C57BL/6 Substrain Differences in Pharmacological Effects after Acute and Repeated Nicotine Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Drugs
2.3. Behavioral Tests
2.3.1. Tail-Flick Test
2.3.2. Hot Plate Test
2.3.3. Locomotor Activity
Acute Nicotine
Repeated Administration
2.3.4. Body Temperature
2.3.5. Elevated Plus-Maze
2.4. Nicotine and Cotinine Plasma Levels
2.4.1. HPLC/MS/MS Analysis of Nicotine and Cotinine
Specimen Extraction
Instrumental Analysis
2.4.2. Pharmacokinetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Acute Nicotine on Thermal Antinociception, Locomotion, and Body Temperature
3.2. Dichotomous Effect of Nicotine on Anxiety-Like Behavior in the EPM Assay
3.3. Impact of Repeated Drug Administration on Locomotor Activity
3.4. Nicotine Metabolism and Kinetics Do not Differ between the B6N and B6J Substrains after Acute Administration of Nicotine
3.5. B6N and B6J Substrains Differ at Basal Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Tobacco Fact Sheet. 2019. Available online: http://www.who.int/mediacentre/factsheets/fs339/en/ (accessed on 31 August 2019).
- Jha, P.; Peto, R. Global Effects of Smoking, of Quitting, and of Taxing Tobacco. N. Engl. J. Med. 2014, 370, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Shariat, S.F. Re: Global Effects of Smoking, of Quitting, and of Taxing Tobacco. Eur. Urol. 2014, 66, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Chaiton, M.O.; Mecredy, G.; Cohen, J. Tobacco retail availability and risk of relapse among smokers who make a quit attempt: A population-based cohort study. Tob. Control 2018, 27, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Hyland, A.; Borland, R.; Li, Q.; Yong, H.-H.; Mcneill, A.; Fong, G.T.; O’Connor, R.J. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tob. Control 2006, 15, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.-H.; Borland, R.; Balmford, J.; Hyland, A.; O’Connor, R.J.; Thompson, M.E.; Spittal, M.J. Heaviness of Smoking Predicts Smoking Relapse Only in the First Weeks of a Quit Attempt: Findings from the International Tobacco Control Four-Country Survey. Nicotine Tob. Res. 2014, 16, 423–429. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Kendler, K.S. The genetic epidemiology of smoking. Nicotine Tob. Res. 1999, 1 (Suppl. S2), S51–S57, discussion S69–S70. [Google Scholar] [CrossRef]
- Swan, G.E.; Baker, T.B.; Chassin, L.; Conti, D.V.; Lerman, C.; Perkins, K.A. Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence; Tobacco Control Monograph; National Cancer Institute: Bethesda, MD, USA, 2009.
- Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 2010, 42, 441–447. [Google Scholar] [CrossRef]
- Thorgeirsson, T.E.; Gudbjartsson, D.F.; Surakka, I.; Vink, J.M.; Amin, N.; Geller, F.; Sulem, P.; Rafnar, T.; Esko, T.; Walter, S.; et al. Sequence variants at CHRNB3–CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 2010, 42, 448–453. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef]
- Lessov, C.; Martin, N.; Statham, D.; Todorov, A.; Slutske, W.; Bucholz, K.; Madden, P. Defining nicotine dependence for genetic research: Evidence from Australian twins. Psychol. Med. 2004, 34, 865–879. [Google Scholar] [CrossRef]
- Flint, J.; Valdar, W.; Shifman, S.; Mott, R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat. Rev. Genet. 2005, 6, 271–286. [Google Scholar] [CrossRef]
- Baud, A.; Flint, J. Identifying genes for neurobehavioural traits in rodents: Progress and pitfalls. Dis. Model. Mech. 2017, 10, 373–383. [Google Scholar] [CrossRef]
- Li, X.C.; Karadsheh, M.S.; Jenkins, P.M.; Brooks, J.C.; Drapeau, J.A.; Shah, M.S.; Lautner, M.A.; Stitzel, J.A. Chromosomal loci that influence oral nicotine consumption in C57BL/6J × C3H/HeJ F2 intercross mice. Genes Brain Behav. 2007, 6, 401–410. [Google Scholar] [CrossRef]
- Boyle, A.E.; Gill, K.J. Genetic analysis of the psychostimulant effects of nicotine in chromosome substitution strains and F2 crosses derived from A/J and C57BL/6J progenitors. Mamm. Genome 2009, 20, 34–42. [Google Scholar] [CrossRef]
- Gill, K.J.; Boyle, A.E. Genetic basis for the psychostimulant effects of nicotine: A quantitative trait locus analysis in AcB/BcA recombinant congenic mice. Genes Brain Behav. 2005, 4, 401–411. [Google Scholar] [CrossRef]
- Keane, T.M.; Goodstadt, L.; Danecek, P.; White, M.A.; Wong, K.; Yalcin, B.; Heger, A.; Agam, A.; Slater, G.; Goodson, M.; et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 2011, 477. [Google Scholar] [CrossRef]
- Yalcin, B.; Wong, K.; Agam, A.; Goodson, M.; Keane, T.M.; Gan, X.; Nellåker, C.; Goodstadt, L.; Nicod, J.; Bhomra, A.; et al. Sequence-based characterization of structural variation in the mouse genome. Nature 2011, 477. [Google Scholar] [CrossRef]
- Bryant, C.D.; Bagdas, D.; Goldberg, L.R.; Khalefa, T.; Reed, E.R.; Kirkpatrick, S.L.; Kelliher, J.C.; Chen, M.M.; Johnson, W.E.; Mulligan, M.K.; et al. C57BL/6 substrain differences in inflammatory and neuropathic nociception and genetic mapping of a major quantitative trait locus underlying acute thermal nociception. Mol. Pain 2019, 15. [Google Scholar] [CrossRef]
- Bryant, C.D. The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann. N. Y. Acad. Sci. 2011, 1245, 31–33. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, K.; Joseph, C.; Kourrich, S.; Yoo, S.-H.; Huang, H.C.; Vitaterna, M.H.; de Villena, F.P.-M.; Churchill, G.; Bonci, A.; et al. C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 2013, 342, 1508–1512. [Google Scholar] [CrossRef]
- Kirkpatrick, S.L.; Goldberg, L.R.; Yazdani, N.; Babbs, K.R.; Wu, J.; Reed, E.R.; Jenkins, D.F.; Bolgioni, A.F.; Landaverde, K.I.; Luttik, K.P.; et al. Cytoplasmic FMR1-Interacting Protein 2 Is a Major Genetic Factor Underlying Binge Eating. Biol. Psychiatry 2017, 81, 757–769. [Google Scholar] [CrossRef]
- Baraona, L.K.; Lovelace, D.; Daniels, J.L.; McDaniel, L. Tobacco Harms, Nicotine Pharmacology, and Pharmacologic Tobacco Cessation Interventions for Women. J. Midwifery Women’s Health 2017, 62, 253–269. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Reducing Tobacco Use: A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2000.
- Marks, M.J. Genetic matters: Thirty years of progress using mouse models in nicotinic research. Biochem. Pharm. 2013, 86, 1105–1113. [Google Scholar] [CrossRef]
- Damaj, M.I.; Fonck, C.; Marks, M.J.; Deshpande, P.; Labarca, C.; Lester, H.A.; Collins, A.C.; Martin, B.R. Genetic approaches identify differential roles for α4β2* nicotinic receptors in acute models of antinociception in mice. J. Pharmacol. Exp. Ther. 2007, 321, 1161–1169. [Google Scholar] [CrossRef]
- D’Amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Dewey, W.L.; Harris, L.S.; Howes, J.S.; Nuite, J.A. The effect of various neurohormonal modulations on the activity of morphine and the narcotic antagonists in tail-flick and phenylquinone test. J. Pharmacol. Exp. Ther. 1970, 175, 435–442. [Google Scholar]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics II. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 358–393. [Google Scholar]
- Atwell, L.; Jacobson, A. The search for less harmful analgesics. Lab. Anim. 1978, 7, 42–47. [Google Scholar]
- AlSharari, S.D.; Akbarali, H.I.; Abdullah, R.A.; Shahab, O.; Auttachoat, W.; Ferreira, G.A.; White, K.L.; Lichtman, A.H.; Cabral, G.A.; Damaj, M.I. Novel insights on the effect of nicotine in a murine colitis model. J. Pharmacol. Exp. Ther. 2013, 344, 207–217. [Google Scholar] [CrossRef]
- Mager, P.; Tallarida, R.J.; Murray, R.B. Manual of Pharmacologic Calculations with Computer Programs; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981; Volume IX, p. 150. [Google Scholar]
- Anderson, S.M.; Brunzell, D.H.; Brunzell, D.H. Anxiolytic-like and anxiogenic-like effects of nicotine are regulated via diverse action at β2*nicotinic acetylcholine receptors. Br. J. Pharmacol. 2015, 172, 2864–2877. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Rosecrans, J.A. Effects of nicotine on behavioral arousal and brain 5-Hydroxytryptamine function in female rats selected for differences in activity. Eur. J. Pharmacol. 1971, 14, 29–37. [Google Scholar] [CrossRef]
- Rosecrans, J.A. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology 1972, 11, 863–870. [Google Scholar] [CrossRef]
- Collins, A.C.; Miner, L.L.; Marks, M.J. Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacol. Biochem. Behav. 1988, 30, 269–278. [Google Scholar] [CrossRef]
- Nestler, E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001, 2, 119–128. [Google Scholar] [CrossRef]
- Anderson, S.M.; Brunzell, D.H. Low dose nicotine and antagonism of β2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PLoS ONE 2012, 7, 11. [Google Scholar] [CrossRef]
- Lasser, K.; Boyd, J.W.; Woolhandler, S.; Himmelstein, D.U.; McCormick, D.; Bor, D.H. Smoking and Mental Illness. JAMA 2000, 284, 2606–2610. [Google Scholar] [CrossRef]
- Siu, E.C.K.; Tyndale, R.F. Characterization and comparison of nicotine and cotinine metabolism in vitro and in vivo in DBA/2 and C57BL/6 mice. Mol. Pharmacol. 2007, 71, 826–834. [Google Scholar] [CrossRef]
- Damaj, M.I.; Fei-Yin, M.; Dukat, M.; Glassco, W.; Glennon, R.A.; Martin, B.R. Antinociceptive Responses to Nicotinic Acetylcholine Receptor Ligands after Systemic and Intrathecal Administration in Mice. J. Pharmacol. Exp. Ther. 1998, 284, 1058–1065. [Google Scholar]
- Bryant, C.D.; Zhang, N.N.; Sokoloff, G.; Fanselow, M.S.; Ennes, H.S.; Palmer, A.A.; McRoberts, J.A. Behavioral differences among C57BL/6 substrains: Implications for transgenic and knockout studies. J. Neurogenet. 2008, 22, 315–331. [Google Scholar] [CrossRef]
- Heiker, J.T.; Kunath, A.; Kosacka, J.; Flehmig, G.; Knigge, A.; Kern, M.; Stumvoll, M.; Kovacs, P.; Blüher, M.; Klöting, N. Identification of genetic loci associated with different responses to high-fat diet-induced obesity in C57BL/6N and C57BL/6J substrains. Physiol. Genom. 2014, 46, 377–384. [Google Scholar] [CrossRef]
- Mahajan, V.S.; Demissie, E.; Mattoo, H.; Viswanadham, V.; Varki, A.; Morris, R.; Pillai, S. Striking Immune Phenotypes in Gene-Targeted Mice Are Driven by a Copy-Number Variant Originating from a Commercially Available C57BL/6 Strain. Cell Rep. 2016, 15, 1901–1909. [Google Scholar] [CrossRef]
- Simecek, P.; Churchill, G.A.; Yang, H.; Rowe, L.B.; Herberg, L.; Serreze, D.V.; Leiter, E.H. Genetic Analysis of Substrain Divergence in Non-Obese Diabetic (NOD) Mice. G3 Genes Genomes Genet. 2015, 5, 771–775. [Google Scholar] [CrossRef]
- Chen, X.; Aggen, S.H.; Chen, J.; Li, L.; Kendler, K.S.; Blank, M.; Eissenberg, T. Genetic risks to nicotine dependence predict negative mood and affect in current non-smokers. Sci. Rep. 2015, 5, 9521. [Google Scholar] [CrossRef]
- Crabbe, J.C.; Wahlsten, D.; Dudek, B.C. Genetics of Mouse Behavior: Interactions with Laboratory. Environ. Sci. 1999, 284, 1670–1672. [Google Scholar] [CrossRef]
- Mulligan, M.K.; Ponomarev, I.; Boehm, S.L.; Owen, J.A.; Levin, P.S.; Berman, A.E.; Blednov, Y.A.; Crabbe, J.C.; Williams, R.W.; Miles, M.F.; et al. Alcohol trait and transcriptional genomic analysis of C57BL/6 substrains. Genes Brain Behav. 2008, 7, 677–689. [Google Scholar] [CrossRef]
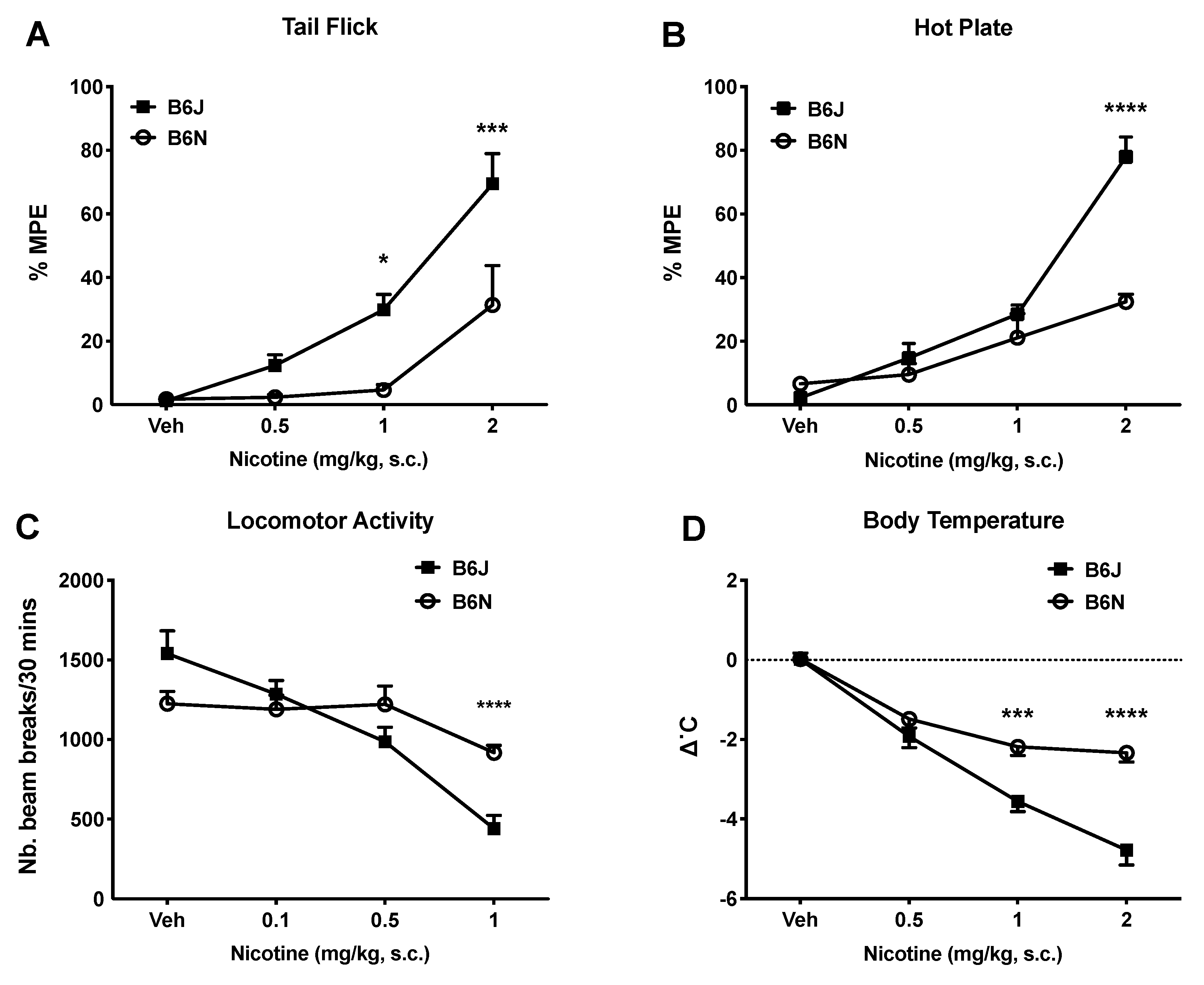
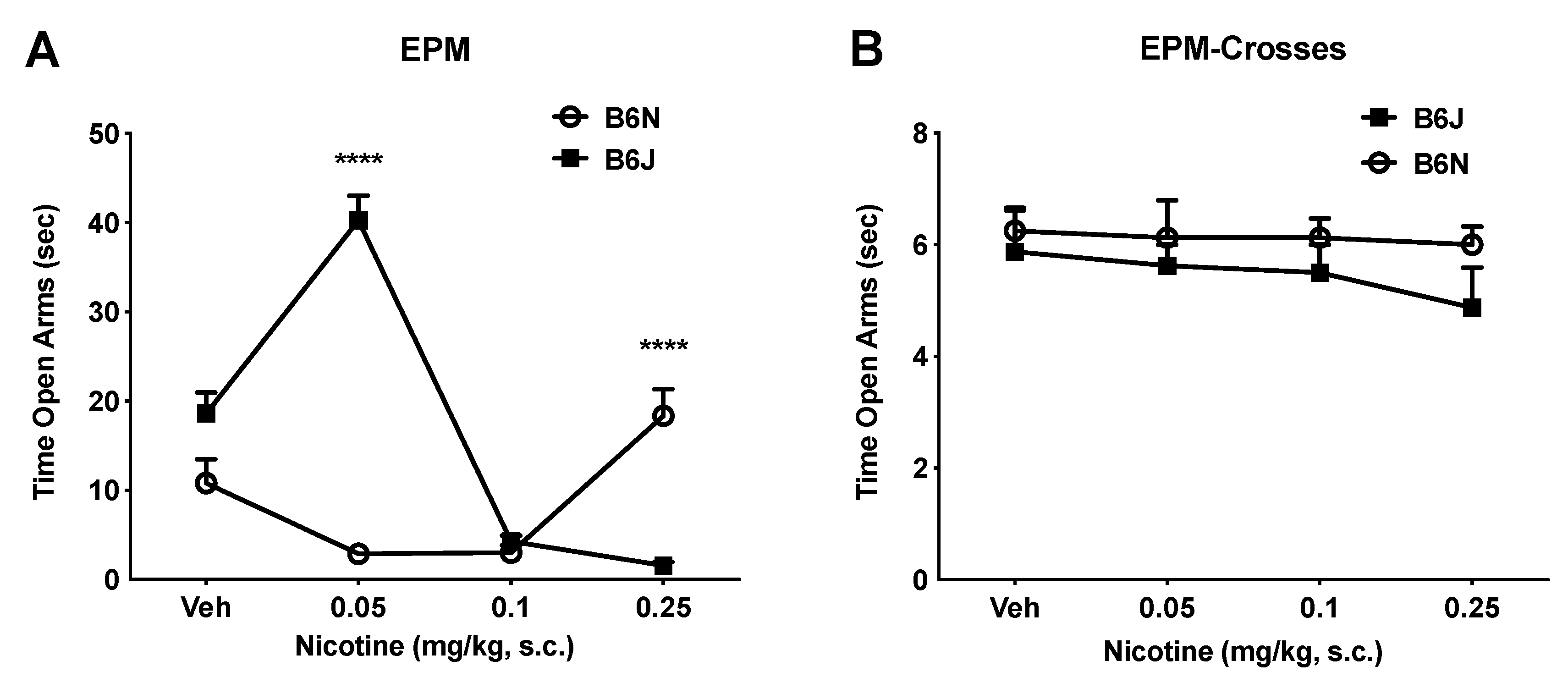
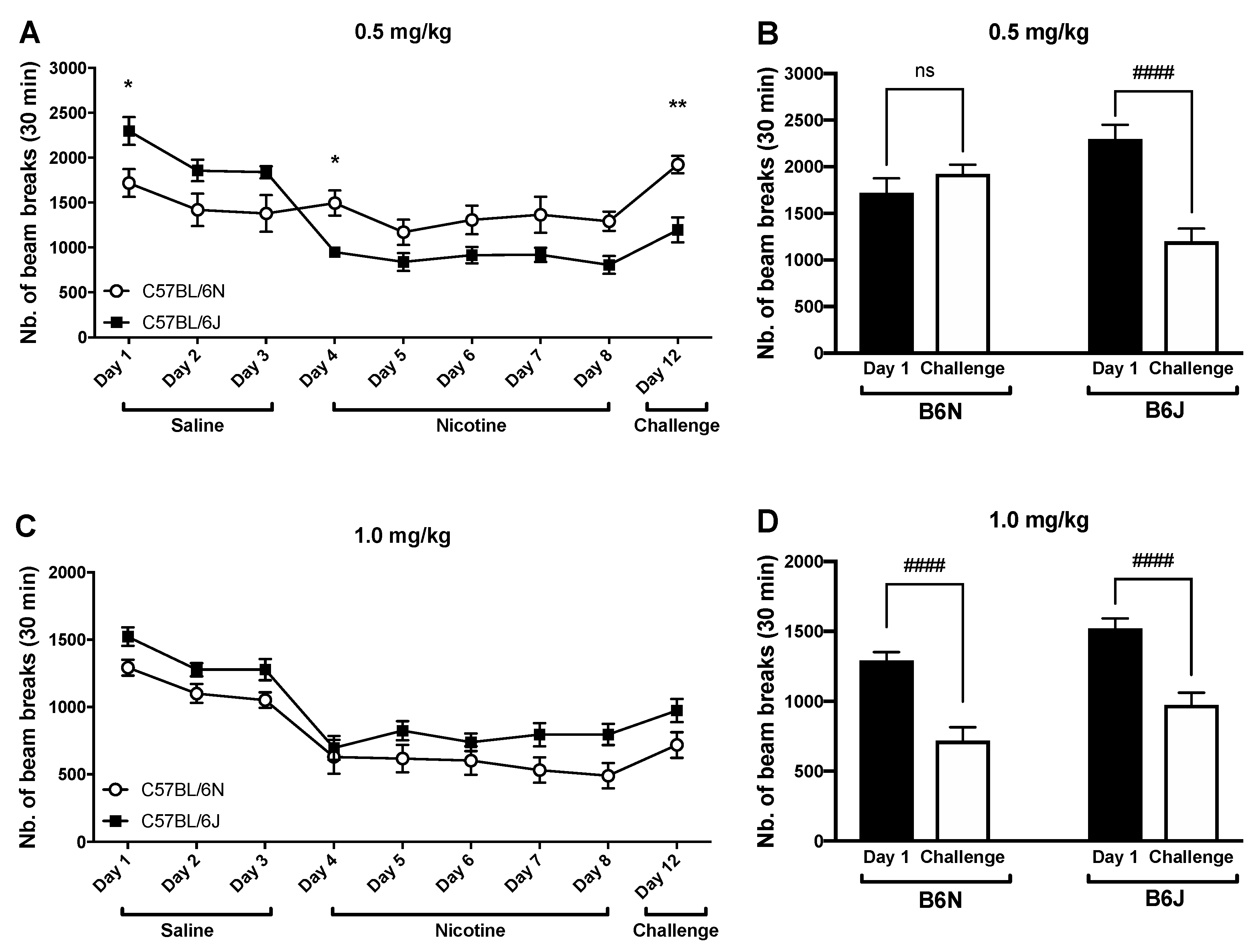
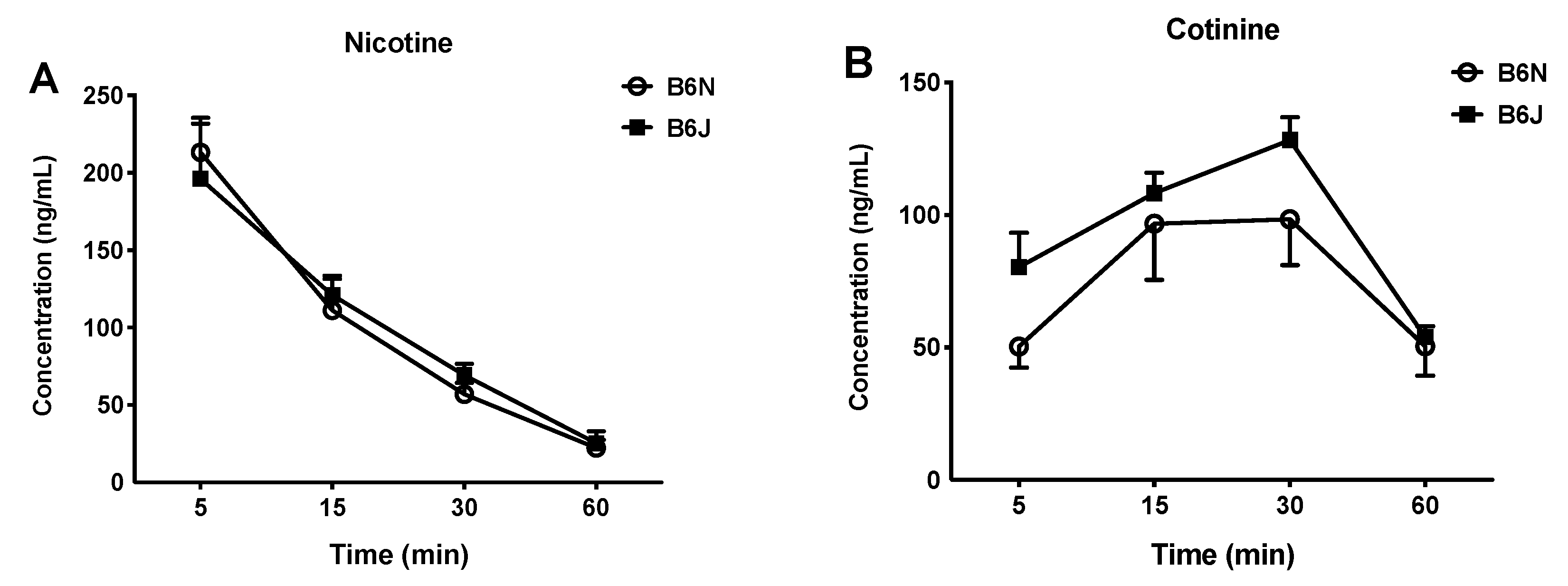

| Test | B6J (mg/kg) | B6N (mg/kg) |
|---|---|---|
| Tail flick | 1.3 (0.56–1.89) | 5.9 (3.1–11.23) * |
| Hot plate | 1.08 (0.83–1.38) | 7.79 (3.56–13.41) * |
| Body temperature | 0.57 (0.37–0.88) | 1.15 (0.96–2.13) * |
| Nicotine | Cotinine | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SE) | AUC0-90 (min ng/mL) | AUC0-inf (min ng/mL) | Cmax (ng/mL) | T1/2 (min) | AUC0-90 (min ng/mL) | AUC0-inf (min ng/mL) | Cmax (ng/mL) |
| 6N | 4610 (126) | 5200 (136) | 216 (20.6) | 17.2 (1.43) | 5740 (384) | 7230 (713) | 129 (12.9) |
| 6J | 4930 (380) | 5880 (741) | 206 (30) | 20.5 3.68 | 7000 (418) | 8780 (506) | 131 (7.16) |
| Comparison | N.S. | N.S. | N.S. | N.S. | P = 0.05 | N.S. | N.S. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinola, L.S.; Mckiver, B.; Toma, W.; Zhu, A.Z.X.; Tyndale, R.F.; Kumar, V.; Damaj, M.I. C57BL/6 Substrain Differences in Pharmacological Effects after Acute and Repeated Nicotine Administration. Brain Sci. 2019, 9, 244. https://doi.org/10.3390/brainsci9100244
Akinola LS, Mckiver B, Toma W, Zhu AZX, Tyndale RF, Kumar V, Damaj MI. C57BL/6 Substrain Differences in Pharmacological Effects after Acute and Repeated Nicotine Administration. Brain Sciences. 2019; 9(10):244. https://doi.org/10.3390/brainsci9100244
Chicago/Turabian StyleAkinola, Lois S., Bryan Mckiver, Wisam Toma, Andy Z. X. Zhu, Rachel F. Tyndale, Vivek Kumar, and M. Imad Damaj. 2019. "C57BL/6 Substrain Differences in Pharmacological Effects after Acute and Repeated Nicotine Administration" Brain Sciences 9, no. 10: 244. https://doi.org/10.3390/brainsci9100244
APA StyleAkinola, L. S., Mckiver, B., Toma, W., Zhu, A. Z. X., Tyndale, R. F., Kumar, V., & Damaj, M. I. (2019). C57BL/6 Substrain Differences in Pharmacological Effects after Acute and Repeated Nicotine Administration. Brain Sciences, 9(10), 244. https://doi.org/10.3390/brainsci9100244






