Hinerfeld, D.A.; Melov, S. Oxidative stress and aging: Beyond correlation. Aging Cell 2002, 1, 117–123. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57, 715S–725S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunca, U.; Saygin, M.; Ozmen, O.; Aslankoc, R.; Yalcin, A. The impact of moderate-intensity swimming exercise on learning and memory in aged rats: The role of Sirtuin-1. Iran. J. Basic Med. Sci. 2021, 24, 1413. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncu, Ş.; Yilmaz, B.; Demir, M.; Tuncer, M.; Bozdağ, Z.; İnce, O.; Bozdayi, M.A.; Ulusal, H.; Taysi, S. Melatonin attenuates ovarian ischemia reperfusion injury in rats by decreasing oxidative stress index and peroxynitrite. Turk. J. Med. Sci. 2020, 50, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 10 November 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Package ‘lmertest’; R Package Version. 2015, Volume 2, p. 734. Available online: http://cran.uib.no/web/packages/lmerTest/lmerTest.pdf (accessed on 10 November 2022).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package Version. 2018, Volume 1, p. 3. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 10 November 2022).
- Francioli, Y.; Thorley, J.; Finn, K.; Clutton-Brock, T.; Zöttl, M. Breeders are less active foragers than non-breeders in wild Damaraland mole-rats. Biol. Lett. 2020, 16, 20200475. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, T.; Ilyukha, V.; Baishnikova, I.; Belkin, V.; Sergina, S.; Antonova, E. Antioxidant defense system in tissues of semiaquatic mammals. J. Evol. Biochem. Physiol. 2017, 53, 282–288. [Google Scholar] [CrossRef]
- Kumar, S. Free radicals and antioxidants: Human and food system. Adv. Appl. Sci. Res. 2011, 2, 129–135. [Google Scholar]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular antioxidant activity of common vegetables. J. Agric. Food Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Sachdeva, M.; Karan, M.; Singh, T.; Dhingra, S. Oxidants and antioxidants in complementary and alternative medicine: A review. Spatula DD 2014, 4, 1–16. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-J.; Liu, H.-B.; Wang, P.; Guan, H.-S. A study on the antioxidant activity and tissues selective inhibition of lipid peroxidation by saponins from the roots of Platycodon grandiflorum. Am. J. Chin. Med. 2009, 37, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Mandal, A.; Chanda, D.; Chakraborti, S. Oxidant, antioxidant and physical exercise. Mol. Cell. Biochem. 2003, 253, 307–312. [Google Scholar] [CrossRef]
- Ji, L.L. Exercise and oxidative stress: Role of the cellular antioxidant systems. Exerc. Sport Sci. Rev. 1995, 23, 135–166. [Google Scholar] [CrossRef] [PubMed]
- Robb, G.; Harrison, A.; Woodborne, S.; Bennett, N. Diet composition of two common mole-rat populations in arid and mesic environments in South Africa as determined by stable isotope analysis. J. Zool. 2016, 300, 257–264. [Google Scholar] [CrossRef]
- Daniels, C.; Rautenbach, F.; Marnewick, J.L.; Valentine, A.; Babajide, O.; Mabusela, W. Environmental stress effect on the phytochemistry and antioxidant activity of a South African bulbous geophyte, Gethyllis multifolia L. Bolus. S. Afr. J. Bot. 2015, 96, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Snijman, D. Gethyllis L. (Amaryllidaceae). Available online: http://opus.sanbi.org/bitstream/20.500.12143/3456/1/Gethyllis_PlantzAfrica.pdf (accessed on 3 November 2022).
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [Green Version]
- Talbi, S.; Romero-Puertas, M.C.; Hernández, A.; Terrón, L.; Ferchichi, A.; Sandalio, L.M. Drought tolerance in a Saharian plant Oudneya africana: Role of antioxidant defences. Environ. Exp. Bot. 2015, 111, 114–126. [Google Scholar] [CrossRef]
- Reed, M.C.; Thomas, R.L.; Pavisic, J.; James, S.J.; Ulrich, C.M.; Nijhout, H.F. A mathematical model of glutathione metabolism. Theor. Biol. Med. Model. 2008, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Costantini, D.; Verhulst, S. Does high antioxidant capacity indicate low oxidative stress? Funct. Ecol. 2009, 23, 506–509. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Marklund, S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984, 222, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.M.; Blount, J.D.; Bennett, N.C. Reproduction is associated with a tissue-dependent reduction of oxidative stress in eusocial female Damaraland mole-rats (Fukomys damarensis). PLoS ONE 2014, 9, e103286. [Google Scholar] [CrossRef] [PubMed]
- Iman, M.M. Effect of aspartame on some oxidative stress parameters in liver and kidney of rats. Afr. J. Pharm. Pharm. 2011, 5, 678–682. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Kaur, J. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin. Chim. Acta 2003, 333, 69–77. [Google Scholar] [CrossRef]
- Liu, J.; Cui, H.; Liu, X.; Peng, X.; Deng, J.; Zuo, Z.; Cui, W.; Deng, Y.; Wang, K. Dietary high vanadium causes oxidative damage-induced renal and hepatic toxicity in broilers. Biol. Trace Elem. Res. 2012, 145, 189–200. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Geihs, M.A.; França, T.F.; Moreira, D.C.; Hermes-Lima, M. Is “preparation for oxidative stress” a case of physiological conditioning hormesis? Front. Physiol. 2018, 9, 945. [Google Scholar] [CrossRef] [Green Version]
- Hood, W.; Zhang, Y.; Mowry, A.; Hyatt, H.; Kavazis, A. Life history trade-offs within the context of mitochondrial hormesis. Integr. Comp. Biol. 2018, 58, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Costantini, D. Variation in oxidative stress threats and hormesis across environments. In Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 75–109. [Google Scholar]
- Luna–López, A.; González-Puertos, V.Y.; López-Diazguerrero, N.E.; Königsberg, M. New considerations on hormetic response against oxidative stress. J. Cell Commun. Signal. 2014, 8, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Urison, N.; Buffenstein, R. Kidney concentrating ability of a subterranean xeric rodent, the naked mole-rat (Heterocephalus glaber). J. Comp. Physiol. B 1994, 163, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Sánchez-Lozada, L.G.; Newman, L.S.; Lanaspa, M.A.; Diaz, H.F.; Lemery, J.; Rodriguez-Iturbe, B.; Tolan, D.R.; Butler-Dawson, J.; Sato, Y. Climate change and the kidney. Ann. Nutr. Metab. 2019, 74, 38–44. [Google Scholar] [CrossRef]
- Kültz, D. Hyperosmolality triggers oxidative damage in kidney cells. Proc. Natl. Acad. Sci. USA 2004, 101, 9177–9178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.G.; Smith, D.W.; Lee, C.J.; Ngo, J.P.; Gardiner, B.S. What makes the kidney susceptible to hypoxia? Anat. Rec. 2020, 303, 2544–2552. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcerczyk, A.; Bartosz, G. Thiols are main determinants of total antioxidant capacity of cellular homogenates. Free Radic. Res. 2003, 37, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Bharti, V.K.; Srivastava, R.; Kumar, H.; Bag, S.; Majumdar, A.; Singh, G.; Pandi-Perumal, S.; Brown, G.M. Effects of melatonin and epiphyseal proteins on fluoride-induced adverse changes in antioxidant status of heart, liver, and kidney of rats. Adv. Pharmacol. Sci. 2014, 2014, 532969. [Google Scholar] [CrossRef] [Green Version]
- Lauterburg, B.H.; Adams, J.D.; Mitchell, J.R. Hepatic glutathione homeostasis in the rat: Efflux accounts for glutathione turnover. Hepatology 1984, 4, 586–590. [Google Scholar] [CrossRef]
- Hellsten, Y.; Svensson, M.; Sjödin, B.; Smith, S.; Christensen, A.; Richter, E.; Bangsbo, J. Allantoin formation and urate and glutathione exchange in human muscle during submaximal exercise. Free. Radic. Biol. Med. 2001, 31, 1313–1322. [Google Scholar] [CrossRef]
- Kasapoglu, M.; Özben, T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp. Gerontol. 2001, 36, 209–220. [Google Scholar] [CrossRef]
- Tiana, L.; Caib, Q.; Wei, H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free. Radic. Biol. Med. 1998, 24, 1477–1484. [Google Scholar] [CrossRef]
- Sani, M.; Sebaï, H.; Gadacha, W.; Boughattas, N.A.; Reinberg, A.; Mossadok, B.A. Catalase activity and rhythmic patterns in mouse brain, kidney and liver. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 145, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Turner, N.; Hinde, J.; Else, P.; Guderley, H. How might you compare mitochondria from different tissues and different species? J. Comp. Physiol. B 2006, 176, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signaling transduction. Annu. Rev. Plant Biol. 2004, 55, 373. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.; Ghanem-Boughanmi, N.; Gadacha, W.; Sebai, H.; Boughattas, N.A.; Reinberg, A.; Ben-Attia, M. Malondialdehyde content and circadian variations in brain, kidney, liver, and plasma of mice. Chronobiol. Int. 2007, 24, 671–685. [Google Scholar] [CrossRef] [PubMed]


| Kidney | Variables Kept in Best Model | R2 | Adjusted R2 | F (df Regression, df Residual) | p (<0.05) |
|---|---|---|---|---|---|
| TOS | Aridity + Sex + Aridity*Sex | 0.22 | 0.06 | 1.34 (5, 24) | 0.28 |
| TAC | Aridity + Sex | 0.41 | 0.35 | 6.13 (3, 26) | 0.003 * |
| OSI | Aridity + Sex | 0.32 | 0.24 | 4.02 (3, 26) | 0.02 * |
| MDA | No variable | 0.13 | −0.15 | 0.46 (7, 22) | 0.86 |
| SOD | Age | 0.11 | 0.08 | 3.53 (1, 28) | 0.07 |
| Liver | Variables Kept in Best Model | R2 | Adjusted R2 | F (df Regression, df Residual) | p (<0.05) |
|---|---|---|---|---|---|
| TOS | No variable | 0.15 | −0.13 | 0.53 (7, 22) | 0.80 |
| TAC | No variable | 0.09 | −0.20 | 0.31 (7, 22) | 0.94 |
| OSI | No variable | 0.09 | −0.20 | 0.32 (7, 22) | 0.94 |
| MDA | No variable | 0.21 | −0.04 | 0.84 (7, 22) | 0.56 |
| SOD | Aridity + Sex + Aridity*Sex | 0.29 | 0.14 | 1.98 (5, 24) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, P.J.; Hart, D.W.; Merchant, H.N.; Janse van Vuuren, A.K.; Faulkes, C.G.; Portugal, S.J.; Van Jaarsveld, B.; Bennett, N.C. Tissue Oxidative Ecology along an Aridity Gradient in a Mammalian Subterranean Species. Antioxidants 2022, 11, 2290. https://doi.org/10.3390/antiox11112290
Jacobs PJ, Hart DW, Merchant HN, Janse van Vuuren AK, Faulkes CG, Portugal SJ, Van Jaarsveld B, Bennett NC. Tissue Oxidative Ecology along an Aridity Gradient in a Mammalian Subterranean Species. Antioxidants. 2022; 11(11):2290. https://doi.org/10.3390/antiox11112290
Chicago/Turabian StyleJacobs, Paul J., Daniel W. Hart, Hana N. Merchant, Andries K. Janse van Vuuren, Chris G. Faulkes, Steven J. Portugal, Barry Van Jaarsveld, and Nigel C. Bennett. 2022. "Tissue Oxidative Ecology along an Aridity Gradient in a Mammalian Subterranean Species" Antioxidants 11, no. 11: 2290. https://doi.org/10.3390/antiox11112290