Multi-Modal Synergistic 99mTc-TRODAT-1 SPECT and MRI for Evaluation of the Efficacy of Hyperbaric Oxygen Therapy in CO-Induced Delayed Parkinsonian and Non-Parkinsonian Syndromes
Abstract
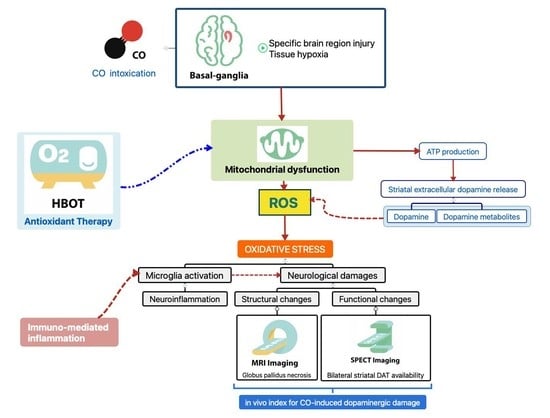
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Study Design
2.3. Hyperbaric Oxygen Therapy
2.4. Imaging Studies
2.4.1. TC-99m TRODAT-1 Brain SPECT
2.4.2. Brain MRI
2.5. Clinical Diagnosis of Parkinsonism
2.6. Statistical Analysis
3. Results
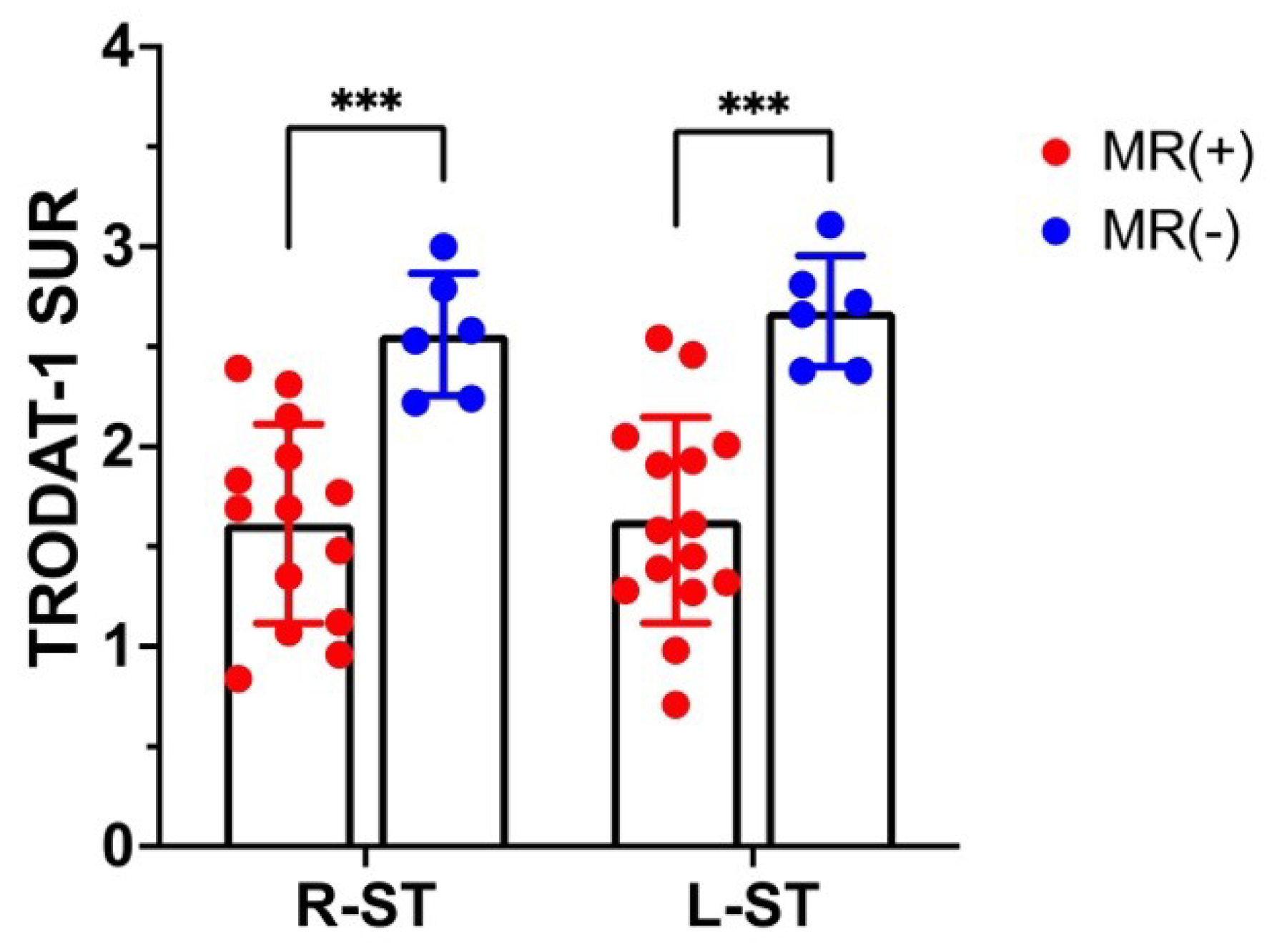
3.1. 99mTC-TRODAT-1 SPECT DAT Availability Correlates with MR Globus Pallidus Lesions
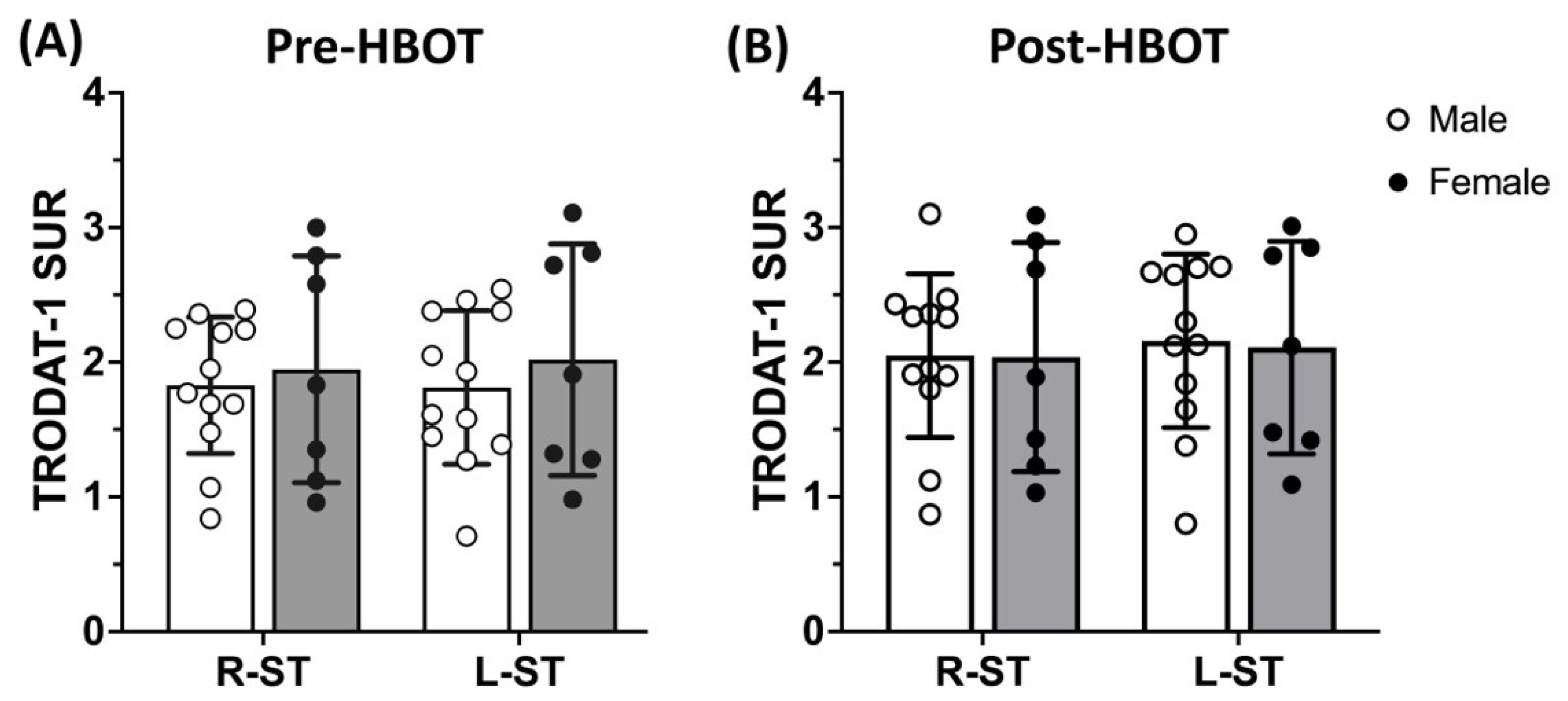
3.2. 99mTC-TRODAT-1 SPECT DAT Availability Is Significantly Reduced in Parkinsonism Syndrome and HBOT Has Limited Effects
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chenoweth, J.A.; Albertson, T.E.; Greer, M.R. Carbon Monoxide Poisoning. Crit. Care Clin. 2021, 37, 657–672. [Google Scholar] [CrossRef]
- National Center for Environmental Health. Carbon Monoxide Poisoning. Available online: https://www.cdc.gov/co/default.htm (accessed on 15 August 2021).
- Long, J.; Sun, Y.; Zhao, J.; Liu, J.; Peng, X. Temporal trends of carbon monoxide poisoning mortality at the global, regional and national levels: A cross-sectional study from the Global Burden of Disease study, 1990 and 2017. BMJ Open 2021, 11, e053240. [Google Scholar] [CrossRef] [PubMed]
- Akyol, S.; Erdogan, S.; Idiz, N.; Celik, S.; Kaya, M.; Ucar, F.; Dane, S.; Akyol, O. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: An in-depth analysis. Redox Rep. 2014, 19, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, S.; Mukai, T.; Kurosaki, K.; Kuriiwa, F.; Endo, T. Characterization of hydroxyl radical generation in the striatum of free-moving rats due to carbon monoxide poisoning, as determined by in vivo microdialysis. Brain Res. 2004, 1016, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Omaye, S.T. Metabolic modulation of carbon monoxide toxicity. Toxicology 2002, 180, 139–150. [Google Scholar] [CrossRef]
- Pepe, G.; Castelli, M.; Nazerian, P.; Vanni, S.; Del Panta, M.; Gambassi, F.; Botti, P.; Missanelli, A.; Grifoni, S. Delayed neuropsychological sequelae after carbon monoxide poisoning: Predictive risk factors in the Emergency Department. A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.I.; Changlai, S.P.; Huang, W.S.; Tsai, C.H.; Lee, C.C.; Kao, C.H. Usefulness of 99mTc ethyl cysteinate dimer brain SPECT to detect abnormal regional cerebral blood flow in patients with acute carbon monoxide poisoning. Nucl. Med. Commun. 2003, 24, 1185–1188. [Google Scholar] [CrossRef]
- Tibbles, P.M.; Perrotta, P.L. Treatment of carbon monoxide poisoning: A critical review of human outcome studies comparing normobaric oxygen with hyperbaric oxygen. Ann. Emerg. Med. 1994, 24, 269–276. [Google Scholar] [CrossRef]
- Oh, S.; Choi, S.C. Acute carbon monoxide poisoning and delayed neurological sequelae: A potential neuroprotection bundle therapy. Neural Regen. Res. 2015, 10, 36–38. [Google Scholar] [CrossRef]
- Mujahid, M.; Shaikh, S.; Dredar, A. Delayed Neuropsychiatric Syndrome in a Patient with Intentional Carbon Monoxide Intoxication. In Proceedings of the Hospital Medicine Meeting, San Diego, CA, USA, 6–9 March 2016. [Google Scholar]
- Choi, I.S. Parkinsonism after carbon monoxide poisoning. Eur. Neurol. 2002, 48, 30–33. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 2018, 46 (Suppl. 1), S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.L.; Maynard, R.L. Effects on health of prolonged exposure to low concentrations of carbon monoxide. Occup. Environ. Med. 2002, 59, 708–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, M.A.; Carraway, M.S.; Piantadosi, C.A.; Reynolds, C.M.; Cherry, A.D.; Wester, T.E.; Natoli, M.J.; Massey, E.W.; Moon, R.E.; Suliman, H.B. Carbon monoxide, skeletal muscle oxidative stress, and mitochondrial biogenesis in humans. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H392–H399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, J.J.; Wang, L.; Xu, Q.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am. J. Respir. Crit. Care Med. 2017, 195, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Bhopale, V.M.; Fisher, D.; Zhang, J.; Gimotty, P. Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc. Natl. Acad. Sci. USA 2004, 101, 13660–13665. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Bhopale, V.M.; Fisher, D. Hyperbaric oxygen reduces delayed immune-mediated neuropathology in experimental carbon monoxide toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 152–159. [Google Scholar] [CrossRef]
- Chen, R.J.; Lee, Y.H.; Chen, T.H.; Chen, Y.Y.; Yeh, Y.L.; Chang, C.P.; Huang, C.C.; Guo, H.R.; Wang, Y.J. Carbon monoxide-triggered health effects: The important role of the inflammasome and its possible crosstalk with autophagy and exosomes. Arch. Toxicol. 2021, 95, 1141–1159. [Google Scholar] [CrossRef]
- Jayanti, S.; Moretti, R.; Tiribelli, C.; Gazzin, S. Bilirubin and inflammation in neurodegenerative and other neurological diseases. Neuroimmunol. Neuroinflamm. 2020, 7, 92–108. [Google Scholar] [CrossRef]
- Mazzoni, P.; Shabbott, B.; Cortes, J.C. Motor control abnormalities in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009282. [Google Scholar] [CrossRef] [Green Version]
- Park, H.K.; Chung, S.J. New perspective on parkinsonism in frontotemporal lobar degeneration. J. Mov. Disord. 2013, 6, 1–8. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The Influence of Vicarious Fear-Learning in “Infecting” Reactive Action Inhibition. Front. Behav. Neurosci. 2022, 16, 946263. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e)motion: Reactive action inhibition when facing valence-independent emotional stimuli. Front. Behav. Neurosci. 2022, 16, 998714. [Google Scholar] [CrossRef] [PubMed]
- Rawji, V.; Rocchi, L.; Foltynie, T.; Rothwell, J.C.; Jahanshahi, M. Ropinirole, a dopamine agonist with high D3 affinity, reduces proactive inhibition: A double-blind, placebo-controlled study in healthy adults. Neuropharmacology 2020, 179, 108278. [Google Scholar] [CrossRef]
- Kim, S.K.; Joe, Y.; Chen, Y.; Ryu, J.; Lee, J.H.; Cho, G.J.; Ryter, S.W.; Chung, H.T. Carbon monoxide decreases interleukin-1beta levels in the lung through the induction of pyrin. Cell. Mol. Immunol. 2017, 14, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, C.P.; Chen, S.Y.; Chou, M.C.; Wang, C.Y.; Lee, K.W.; Hsueh, C.J.; Chen, C.Y.; Huang, K.L.; Huang, G.S. Diffusion-tensor MR imaging for evaluation of the efficacy of hyperbaric oxygen therapy in patients with delayed neuropsychiatric syndrome caused by carbon monoxide inhalation. Eur. J. Neurol. 2007, 14, 777–782. [Google Scholar] [CrossRef]
- Kao, H.W.; Cho, N.Y.; Hsueh, C.J.; Chou, M.C.; Chung, H.W.; Liou, M.; Chiang, S.W.; Chen, S.Y.; Juan, C.J.; Huang, G.S.; et al. Delayed parkinsonism after CO intoxication: Evaluation of the substantia nigra with inversion-recovery MR imaging. Radiology 2012, 265, 215–221. [Google Scholar] [CrossRef]
- Yang, K.C.; Wang, S.J.; Hsieh, W.C.; Lirng, J.F.; Yang, C.C.; Deng, J.F.; Lin, C.L.; Chou, Y.H. Longitudinal changes in the dopamine transporter and cognition in suicide attempters with charcoal burning. Psychiatry Res. 2015, 231, 160–167. [Google Scholar] [CrossRef]
- Sun, T.K.; Chen, Y.Y.; Huang, S.H.; Hsu, S.W.; Lee, C.C.; Chang, W.N.; Huang, C.W.; Lui, C.C.; Lien, C.Y.; Cheng, J.L.; et al. Neurotoxicity of carbon monoxide targets caudate-mediated dopaminergic system. Neurotoxicology 2018, 65, 272–279. [Google Scholar] [CrossRef]
- Weaver, L.K.; Hopkins, R.O.; Chan, K.J.; Churchill, S.; Elliott, C.G.; Clemmer, T.P.; Orme, J.F., Jr.; Thomas, F.O.; Morris, A.H. Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 2002, 347, 1057–1067. [Google Scholar] [CrossRef]
- Liao, S.C.; Shao, S.C.; Yang, K.J.; Yang, C.C. Real-world effectiveness of hyperbaric oxygen therapy for delayed neuropsychiatric sequelae after carbon monoxide poisoning. Sci. Rep. 2021, 11, 19212. [Google Scholar] [CrossRef]
- Huang, C.C.; Ho, C.H.; Chen, Y.C.; Lin, H.J.; Hsu, C.C.; Wang, J.J.; Su, S.B.; Guo, H.R. Hyperbaric Oxygen Therapy Is Associated With Lower Short- and Long-Term Mortality in Patients With Carbon Monoxide Poisoning. Chest 2017, 152, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Chadda, K.; Gajdos, P.; Jars-Guincestre, M.C.; Chevret, S.; Raphael, J.C. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: Two randomized controlled trials. Intensive Care Med. 2011, 37, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Todani, M.; Kaneda, K.; Suzuki, S.; Wakai, S.; Kikuta, S.; Sasaki, S.; Hattori, N.; Yagishita, K.; Kuwata, K.; et al. Use of hyperbaric oxygen therapy for preventing delayed neurological sequelae in patients with carbon monoxide poisoning: A multicenter, prospective, observational study in Japan. PLoS ONE 2021, 16, e0253602. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Si, Z.; Liu, J.; Lin, Y.; Yuan, J.; Xu, S.; He, Y.; Zhang, T.; Wang, A. Clinical and Imaging Prognosis in Patients with Delayed Encephalopathy after Acute Carbon Monoxide Poisoning. Behav. Neurol. 2020, 2020, 1719360. [Google Scholar] [CrossRef]
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I.; Brooks, D.J.; Darcourt, J.; Dickson, J.C.; Douglas, D.; et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1885–1912. [Google Scholar] [CrossRef]
- Huang, W.S.; Lin, S.Z.; Lin, J.C.; Wey, S.P.; Ting, G.; Liu, R.S. Evaluation of early-stage Parkinson’s disease with 99mTc-TRODAT-1 imaging. J. Nucl. Med. 2001, 42, 1303–1308. [Google Scholar]
- Yeh, S.H.; Tsai, C.J.; Yu, T.H.; Chiang, Y.H.; Lin, S.Z.; Peng, N.J.; Huang, W.S. 99mTc-TRODAT-1 SPECT Revealed That Striatal Dopamine Transport Availability Significantly Decreases in Late Mid-Aged Healthy Taiwanese and Then Remains Stable. Clin. Nucl. Med. 2022, 47, 201–208. [Google Scholar] [CrossRef]
- Yeh, C.B.; Chou, Y.H.; Cheng, C.Y.; Lee, M.S.; Wang, J.J.; Lee, C.H.; Shiue, C.Y.; Su, T.P.; Huang, W.S. Reproducibility of brain dopamine transporter binding with Tc-99m TRODAT-1 SPECT in healthy young men. Psychiatry Res. 2012, 201, 222–225. [Google Scholar] [CrossRef]
- Chang, C.C.; Hsu, J.L.; Chang, W.N.; Huang, S.H.; Huang, C.W.; Chang, Y.T.; Chen, N.C.; Lui, C.C.; Lee, C.C.; Hsu, S.W. Metabolic Covariant Network in Relation to Nigrostriatal Degeneration in Carbon Monoxide Intoxication-Related Parkinsonism. Front. Neurosci. 2016, 10, 187. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.C.; Yang, B.H.; Lirng, J.F.; Liu, M.N.; Hu, L.Y.; Liou, Y.J.; Chan, L.A.; Chou, Y.H. Interaction of dopamine transporter and metabolite ratios underpinning the cognitive dysfunction in patients with carbon monoxide poisoning: A combined SPECT and MRS study. Neurotoxicology 2021, 82, 26–34. [Google Scholar] [CrossRef]
- Chou, M.C.; Lai, P.H.; Li, J.Y. Early white matter injuries associated with dopamine transporter dysfunction in patients with acute CO intoxication: A diffusion kurtosis imaging and Tc-99m TRODAT-1 SPECT study. Eur. Radiol. 2019, 29, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chang, W.N.; Lui, C.C.; Huang, S.H.; Lee, C.C.; Chen, C.; Wang, J.J. Clinical significance of the pallidoreticular pathway in patients with carbon monoxide intoxication. Brain 2011, 134, 3632–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondziella, D.; Danielsen, E.R.; Hansen, K.; Thomsen, C.; Jansen, E.C.; Arlien-Soeborg, P. 1H MR spectroscopy of gray and white matter in carbon monoxide poisoning. J. Neurol. 2009, 256, 970–979. [Google Scholar] [CrossRef]
- Almeida, A.S.; Figueiredo-Pereira, C.; Vieira, H.L. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front. Physiol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teksam, O.; Sabuncuoglu, S.; Girgin, G.; Ozgunes, H. Evaluation of oxidative stress and antioxidant parameters in children with carbon monoxide poisoning. Hum. Exp. Toxicol. 2019, 38, 1235–1243. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Buboltz, J.B.; Robins, M. Hyperbaric treatment of carbon monoxide toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pan, X.; Chen, C.; Huang, J.; Wei, H.; Fan, Q. Neuroprotective effect of combined therapy with hyperbaric oxygen and madopar on 6-hydroxydopamine-induced Parkinson’s disease in rats. Neurosci. Lett. 2015, 600, 220–225. [Google Scholar] [CrossRef]
- Kusuda, Y.; Takemura, A.; Nakano, M.; Ishihara, A. Mild hyperbaric oxygen inhibits the decrease of dopaminergic neurons in the substantia nigra of mice with MPTP-induced Parkinson’s disease. Neurosci. Res. 2018, 132, 58–62. [Google Scholar] [CrossRef]
- Hampson, N.B.; Piantadosi, C.A.; Thom, S.R.; Weaver, L.K. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am. J. Respir. Crit. Care Med. 2012, 186, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, H.; Fujihara, K.; Kushimoto, S.; Aoki, M. Novel clinical grading of delayed neurologic sequelae after carbon monoxide poisoning and factors associated with outcome. Neurotoxicology 2015, 48, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.T.; Lu, J.J.; Valento, M.; Bryant, S.M. Variability in hyperbaric oxygen treatment for acute carbon monoxide poisoning. Undersea Hyperb. Med. 2012, 39, 627–638. [Google Scholar] [PubMed]
- Uzun, G.; Mutluoglu, M.; Metin, S. The use of hyperbaric oxygen treatment for sudden sensorineural hearing loss in Europe. Diving Hyperb. Med. 2016, 46, 43–46. [Google Scholar] [PubMed]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D’Ausilio, A.; Borgomaneri, S. Frozen in (e)motion: How reactive motor inhibition is influenced by the emotional content of stimuli in healthy and psychiatric populations. Behav. Res. Ther. 2021, 146, 103963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, Z.; McClure, M.A.; He, L.; Mu, Q. Effect of rTMS on Parkinson’s cognitive function: A systematic review and meta-analysis. BMC Neurol. 2020, 20, 377. [Google Scholar] [CrossRef]



| Male | Female | All | |
|---|---|---|---|
| Sample size (n) | 14 | 7 | 21 |
| Age (yr) | 38.6 ± 12.5 | 37.6 ± 11.0 | 38.6 ± 11.4 |
| Initial COHb (%) | 32.6 ± 13.1 | 28.9 ± 5.3 | 30.0 ± 10.8 |
| Before HBOT (baseline) | |||
| Interval from Dx of CO to MRI (days) | 21.9 ± 18.7 | 16.0 ± 20.2 | 20.1 ± 21.9 |
| Interval from Dx of CO to TRODAT-1 (days) | 34.9 ± 23.3 | 27.7 ± 24.3 | 32.3 ± 23.3 |
| After HBOT (3-month follow-up) | |||
| Interval from Dx of CO to TRODAT-1 after HBOT (days) | 72.3 ± 28.2 | 106.3 ± 30.0 | 84.6 ± 32.3 |
| Male | Female | All | |
|---|---|---|---|
| Clinical diagnosis of DNS | |||
| DNS Symptom | 100% (14/14) | 100% (7/7) | 100% (21/21) |
| MR Findings | |||
| Incidence of Globus pallidus lesion | 78.6% (11/14) | 57.1% (4/7) | 71.4% (15/21) |
| Clinical diagnosis of Parkinsonism | |||
| Incidence of non-Parkinsonian | 25.0% (3/12) | 42.9% (3/7) | 31.6% (6/19) |
| Incidence of Parkinsonian | 75.0% (9/12) a | 57.1% (4/7) | 68.4% (13/19) a |
| Gender difference | NS | ||
| Parkinsonian correlates with MR findings | |||
| Parkinsonian with GP (+) | 81.8% (9/9) | 57.1%% (4/4) | 100.0% (13/13) |
| Parkinsonian with GP (−) | 0.0% (0/9) | 0.0% (0/4) | 0.0% (0/4) |
| Gender difference | NS |
| HC | Non-Parkinsonian (NP) | Parkinsonian | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (n) | 21 | 6 | 13 | ||||||||||||
| DAT availability | Pre-HBOT | p vs. HC | Post-HBOT | p vs. HC | p vs. pre-HBOT | Change % | Pre-HBOT | p vs. HC | p vs. non-PS | Post-HBOT | p vs. HC | p vs. pre-HBOT | p vs. non-PS | Change % | |
| Both sides of striatum | 2.36 ± 0.22 | 2.38 ± 0.55 | NS | 2.51 ± 0.49 | NS | NS | 5.74% ± 5.74% | 1.70 ± 0.60 | **** | # | 1.92 ± 0.65 | ** | NS | # | 10.46% ± 11.81% |
| Right striatum | 2.35 ± 0.48 | --- | 2.45 ± 0.48 | --- | NS | 4.56% ± 1.39% | 1.70 ± 0.60 | --- | # | 1.90 ± 0.70 | --- | NS | 0.0603 | 8.29% ± 13.30% | |
| Left striatum | 2.41 ± 0.61 | --- | 2.57 ± 0.53 | --- | NS | 6.92% ± 6.56% | 1.70 ± 0.61 | --- | # | 1.98 ± 0.67 | --- | NS | # | 12.64% ± 11.18% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, S.H.-H.; Chiu, C.-H.; Kao, H.-W.; Lin, C.-P.; Lai, Y.-H.; Huang, W.-S. Multi-Modal Synergistic 99mTc-TRODAT-1 SPECT and MRI for Evaluation of the Efficacy of Hyperbaric Oxygen Therapy in CO-Induced Delayed Parkinsonian and Non-Parkinsonian Syndromes. Antioxidants 2022, 11, 2289. https://doi.org/10.3390/antiox11112289
Yeh SH-H, Chiu C-H, Kao H-W, Lin C-P, Lai Y-H, Huang W-S. Multi-Modal Synergistic 99mTc-TRODAT-1 SPECT and MRI for Evaluation of the Efficacy of Hyperbaric Oxygen Therapy in CO-Induced Delayed Parkinsonian and Non-Parkinsonian Syndromes. Antioxidants. 2022; 11(11):2289. https://doi.org/10.3390/antiox11112289
Chicago/Turabian StyleYeh, Skye Hsin-Hsien, Chuang-Hsin Chiu, Hung-Wen Kao, Ching-Po Lin, Yu-Hus Lai, and Wen-Sheng Huang. 2022. "Multi-Modal Synergistic 99mTc-TRODAT-1 SPECT and MRI for Evaluation of the Efficacy of Hyperbaric Oxygen Therapy in CO-Induced Delayed Parkinsonian and Non-Parkinsonian Syndromes" Antioxidants 11, no. 11: 2289. https://doi.org/10.3390/antiox11112289