Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites
Abstract
:1. Introduction
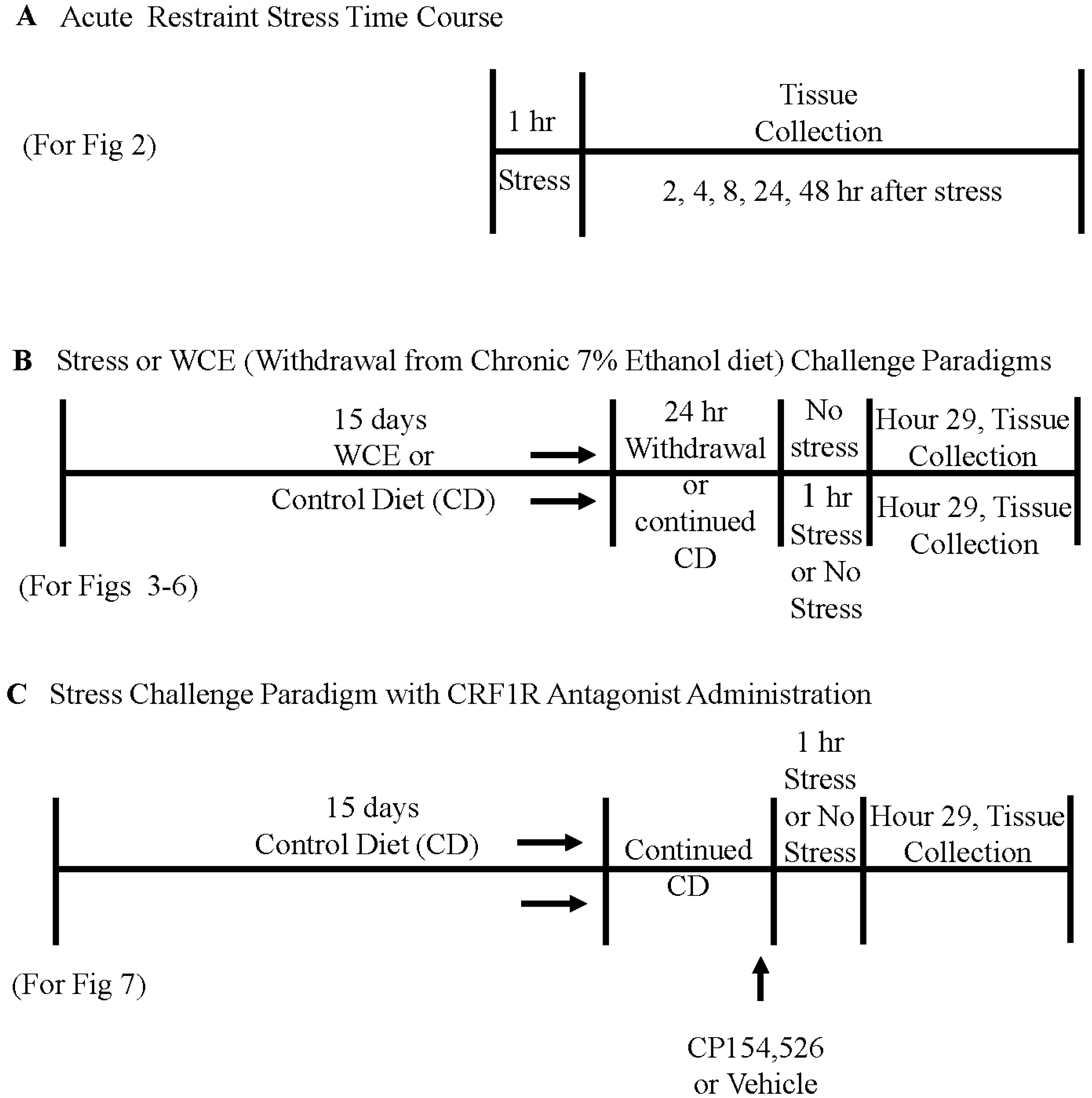
2. Materials and Methods
2.1. Animals
2.2. Liquid Diet for Controls and for Chronic Ethanol Exposure
2.3. Restraint Stress in Controls and after Withdrawal from Chronic Ethanol Exposure
2.4. Brain Tissue Collection and Real-Time PCR Analysis for Tissue mRNA
2.5. Enzyme-linked Immunosorbent Assay (ELISA) for Cytokines
2.6. CRF Receptor Antagonist Administration
2.7. Statistical Analysis
3. Results
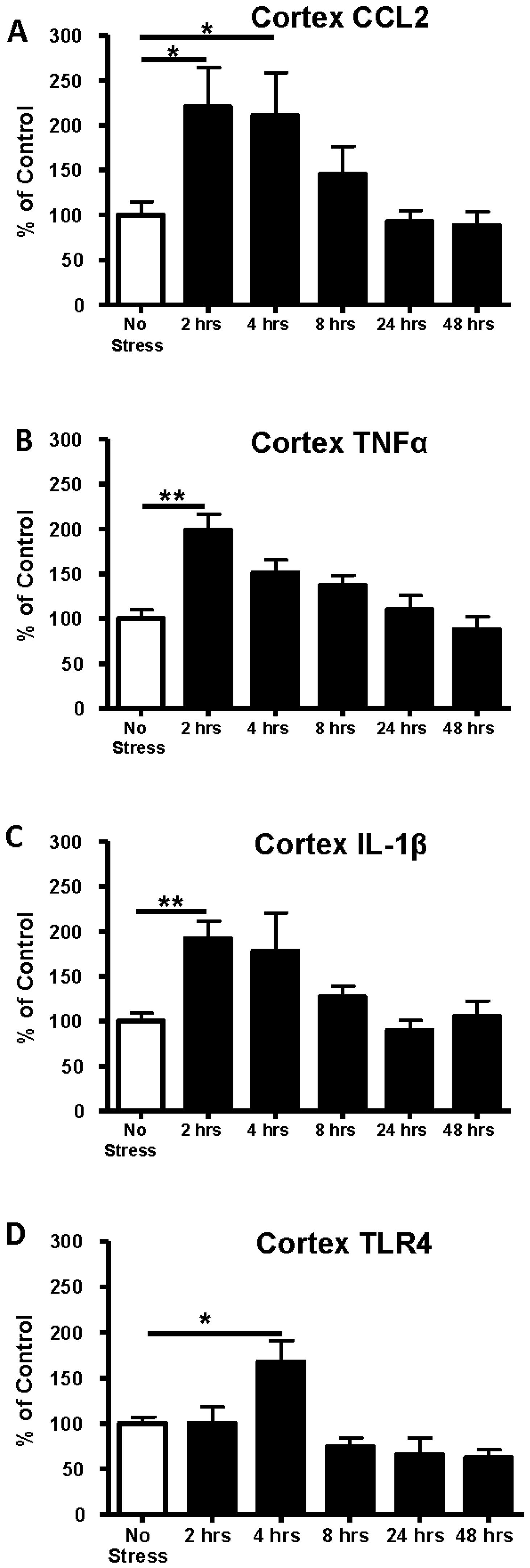
3.1. Time Course of Expression of Cytokine and TLR4 mRNAs in Cortex Following 1-Hour of Restraint Stress in Sprague–Dawley (S–D) Rats
3.2. Determination of Cytokine Protein Levels in Cortex after Restraint Stress
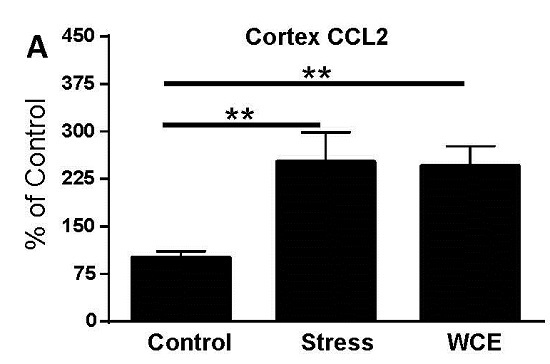
3.3. Effect of Stress or WCE on Selected Cytokine and TLR4 mRNAs in Cortex
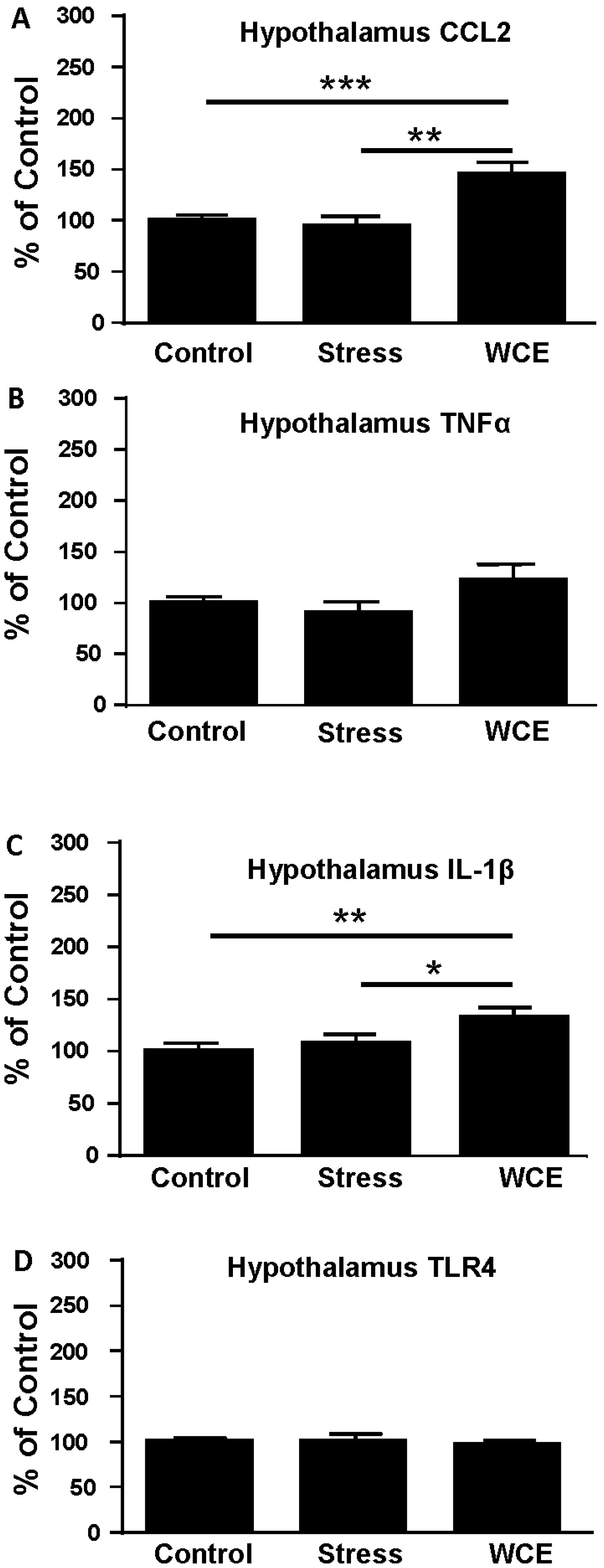
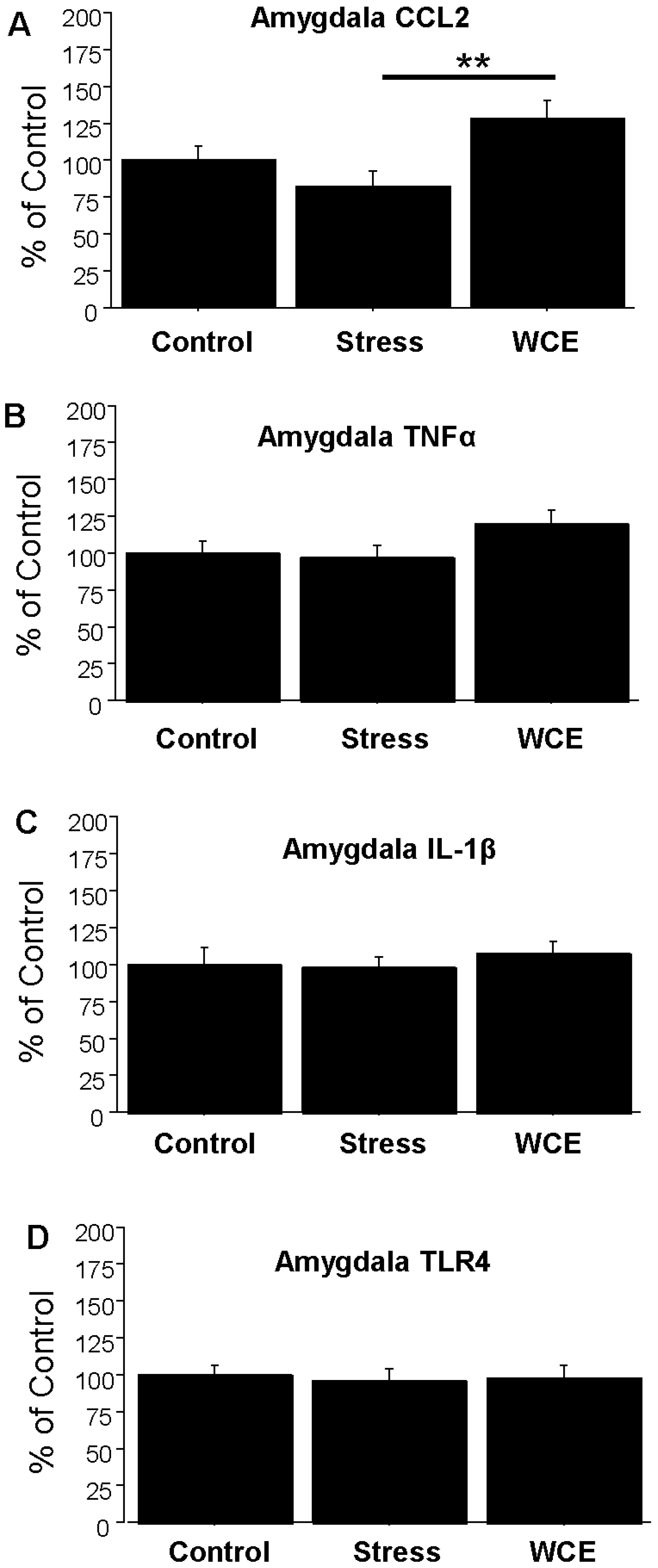
3.4. Effect of Stress or WCE on Selected Cytokine mRNAs in Hypothalamus, Hippocampus, and Amygdala after WCE
3.5. Effect of the CRF1R Antagonist CP154,526 on Cortical Cytokine mRNAs Following Stress
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CCL2 | chemokine (C-C motif) ligand 2 |
| CRF | corticotropin releasing hormone |
| IL-1β | interleukin-1 beta |
| mRNA | messenger ribonucleic acid |
| S-D | Sprague-Dawley |
| TLR4 | toll-like receptor 4 |
| TNFα | tumor necrosis factor-alpha |
| WCE | withdrawal from chronic ethanol |
| qPCR | quantitative polymerase chain reaction |
References
- Overstreet, D.H.; Knapp, D.J.; Breese, G.R. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol. Clin. Exp. Res. 2002, 26, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Rassnick, S.; Heinrichs, S.C.; Britton, K.T.; Koob, G.F. Microinjection of CRF antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993, 605, 25–32. [Google Scholar] [CrossRef]
- Valdez, G.R.; Zorrilla, E.P.; Roberts, A.J.; Koob, G.F. Antagonism of corticotropin releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol 2003, 29, 55–60. [Google Scholar] [CrossRef]
- Breese, G.R.; Knapp, D.J.; Overstreet, D.H. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: Inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A receptor agonist. Neuropsychopharmacology 2004, 29, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Breese, G.R.; Overstreet, D.H.; Knapp, D.J.; Navarro, M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: Inhibition by CRF-1 and benzodiazepine-receptor antagonists and a 5-HT1A receptor antagonists. Neuropsychopharmacology 2005, 30, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Breese, G.R.; Sinha, R.; Heilig, M. Chronic ethanol neuroadaptation and stress contribute to susceptibility for ethanol craving and relapse. Pharmacol. Ther. 2011, 129, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R. How does stress increase risk of drug abuse and relapse? Psychopharmacology 2001, 158, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Breese, G.R.; Knapp, D.J.; Overstreet, D.H.; Navarro, M.; Wills, T.A.; Angel, R.A. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology 2008, 33, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.J.; Whitman, B.A.; Wills, T.A.; Angel, R.A.; Overstreet, D.H.; Criswell, H.E.; Ming, Z.; Breese, G.R. Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain Behav. Immun. 2011, 25, S146–S154. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.M.; Overstreet, D.H.; Knapp, D.J.; Angel, R.; Wills, T.A.; Navarro, M.; Rivier, J.; Vale, W.; Breese, G.R. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is site specific and mediated by CRF-1 receptors: Relation to stress-induced sensitization. J. Pharmacol. Exp. Ther. 2010, 332, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Tracey, K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Ann. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMLPs), microbial associated molecular patterns (MAMPS) and the inflammasome. Brain Behav. Immun. 2013, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Shintani, F.; Kanba, S.; Asai, M.; Nakaki, T. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cell Mol. Neurobiol. 1997, 17, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.Y.; Strassheim, D.; Ishizaka, A.; Esward, A. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004, 279, 7370–7377. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Gamboni-Robertson, F.; He, Q.; Svetkauskaite, D.; Kim, J.Y.; Strassheim, D.; Sohn, J.W.; Yamada, S.; Maruyama, I.; Banerjee, A.; et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 2006, 290, C917–C924. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. HMGB1 Loves Company. J. Leukoc. Biol. 2009, 86, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Barnum, C.J.; Blandino, P., Jr.; Deak, T. Social status modulates basal IL-1 concentrations in the hypothalamus of pair-housed rats and influences certain features of stress reactivity. Brain Behav. Immun. 2008, 22, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Blandino, P.; Barnum, C.J.; Solomon, L.G.; Larish, Y.; Lankow, B.S.; Deak, T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav. Immun. 2009, 23, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Deak, T.; Bordner, K.A.; McElderry, N.K.; Barnum, C.J.; Blandino, P., Jr.; Deak, M.M.; Tammariello, S.P. Stress-induced increases in hypothalamic IL-1: A systematic analysis of multiple stressor paradigms. Brain Res. Bull. 2005, 64, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.; Donegan, J.J.; Morilak, D.A. Chronic intermittent cold stress sensitizes neuroimmune reactivity in the rat brain. Psychoneuroendocrinology 2011, 36, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Hueston, C.M.; Barnum, C.J.; Eberle, J.A.; Ferraioli, F.J.; Buck, H.M.; Deak, T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol. Behav. 2011, 104, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Greenwood, B.N.; Fleshner, M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Kuraishi, Y.; Yamaguchi, T.; Nakai, S.; Hirai, Y.; Satoh, M. Immobilization stress induces interleukin-1 beta mRNA in the rat hypothalamus. Neurosci. Lett. 1991, 123, 254–256. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Deak, T.; Owens, S.M.; Kohno, T.; Fleshner, M.; Watkins, L.R.; Maier, S.F. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J. Neurosci. 1998, 18, 2239–2246. [Google Scholar] [PubMed]
- Nguyen, K.T.; Deak, T.; Will, M.J.; Hansen, M.K.; Hunsaker, B.N.; Fleshner, M.; Watkins, L.R.; Maier, S.F. Time course and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000, 859, 193–201. [Google Scholar] [CrossRef]
- Vecchiarelli, H.A.; Gandhi, C.P.; Gray, J.M.; Morena, M.; Hassan, K.I.; Hill, M.N. Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain Behav. Immun. 2016, 51, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Zou, J.; Qin, L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav. Immun. 2011, 25, S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Qin, L.; Sheedy, D.; Vetreno, R.P.; Zou, J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol. Psychiatry 2013, 73, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Maes, M.; Anderson, G.; Dean, O.M.; Moylan, S.; Berk, M. Putative neuroprotective agents in neuropsychiatric disorders. Prog. Neuropsychopharm. Biol. Psychiatry 2013, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C. Stress and Depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 2008, 56, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Rector, D.M.; Churchill, L. Sleep and cytokines. Sleep Med. Clin. 2007, 2, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiat. 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Frye, G.D.; McCown, T.J.; Breese, G.R. Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. J. Pharmacol. Exp. Ther. 1983, 227, 663–670. [Google Scholar] [PubMed]
- McCown, T.J.; Breese, G.R. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: Evidence for kindling of seizures associated with alcoholism. Alcohol. Clin. Exp. Res. 1990, 14, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Whitman, B.A.; Knapp, D.J.; Werner, D.F.; Crews, F.T.; Breese, G.R. The cytokine-mRNA Increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 Release. Alcohol. Clin. Exp. Res. 2013, 37, 2086–2097. [Google Scholar] [CrossRef] [PubMed]
- Porterfield, V.M.; Zimomra, Z.R.; Caldwell, E.A.; Camp, R.M.; Gabella, K.M.; Johnson, J.D. Rat strain differences in restraint stress-induced brain cytokines. Neuroscience 2011, 188, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.H.; Knapp, D.J.; Breese, G.R. Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin. Exp. Res. 2007, 31, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Fossati, S.; Bianchi, M.E.; Patrone, M.; Pedrazzi, M.; Sparatore, B.; Moroni, F.; Chiarugi, A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J. Neurochem. 2007, 103, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signaling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Maruyama, I. HMGB1, a novel inflammatory cytokine. Clin. Chim. Acta 2007, 375, 36–42. [Google Scholar] [PubMed]
- Yang, H.; Wang, H.; Czura, C.J.; Tracey, K.J. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005, 78, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, H.; Ding, A.; Golenbock, D.T.; Latz, E.; Czura, C.J.; Fenton, M.J.; Tracey, K.J.; Yang, H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006, 26, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.J.; Overstreet, D.H.; Breese, G.R. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin. Exp. Res. 2005, 29, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Shizuya, K.; Komori, T.; Fujiwara, R.; Miyahara, S.; Ohmori, M.; Nomura, J. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sci. 1997, 61, 135–140. [Google Scholar] [CrossRef]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Baliño, P.; Aragón, C.M.; Guerri, C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of TLR4 and TLR2. Neuropharmacology 2015, 89, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Vetreno, R.P. Neuroimmune basis of alcoholic brain damage. Int. Rev. Neurobiol. 2014, 118, 315–357. [Google Scholar] [PubMed]
- Crews, F.T.; Vetreno, R.P. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl.) 2016, 333, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, L.; Wilson, B.; Wu, X.; Qian, L.; Granholm, A.C.; Crews, F.T.; Hong, J.S. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology 2008, 29, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.K.; O’Dell, L.E.; Crawford, E.F.; Koob, G.F. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 2006, 26, 11324–11332. [Google Scholar] [CrossRef] [PubMed]
- Buck, H.M.; Hueston, C.M.; Bishop, C.; Deak, T. Enhancement of the hypothalamic-pituitary-adrenal axis but not cytokine responses to stress challenges imposed during withdrawal from acute alcohol exposure in Sprague-Dawley rats. Psychopharmacology 2011, 218, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.A.; Rassnick, S.; Rivier, J.; Koob, G.F.; Britton, K.T. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology 1991, 103, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Criswell, H.E.; Breese, G.R. Evidence for TNFα action on excitatory and inhibitory neurotransmission in the central amygdala: A brain site influenced by stress. Brain Behav. Immun. 2013, 33, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Breese, G.R.; Knapp, D.J. Persistent adaptation by chronic alcohol is facilitated by neuroimmune activation linked to stress and CRF. Alcohol 2016, 52, 9–23. [Google Scholar] [CrossRef] [PubMed]
- June, H.L.; Liu, J.; Warnock, K.T.; Bell, K.A.; Balan, I.; Bollion, D.; Puche, A.; Aurelian, L. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 2015, 40, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Brureau, A.; Vadigepalli, R.; Staehle, M.M.; Brureau, M.M.; Gonye, G.E.; Hoek, J.B.; Hooper, D.C.; Schwaber, J.S. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. J. Neuroinflamm. 2012, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Breese, G.R.; Overstreet, D.H.; Knapp, D.J. Conceptual framework for the etiology of alcoholism: A “kindling”/stress hypothesis. Psychopharmacology 2005, 178, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; Koob, G.F. A key role of corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007, 30, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Bergeson, S.E.; Walker, D.; Ferreira, V.M.M.; Kuziel, W.A.; Harris, R.A. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav. Brain Res. 2005, 165, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Ponomarev, I.; Geil, C.; Bergeson, S.; Koob, G.F.; Harris, R.A. Neuroimmune regulation of alcohol consumption: Behavioral validation of genes obtained from genomic studies. Addict. Biol. 2012, 17, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.; Most, D.; Ferguson, L.B.; Mayfield, J.; Harris, R.A.; Blednov, Y.A. Neuroimmune pathways in alcohol consumption: Evidence from behavioral and genetic studies in rodents and humans. Int. Rev. Neurobiol. 2014, 118, 13–39. [Google Scholar] [PubMed]
- Valenta, J.P.; Gonzales, R.A. Chronic Intracerebroventricular Infusion of Monocyte Chemoattractant Protein-1 leads to a persistent increase in sweetened ethanol consumption during operant self-administration but does not influence sucrose consumption in Long-Evans rats. Alcohol. Clin. Exp. Res. 2016, 40, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lotrich, F.E. Psychiatric clearance for patients started on interferon-alpha-based therapies. Am. J. Psychiatry 2013, 170, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. Malaise, melancholia and madness: The evolutionary legacy of an inflammatory bias. Brain Behav. Immun. 2013, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ota, K.T.; Duman, R.S. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013, 31, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Fox, H.C.; Hong, K.I.; Hansen, J.; Tuit, K.; Kreek, M.J. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent ethanol relapse and treatment outcomes. Arch. Gen. Psychiat. 2011, 68, 942–952. [Google Scholar] [CrossRef] [PubMed]
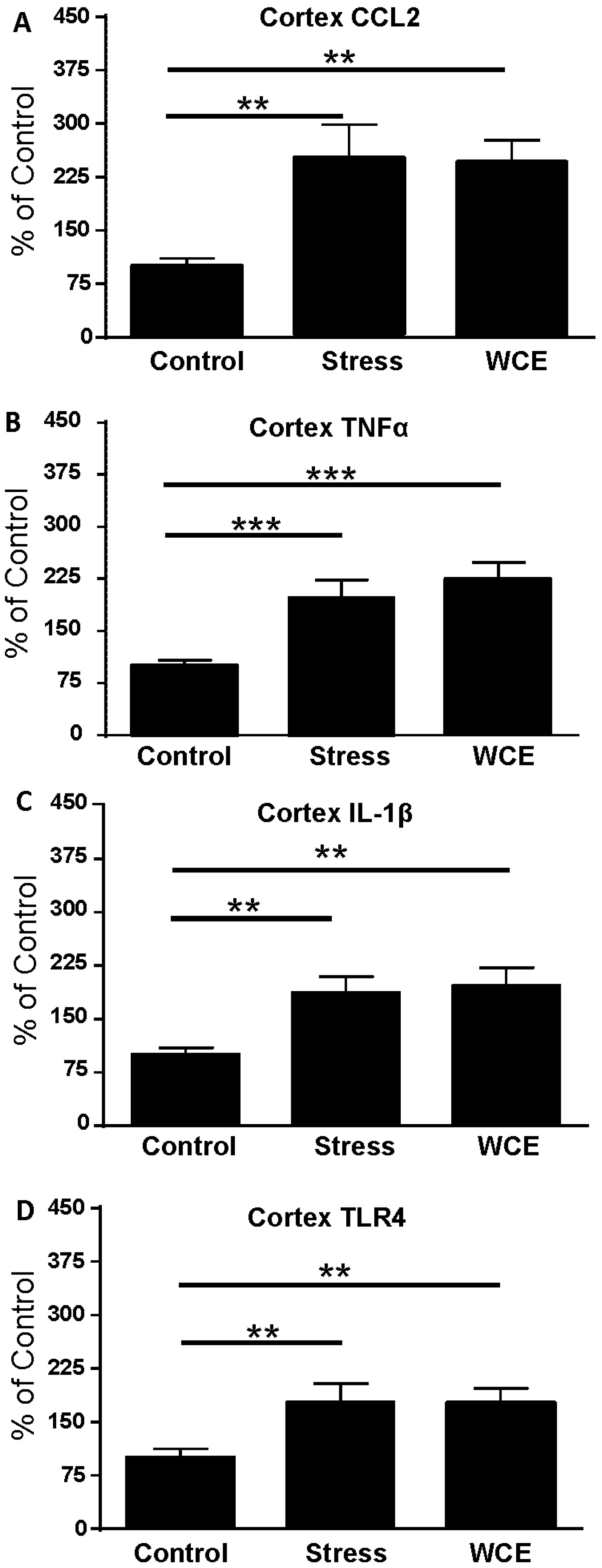


| Group | CCL2 | IL-1β | TNFα |
|---|---|---|---|
| S-D Non-Stressed | 12.9 (0.3) | 0.37 (0.05) | ND |
| S-D Stressed | 13.7 (0.6) | 0.28 (0.02) | ND |
| Wistar Non-Stressed | 42.2 (2.3) | 1.48 (0.22) | 0.11 (0.03) |
| Wistar Stressed | 58.1 (15.4) | 1.05 (0.84) | 0.12 (0.03) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapp, D.J.; Harper, K.M.; Whitman, B.A.; Zimomra, Z.; Breese, G.R. Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites. Brain Sci. 2016, 6, 25. https://doi.org/10.3390/brainsci6030025
Knapp DJ, Harper KM, Whitman BA, Zimomra Z, Breese GR. Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites. Brain Sciences. 2016; 6(3):25. https://doi.org/10.3390/brainsci6030025
Chicago/Turabian StyleKnapp, Darin J., Kathryn M. Harper, Buddy A. Whitman, Zachary Zimomra, and George R. Breese. 2016. "Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites" Brain Sciences 6, no. 3: 25. https://doi.org/10.3390/brainsci6030025