Left-Hemispheric Asymmetry for Object-Based Attention: an ERP Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
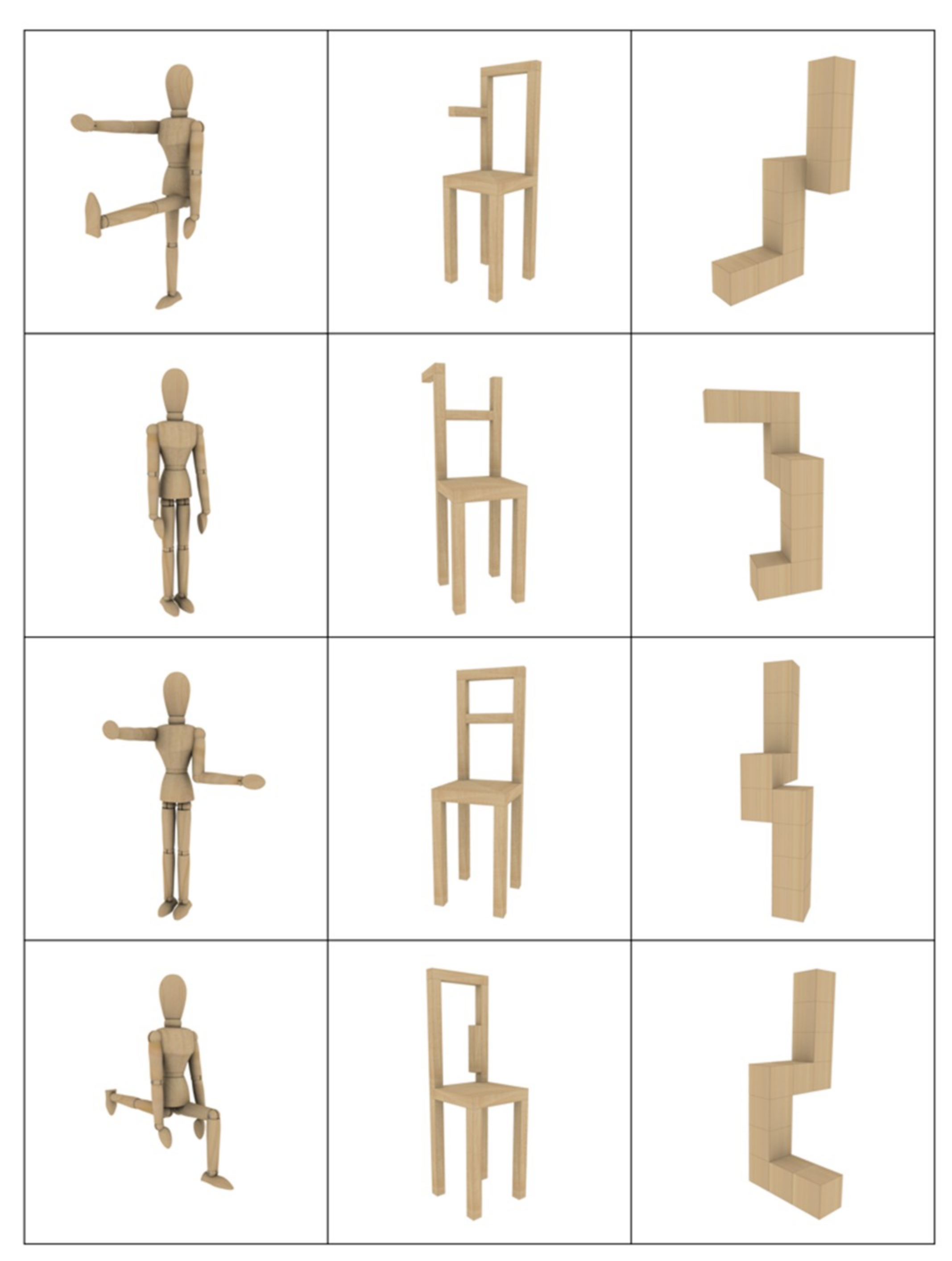
2.2. Stimuli
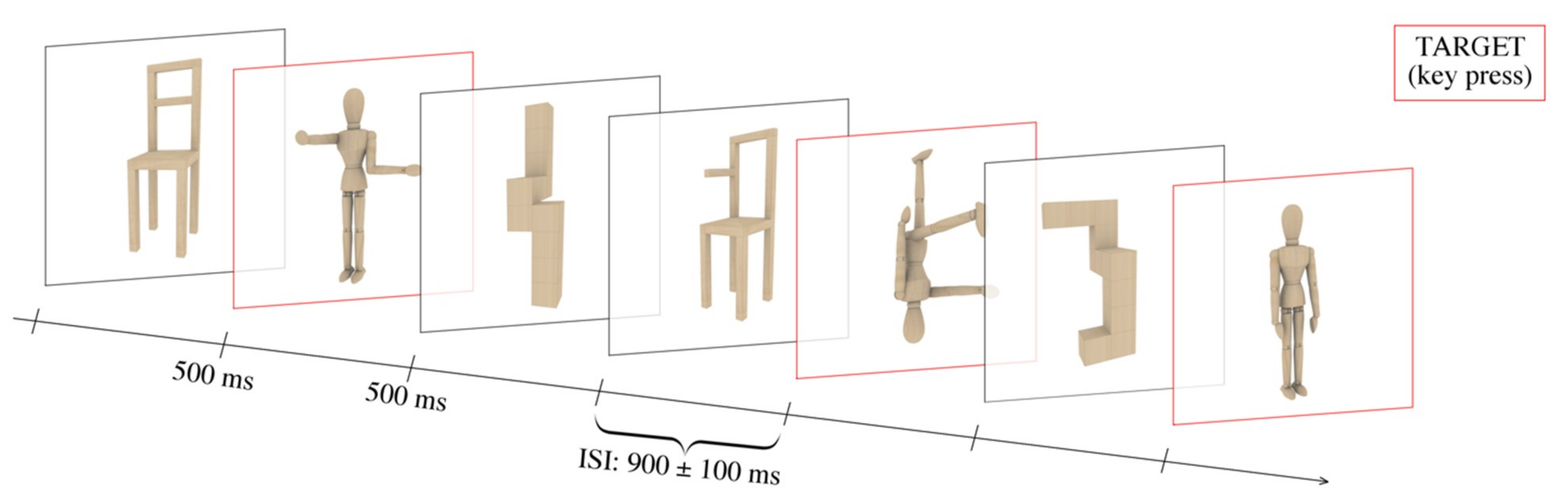
2.3. Task and Procedure
2.4. EEG Recording and Data Analysis
3. Results
3.1. Behavioral Results
3.1.1. Accuracy
3.1.2. Reaction Times
3.2. Electrophysiological Results
3.2.1. Anterior N2 (225–265 ms)
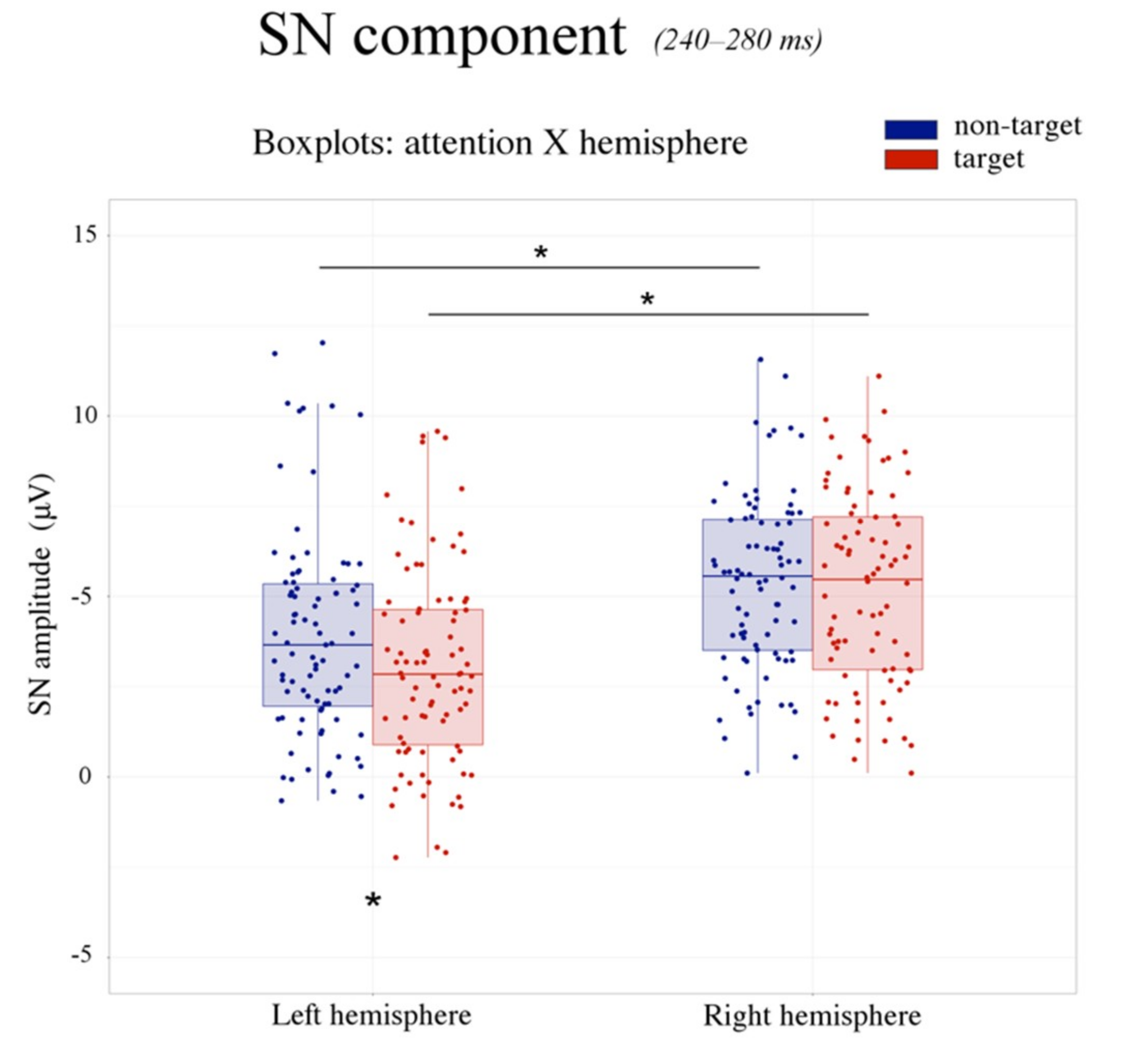
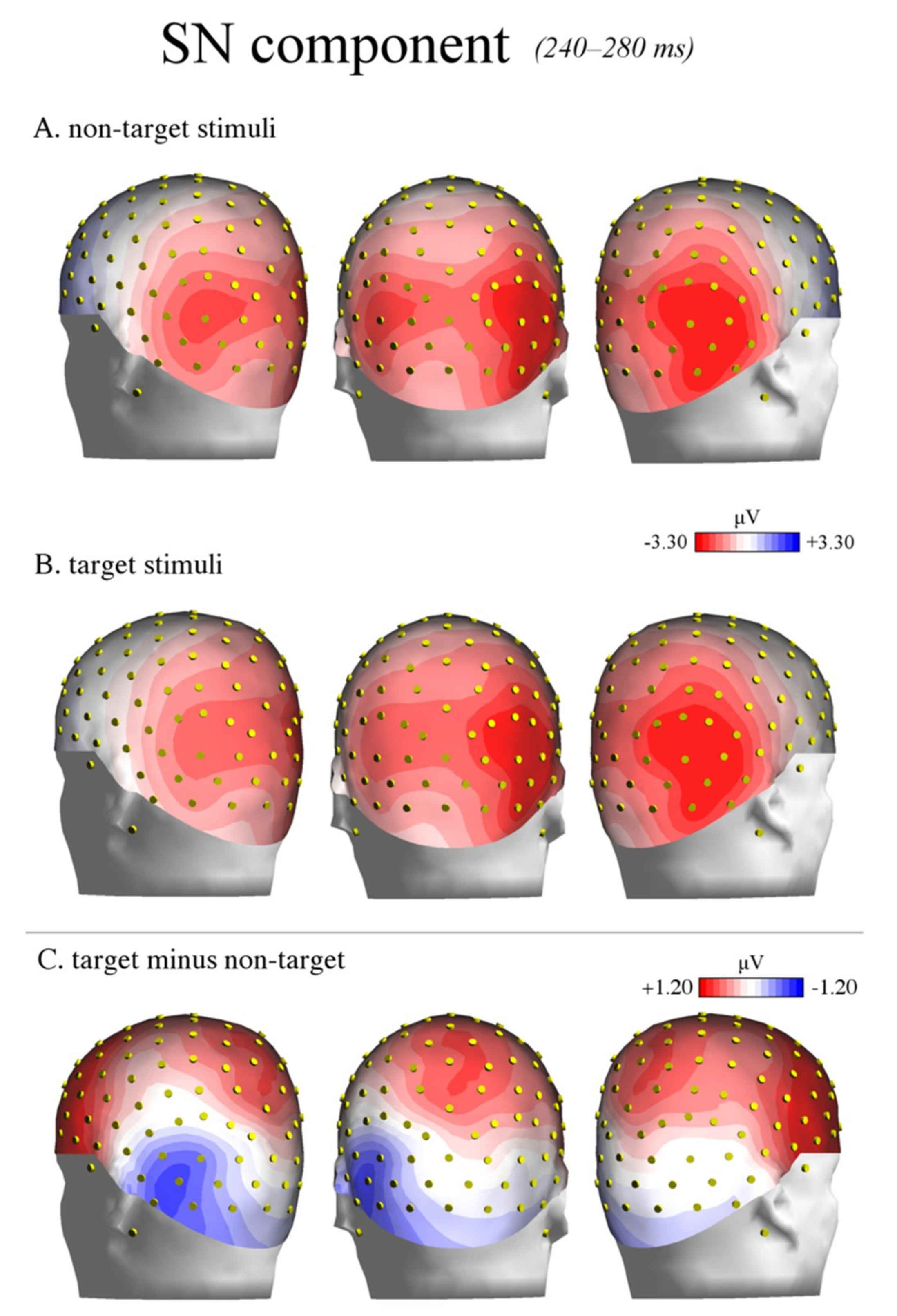
3.2.2. Selection Negativity (240–280 ms)
3.2.3. P300 (350–450 ms)
3.2.4. swLORETA Source Reconstruction (240–280 ms)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mangun, G.R. Neural mechanisms of visual selective attention. Psychophysiology 1995, 32, 4–18. [Google Scholar] [CrossRef]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef]
- O’Craven, K.M.; Downing, P.E.; Kanwisher, N. fMRI evidence for objects as the units of attentional selection. Nature 1999, 401, 584. [Google Scholar] [CrossRef] [PubMed]
- Serences, J.T.; Schwarzbach, J.; Courtney, S.M.; Golay, X.; Yantis, S. Control of object-based attention in human cortex. Cereb. Cortex 2004, 14, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Blaser, E.; Pylyshyn, Z.W.; Holcombe, A.O. Tracking an object through feature space. Nature 2000, 408, 196. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Miezin, F.M.; Dobmeyer, S.; Shulman, G.L.; Petersen, S.E. Attentional modulation of neural processing of shape, color, and velocity in humans. Science 1990, 248, 1556–1559. [Google Scholar] [CrossRef] [PubMed]
- Desimone, R.; Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995, 18, 193–222. [Google Scholar] [CrossRef]
- Schoenfeld, M.A.; Hopf, J.M.; Martinez, A.; Mai, H.M.; Sattler, C.; Gasde, A.; Heinze, H.-J.; Hillyard, S.A. Spatio-temporal analysis of feature-based attention. Cereb. Cortex 2007, 17, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201. [Google Scholar] [CrossRef]
- Hopfinger, J.B.; Buonocore, M.H.; Mangun, G.R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000, 3, 284. [Google Scholar] [CrossRef]
- Stoppel, C.M.; Boehler, C.N.; Sabelhaus, C.; Heinze, H.J.; Hopf, J.M.; Schoenfeld, M.A. Neural mechanisms of spatial-and feature-based attention: A quantitative analysis. Brain Res. 2007, 1181, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, D.R.; Nobre, A.C.; Parrish, T.B.; LaBar, K.S.; Kim, Y.H.; Meyer, J.R.; Mesulam, M.M. A large-scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain 1999, 122, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Giesbrecht, B.; Woldorff, M.G.; Song, A.W.; Mangun, G.R. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage 2003, 19, 496–512. [Google Scholar] [CrossRef]
- Baldauf, D.; Desimone, R. Neural mechanisms of object-based attention. Science 2014, 344, 424–427. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef]
- Epstein, R.; Harris, A.; Stanley, D.; Kanwisher, N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron 1999, 23, 115–125. [Google Scholar] [CrossRef]
- Grossman, E.; Donnelly, M.; Price, R.; Pickens, D.; Morgan, V.; Neighbor, G.; Blake, R. Brain areas involved in perception of biological motion. J. Cognit. Neurosci. 2000, 12, 711–720. [Google Scholar] [CrossRef]
- Corradi-Dell’Acqua, C.; Fink, G.R.; Weidner, R. Selecting category specific visual information: Top-down and bottom-up control of object based attention. Conscious. Cogn. 2015, 35, 330–341. [Google Scholar] [CrossRef]
- Liu, T. Neural representation of object-specific attentional priority. Neuroimage 2016, 129, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Valdes-Sosa, M.; Bobes, M.A.; Rodriguez, V.; Pinilla, T. Switching attention without shifting the spotlight: Object-based attentional modulation of brain potentials. J. Cognit. Neurosci. 1998, 10, 137–151. [Google Scholar] [CrossRef]
- Corbetta, M.; Kincade, M.J.; Lewis, C.; Snyder, A.Z.; Sapir, A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat. Neurosci. 2005, 8, 1603. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Spatial neglect and attention networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef] [PubMed]
- De Schotten, M.T.; Dell’Acqua, F.; Forkel, S.J.; Simmons, A.; Vergani, F.; Murphy, D.G.; Catani, M. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011, 14, 1245. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.L.; Pope, D.L.; Astafiev, S.V.; McAvoy, M.P.; Snyder, A.Z.; Corbetta, M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J. Neurosci. 2010, 30, 3640–3651. [Google Scholar] [CrossRef]
- Pardo, J.V.; Fox, P.T.; Raichle, M.E. Localization of a human system for sustained attention by positron emission tomography. Nature 1991, 349, 61. [Google Scholar] [CrossRef]
- Whitehead, R. Right hemisphere processing superiority during sustained visual attention. J. Cognit. Neurosci. 1991, 3, 329–334. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; Whang, K.; Georgopoulos, M.A.; Tagaris, G.A.; Amirikian, B.; Richter, W.; Kim, S.; Uǧurbil, K. Functional magnetic resonance imaging of visual object construction and shape discrimination: Relations among task, hemispheric lateralization, and gender. J. Cognit. Neurosci. 2001, 13, 72–89. [Google Scholar] [CrossRef]
- Milham, M.P.; Banich, M.T.; Webb, A.; Barad, V.; Cohen, N.J.; Wszalek, T.; Kramer, A.F. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognit. Brain Res. 2001, 12, 467–473. [Google Scholar] [CrossRef]
- Proverbio, A.M. Left and Right Hemisphere Role for Selective and Sustained Attention: An Electrophysiological Approach. Ph.D. Thesis, University of Padua, Padua, India, 1993. [Google Scholar]
- Proverbio, A.M.; Burco, F.; del Zotto, M.; Zani, A. Blue piglets? Electrophysiological evidence for the primacy of shape over color in object recognition. Cognit. Brain Res. 2004, 18, 288–300. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Zani, A. Electrophysiological indexes of illusory contours perception in humans. Neuropsychologia 2002, 40, 479–491. [Google Scholar] [CrossRef]
- Gable, P.A.; Poole, B.D.; Cook, M.S. Asymmetrical hemisphere activation enhances global-local processing. Brain Cognit. 2013, 83, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Navon, D. Forest before trees: The precedence of global features in visual perception. Cognit. Psychol. 1977, 9, 353–383. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Minniti, A.; Zani, A. Electrophysiological evidence of a perceptual precedence of global vs. local visual information. Cognit. Brain Res. 1998, 6, 321–334. [Google Scholar] [CrossRef]
- Van Kleeck, M.H. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: New data and a meta-analysis of previous studies. Neuropsychologia 1989, 27, 1165–1178. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamagata, S.; Kobayashi, S. Cerebral asymmetry of the “top-down” allocation of attention to global and local features. J. Neurosci. 2000, 20, RC72. [Google Scholar] [CrossRef]
- Fink, G.R.; Halligan, P.W.; Marshall, J.C.; Frith, C.D.; Frackowiak, R.S.J.; Dolan, R.J. Where in the brain does visual attention select the forest and the trees? Nature 1996, 382, 626. [Google Scholar] [CrossRef]
- Baas, J.M.; Kenemans, J.L.; Mangun, G.R. Selective attention to spatial frequency: An ERP and source localization analysis. Clin. Neurophysiol. 2002, 113, 1840–1854. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Zani, A.; Avella, C. Differential activation of multiple current sources of foveal VEPs as a function of spatial frequency. Brain Topogr. 1996, 9, 59–68. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Zani, A.; Avella, C. Hemispheric asymmetries for spatial frequency discrimination in a selective attention task. Brain Cognit. 1997, 34, 311–320. [Google Scholar] [CrossRef]
- Martınez, A.; Di Russo, F.; Anllo-Vento, L.; Hillyard, S.A. Electrophysiological analysis of cortical mechanisms of selective attention to high and low spatial frequencies. Clin. Neurophysiol. 2001, 112, 1980–1998. [Google Scholar] [CrossRef]
- Robertson, L.C.; Lamb, M.R. Neuropsychological contributions to theories of part/whole organization. Cognit. Psychol. 1991, 23, 299–330. [Google Scholar] [CrossRef]
- Robertson, L.C.; Lamb, M.R.; Knight, R.T. Effects of lesions of temporal-parietal junction on perceptual and attentional processing in humans. J. Neurosci. 1988, 8, 3757–3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannes, S.; Wieringa, B.M.; Matzke, M.; Münte, T.F. Hierarchical visual stimuli: Electrophysiological evidence for separate left hemispheric global and local processing mechanisms in humans. Neurosci. Lett. 1996, 210, 111–114. [Google Scholar] [CrossRef]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the event-related potential. Int. J. Med. Sci. 2005, 2, 147. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, S.A.; Anllo-Vento, L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. USA 1998, 95, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Anllo-Vento, L.; Sereno, M.I.; Frank, L.R.; Buxton, R.B.; Dubowitz, D.J.; Wong, E.C.; Hinrichs, H.; Heinze, H.J.; Hillyard, S.A. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat. Neurosci. 1999, 2, 364. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Del Zotto, M.; Zani, A. Electrical neuroimaging evidence that spatial frequency-based selective attention affects V1 activity as early as 40–60 ms in humans. BMC Neurosci. 2010, 11, 59. [Google Scholar] [CrossRef]
- Zani, A.; Proverbio, A.M. Selective attention to spatial frequency gratings affects visual processing as early as 60 msec. poststimulus. Percept. Motor Skill. 2009, 109, 140–158. [Google Scholar] [CrossRef]
- Orlandi, A.; Proverbio, A.M. ERP indices of an orientation-dependent recognition of the human body schema. Neuropsychologia. under review.
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Picton, T.W.; Bentin, S.; Berg, P.; Donchin, E.; Hillyard, S.A.; Johnson, R.; Miller, G.A.; Ritter, W.; Ruchkin, D.S.; Rugg, M.D.; et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 2000, 37, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.L.; Stone, V.E.; Bozova, S.; Tanaka, J. The body-inversion effect. Psychol. Sci. 2003, 14, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.L.; Stone, V.E.; Grubb, J.D.; McGoldrick, J.E. Turning configural processing upside down: Part and whole body postures. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 73. [Google Scholar] [CrossRef] [PubMed]
- Palmero-Soler, E.; Dolan, K.; Hadamschek, V.; Tass, P.A. swLORETA: A novel approach to robust source localization and synchronization tomography. Phys. Med. Biol. 2007, 52, 1783–1800. [Google Scholar] [CrossRef]
- Angelini, M.; Calbi, M.; Ferrari, A.; Sbriscia-Fioretti, B.; Franca, M.; Gallese, V.; Umiltà, M.A. Motor inhibition during overt and covert actions: An electrical neuroimaging study. PLoS ONE 2015, 10, e0126800. [Google Scholar] [CrossRef]
- Bekker, E.M.; Kenemans, J.L.; Verbaten, M.N. Source analysis of the N2 in a cued Go/NoGo task. Cognit. Brain Res. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Beste, C.; Saft, C.; Andrich, J.; Gold, R.; Falkenstein, M. Response inhibition in Huntington’s disease—A study using ERPs and sLORETA. Neuropsychologia 2008, 46, 1290–1297. [Google Scholar] [CrossRef]
- MacDonald, A.W.; Cohen, J.D.; Stenger, V.A.; Carter, C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef]
- Donkers, F.C.; Van Boxtel, G.J. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cognit. 2004, 56, 165–176. [Google Scholar] [CrossRef]
- Folstein, J.R.; Van Petten, C.; Rose, S.A. Novelty and conflict in the categorization of complex stimuli. Psychophysiology 2008, 45, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Proverbio, A.M.; Del Zotto, M.; Zani, A. The emergence of semantic categorization in early visual processing: ERP indices of animal vs. artifact recognition. BMC Neurosci. 2007, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Zani, A.; Marsili, G.; Senerchia, A.; Orlandi, A.; Citron, F.M.; Rizzi, E.; Proverbio, A.M. ERP signs of categorical and supra-categorical processing of visual information. Biol. Psychol. 2015, 104, 90–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, A.M.; Del Zotto, M.; Crotti, N.; Zani, A. A no-go related prefrontal negativity larger to irrelevant stimuli that are difficult to suppress. Behav. Brain Funct. 2009, 5, 25. [Google Scholar] [CrossRef]
- Harper, J.; Malone, S.M.; Bernat, E.M. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clin. Neurophysiol. 2014, 125, 124–132. [Google Scholar] [CrossRef]
- Bokura, H.; Yamaguchi, S.; Kobayashi, S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin. Neurophysiol. 2001, 112, 2224–2232. [Google Scholar] [CrossRef]
- Anllo-Vento, L.; Hillyard, S.A. Selective attention to the color and direction of moving stimuli: Electrophysiological correlates of hierarchical feature selection. Percept. Psychophys. 1996, 58, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.M.; Keil, A. Neuronal synchronization and selective color processing in the human brain. J. Cognit. Neurosci. 2004, 16, 503–522. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Esposito, P.; Zani, A. Early involvement of the temporal area in attentional selection of grating orientation: An ERP study. Cognit. Brain Res. 2002, 13, 139–151. [Google Scholar] [CrossRef]
- Zani, A.; Proverbio, A.M. ERP signs of early selective attention effects to check size. Electroen. Clin. Neurophysiol. 1995, 95, 277–292. [Google Scholar] [CrossRef]
- Smid, H.G.; Jakob, A.; Heinze, H.J. An event-related brain potential study of visual selective attention to conjunctions of color and shape. Psychophysiology 1999, 36, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Eimer, M. An event-related potential (ERP) study of transient and sustained visual attention to color and form. Biol. Psychol. 1997, 44, 143–160. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Van Den Wildenberg, W.P.; Segalowitz, S.J.; Carter, C.S. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognit. 2004, 56, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.; Nystrom, L.E.; Fissell, K.; Carter, C.S.; Cohen, J.D. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 1999, 402, 179. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.W.; Schmidt, L.A. Neuroanatomy of the human affective system. Brain Cognit. 2003, 52, 24–26. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Cozzi, M.; Orlandi, A.; Carminati, M. Error-related negativity in the skilled brain of pianists reveals motor simulation. Neuroscience 2017, 346, 309–319. [Google Scholar] [CrossRef]
- Vocks, S.; Busch, M.; Grönemeyer, D.; Schulte, D.; Herpertz, S.; Suchan, B. Neural correlates of viewing photographs of one’s own body and another woman’s body in anorexia and bulimia nervosa: An fMRI study. J. Psychiatr. Neurosci. 2010, 35, 163. [Google Scholar] [CrossRef]
- Rubia, K.; Smith, A.B.; Brammer, M.J.; Taylor, E. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol. Psychiatr. 2007, 62, 999–1006. [Google Scholar] [CrossRef]
- Visser, M.; Jefferies, E.; Embleton, K.V.; Lambon Ralph, M.A. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: Distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. J. Cognit. Neurosci. 2012, 24, 1766–1778. [Google Scholar] [CrossRef]
- Tyler, L.K.; Stamatakis, E.A.; Dick, E.; Bright, P.; Fletcher, P.; Moss, H. Objects and their actions: Evidence for a neurally distributed semantic system. Neuroimage 2003, 18, 542–557. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Eimer, M. The N2pc component as an indicator of attentional selectivity. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 225–234. [Google Scholar] [CrossRef]
- Bledowski, C.; Prvulovic, D.; Hoechstetter, K.; Scherg, M.; Wibral, M.; Goebel, R.; Linden, D.E. Localizing P300 generators in visual target and distractor processing: A combined event-related potential and functional magnetic resonance imaging study. J. Neurosci. 2004, 24, 9353–9360. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Proverbio, A.M. Bilateral engagement of the occipito-temporal cortex in response to dance kinematics in experts. Sci. Rep. 2019, 9, 1000. [Google Scholar] [CrossRef]
- Picton, T.W. The P300 wave of the human event-related potential. J. Clin. Neurophysiol. 1992, 9, 456–479. [Google Scholar] [CrossRef]
- Azizian, A.; Freitas, A.L.; Watson, T.D.; Squires, N.K. Electrophysiological correlates of categorization: P300 amplitude as index of target similarity. Biol. Psychol. 2006, 71, 278–288. [Google Scholar] [CrossRef]
- Nisiyama, M.; Ribeiro-do-Valle, L.E. Relative performance of the two hands in simple and choice reaction time tasks. Braz. J. Med. Biol. Res. 2014, 47, 80–89. [Google Scholar] [CrossRef]
- Johnson-Frey, S.H.; Newman-Norlund, R.; Grafton, S.T. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb. Cortex 2004, 15, 681–695. [Google Scholar] [CrossRef]
- Schluter, N.D.; Krams, M.; Rushworth, M.F.S.; Passingham, R.E. Cerebral dominance for action in the human brain: The selection of actions. Neuropsychologia 2001, 39, 105–113. [Google Scholar] [CrossRef]
- Corballis, M.C. Hemispheric interactions in simple reaction time. Neuropsychologia 2002, 40, 423–434. [Google Scholar] [CrossRef]
- Marzi, C.A.; Bisiacchi, P.; Nicoletti, R. Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia 1991, 29, 1163–1177. [Google Scholar] [CrossRef]
- Weber, B.; Treyer, V.; Oberholzer, N.; Jaermann, T.; Boesiger, P.; Brugger, P.; Regard, M.; Buck, A.; Savazzi, S.; Marzi, C.A. Attention and interhemispheric transfer: A behavioral and fMRI study. J. Cognit. Neurosci. 2005, 17, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Blanco, M.J. Spatial attention and object-based attention: A comparison within a single task. Vis. Res. 2004, 44, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, W.; Yund, E.W.; Woods, D.L. Interactions between spatial attention and global/local feature selection: An ERP study. Neuroreport 2000, 11, 2753–2758. [Google Scholar] [CrossRef]
- Zani, A.; Proverbio, A.M. Cognitive Electrophysiology of Mind and Brain. In The Cognitive Electrophysiology of Mind and Brain; Elsevier-Academic Press: Amsterdam, The Netherlands; San Diego, CA, USA, 2003; pp. 3–12. [Google Scholar]
- Grech, R.; Cassar, T.; Muscat, J.; Camilleri, K.P.; Fabri, S.G.; Zervakis, M.; Xanthopoulos, P.; Sakkalis, V.; Vanrumste, B. Review on solving the inverse problem in EEG source analysis. J. Neuroeng. Rehabil. 2008, 5, 25. [Google Scholar] [CrossRef]
- Boughariou, J.; Jallouli, N.; Zouch, W.; Slima, M.B.; Hamida, A.B. Spatial resolution improvement of EEG source reconstruction using swLORETA. IEEE Trans. Nanobiosci. 2015, 14, 734–739. [Google Scholar] [CrossRef]
- Cebolla, A.M.; Palmero-Soler, E.; Leroy, A.; Cheron, G. EEG spectral generators involved in motor imagery: A swLORETA Study. Front. Psychol. 2017, 8, 2133. [Google Scholar] [CrossRef]





| Magnitude | T-x (mm) | T-y (mm) | T-z (mm) | Hem | Lobe | Gyrus | BA | Function |
|---|---|---|---|---|---|---|---|---|
| 18.2 | 1.5 | 38.2 | −17.9 | R | F | MedFG | 11 | Attentive selection |
| 15.3 | 1.5 | 35.3 | 5.3 | R | Lim | ACC | 24 | |
| 5.5 | −38.5 | 2.4 | 29.4 | L | F | IFG/PrecGyrus | 6/9 | |
| 16.0 | −28.5 | −8 | −28.9 | L | Lim | Uncus | 28 | Affective response |
| 15.8 | 21.2 | −0.6 | −28.2 | R | Lim | Uncus | 36 | |
| 12.8 | −48.5 | −36.6 | −1.3 | L | T | MTG/STG | 22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, A.; Proverbio, A.M. Left-Hemispheric Asymmetry for Object-Based Attention: an ERP Study. Brain Sci. 2019, 9, 315. https://doi.org/10.3390/brainsci9110315
Orlandi A, Proverbio AM. Left-Hemispheric Asymmetry for Object-Based Attention: an ERP Study. Brain Sciences. 2019; 9(11):315. https://doi.org/10.3390/brainsci9110315
Chicago/Turabian StyleOrlandi, Andrea, and Alice Mado Proverbio. 2019. "Left-Hemispheric Asymmetry for Object-Based Attention: an ERP Study" Brain Sciences 9, no. 11: 315. https://doi.org/10.3390/brainsci9110315






