Sex-Specific Brain Deficits in Auditory Processing in an Animal Model of Cocaine-Related Schizophrenic Disorders
Abstract
:1. Introduction
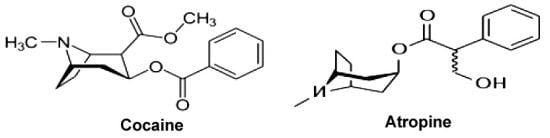
1.1. Cocaine
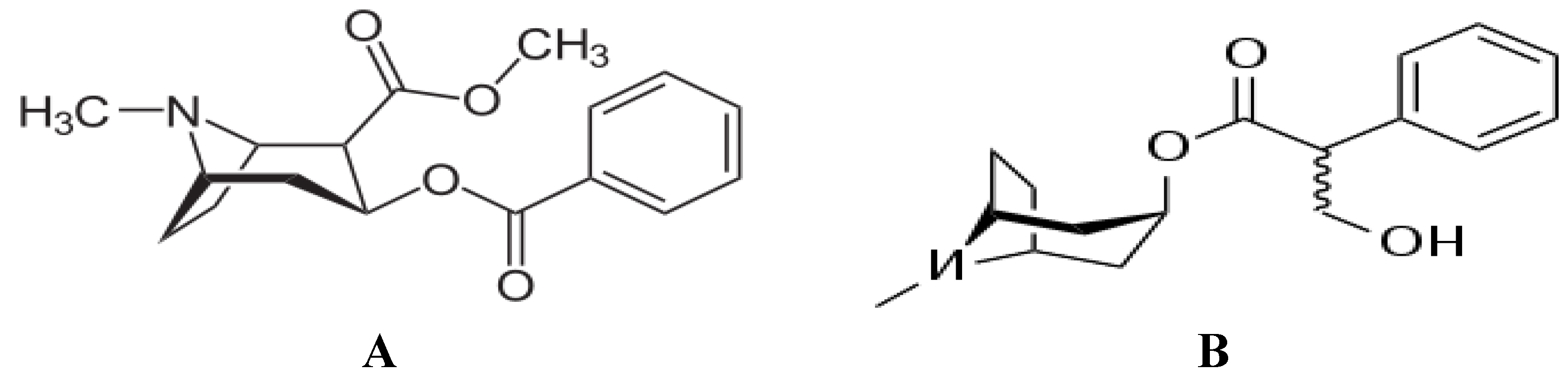
1.2. Schizophrenia and Cocaine Psychoses
1.3. Responses to Auditory Stimuli, Startle and Pre-Pulse Inhibition (PPI)
1.4. Auditory Stimuli-Startle Tests: Sex-Related Differences
2. Experimental Section
2.1. Animals
| Type | No Stimulus | Prepulse | Pulse |
|---|---|---|---|
| 1 | 75 decibels (broadband) | - | - |
| 2 | - | - | 120 decibels |
| 3 | - | 95 decibels | 120 decibels |
| 4 | - | 105 decibels | 120 decibels |
| 5 | - | 115 decibels | 120 decibels |
2.2. Data Analysis
3. Results
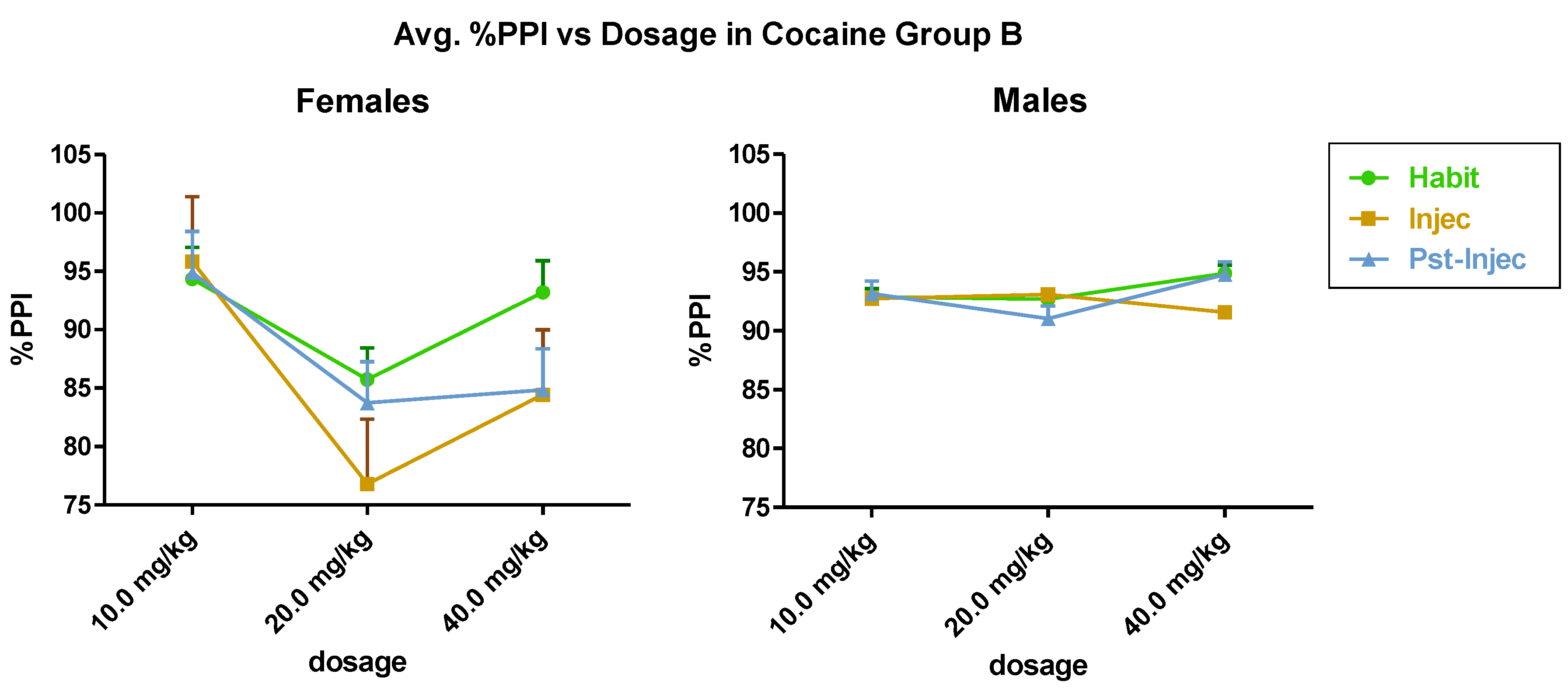
3.1. Sex Differences in Responses during the Acoustic Startle Paradigm after Cocaine


3.2. Saline Control
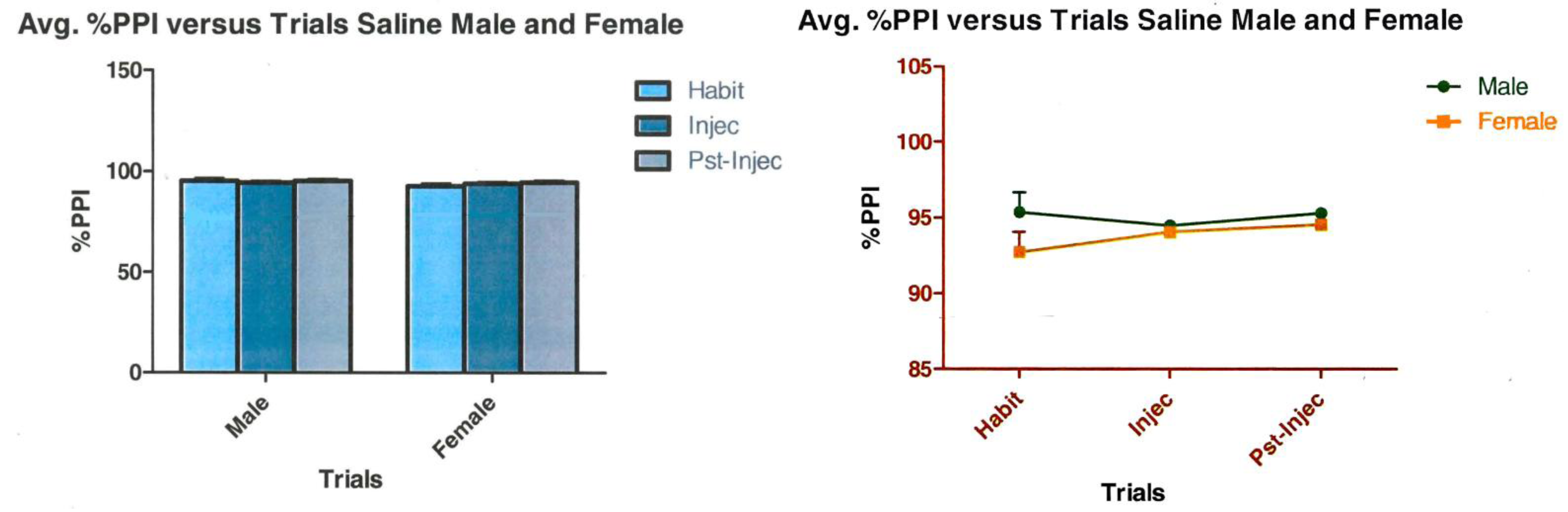
3.3. Summary of Groups A and B: Are There Seasonal Sex Differences in Acoustic Startle Responses?
4. Discussion
4.1. Sex Differences, Cocaine and the Estrous Cycle
4.2. Pharmacological Manipulation of Startle
4.3. Brain Circuitry Mechanisms for the Auditory Startle Stimulus and Response
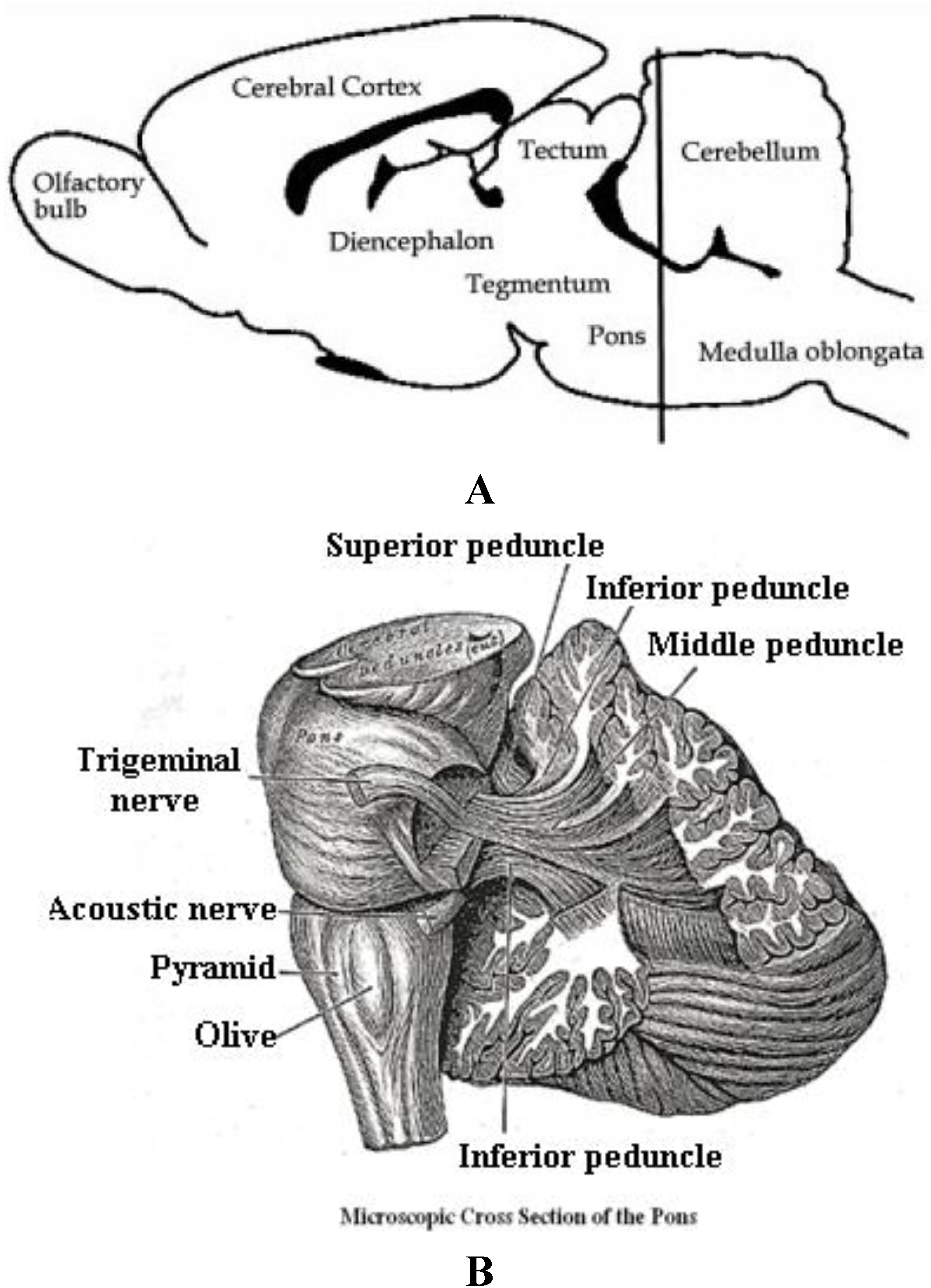
5. Conclusions
Acknowledgements
References
- Swerdlow, N.R.; Hartman, P.L.; Auerbach, P.P. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuropsychiatric disorders. Biol. Psychiatry 1997, 41, 452–460. [Google Scholar] [CrossRef]
- Geyer, M.A.; Kerbs-Thomson, K.; Braff, D.L.; Swerdlow, N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology 2001, 156, 117–154. [Google Scholar] [CrossRef]
- Atropine. Available online: http://www.newworldencyclopedia.org/entry/Atropine (accessed on 28 December 2012).
- Goodman, L.S.; Gilman, A.G.; Limbird, L.E.; Milinoff, P.B.; Ruddon, R.W. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics, 9th ed; Mcgraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Parnas, M.L.; Gaffaney, J.D.; Zou, M.F.; Lever, J.R.; Newman, A.H.; Vaughan, R.A. Labeling of Dopamine Transporter Transmembrane Domain 1 with the Tropane Ligan N-[4-Azido-3-[125I]iodophenyl)butyl]-2β-carbomethoxy-3β-(4-chlorophenyl)tropane Implicates Proximity of Cocaine and Substrate Active Sites. Mol. Pharmacol. 2008, 73, 1141–1150. [Google Scholar] [CrossRef]
- Kopaitic, T.A.; Liu, Y.; Surratt, C.K.; Donavan, D.M.; Newman, A.H.; Katz, J.L. Dopamine Transporter-Dependent and -Independent Striatal Binding of the Benztropine Analog JHW 007, a cocaine Antagonist with Low Abuse Liability. J. Pharmacol. Exp. Ther. 2010, 335, 703–714. [Google Scholar] [CrossRef]
- Broderick, P.A. Cocaine: On-line analysis of an accumbens amine neural basis for psychomotor behavior. Pharmacol. Biochem. Behav. 1991, 40, 959–968. [Google Scholar] [CrossRef]
- Broderick, P.A. In vivo voltammetric studies on release mechanisms for cocaine with γ-butyrolactone. Pharmacol. Biochem. Behav. 1991, 40, 969–975. [Google Scholar] [CrossRef]
- Broderick, P.A. Cocaine’s colocalized effects on synaptic serotonin and dopamine in ventral tegmentum in a reinforcement paradigm. Pharmacol. Biochem. Behav. 1992, 42, 889–898. [Google Scholar] [CrossRef]
- Broderick, P.A. Distinguishing effects of cocaine (IV) and (SC) on mesoaccumbens dopamine and serotonin release with chloral hydrate anesthesia. Pharmacol. Biochem. Behav. 1992, 43, 929–937. [Google Scholar] [CrossRef]
- Broderick, P.A. In vivo electrochemical studies of gradient effects of (SC) cocaine on dopamine and serotonin release in dorsal striatum of conscious rats. Pharmacol. Biochem. Behav. 1993, 46, 973–984. [Google Scholar] [CrossRef]
- Broderick, P.A.; Kornak, E.P.; Eng, F.; Wechsler, R.W. Real time detection of acute (IP) cocaine-enhanced dopamine and serotonin release in ventrolateral nucleus accumbens of the behaving Norway rat. Pharmacol. Biochem. Behav. 1993, 46, 715–722. [Google Scholar] [CrossRef]
- Broderick, P.A.; Hope, O.; Okonji, C.; Rahni, D.N.; Zhou, Y. Clozapine and cocaine effects on dopamine and serotonin release in nucleus accumbens during psychostimulant behavior and withdrawal. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 157–171. [Google Scholar] [CrossRef]
- Broderick, P.A.; Olabisi, O.A.; Rahni, D.N.; Zhou, Y. Cocaine acts on accumbens monoamines and locomotor behavior via a 5-HT2A/2C receptor mechanism as shown by ketanserin: 24 hr follow-up studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 547–557. [Google Scholar] [CrossRef]
- Broderick, P.A.; Hope, O. Monoamines and motor responses are co-deficient in the Fawn-Hooded depressed animal model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 887–898. [Google Scholar] [CrossRef]
- Humby, T.; Wilkinson, L.S.; Robbins, T.W.; Geyer, M.A. Prepulses inhibit startle-induced reductions of extracellular dopamine in the nucleus accumbens of rat. J. Neurosci. 1996, 16, 2149–2156. [Google Scholar]
- Bradberry, C.W.; Nobiletti, J.B.; Elsworth, J.D.; Murphy, B.; Jatlow, P.; Roth, R.H. Cocaine and cocaethylene: Microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J. Neurochem. 1993, 60, 1429–1435. [Google Scholar] [CrossRef]
- Parsons, L.H.; Justice, J.B., Jr. Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J. Neurochem. 1993, 61, 1611–1619. [Google Scholar] [CrossRef]
- Morgan, A.E.; Porter, S.P.; Clarkson, F.A.; Volkow, N.D.; Fowler, J.S.; Dewey, S.L. Direct approach for attenuating cocaine’s effects on extracellular dopamine: Targeting the dopamine transporter. Synapse 1997, 26, 423–427. [Google Scholar] [CrossRef]
- Chapman, T.; Freeman, T.; McGhie, A. Clinical research in schizophrenia; the psychotherapeutic approach. Br. J. Med. Psychol. 1959, 32, 75–85. [Google Scholar] [CrossRef]
- Bleuler, M. The concept of schizophrenia. Am. J. Psychiatry 1954, 5, 382–383. [Google Scholar]
- Freeman, T.; McGhie, A. The relevance of genetic psychology for the psychopathology of schizophrenia. Br. J. Med. Psychol. 1957, 30, 176–187. [Google Scholar] [CrossRef]
- McGhie, A.; Chapman, J.; Lawson, J.S. Disturbances in Selective Attention in Schizophrenia. Proc. R. Soc. Med. 1964, 57, 419–422. [Google Scholar]
- McGhie, A.; Chapman, J. Disorders of attention and perception in early schizophrenia. Br. J. Med. Psychol. 1961, 34, 103–116. [Google Scholar] [CrossRef]
- Hetrick, W.P.; Smith, D.A.; Spuriell, E.; Sandman, C.A.; Bunney, W.E. The putative phenomenological correlates of sensory gating: Factor analyses and a self-report rating scale. Schizophr. Res. 1997, 24, 17. [Google Scholar]
- Brady, K.T.; Lydiard, R.B.; Malcolm, R.; Ballenger, J.C. Cocaine-induced psychosis. J. Clin. Psychiatry 1991, 52, 509–512. [Google Scholar]
- Harris, D.; Batki, S.L. Stimulant psychosis: Symptom profile and acute clinical course. Am. J. Addict. 2000, 9, 28–37. [Google Scholar] [CrossRef]
- Lysaker, P.; Bell, M.; Beam-Goulet, J.; Milstein, R. Relationship of positive and negative symptoms to cocaine abuse in schizophrenia. J. Nerv. Ment. Dis. 1994, 182, 109–112. [Google Scholar] [CrossRef]
- Mendoza, R.; Miller, B.L.; Mena, I. Emergency room evaluation of cocaine-associated neuropsychiatric disorders. Recent Dev. Alcohol. 1992, 10, 73–87. [Google Scholar]
- Miller, B.L.; Mena, I.; Giombetti, R.; Villanueve-Meyer, J.; Djenderdjian, A.H. Neuropsychiatric effects of cocaine: SPECT Measurements. J. Addict. Dis. 1992, 11, 47–58. [Google Scholar]
- Mitchell, J.; Vierkant, A.D. Delusions and hallucinations of cocaine abusers and paranoid schizophrenics: A comparative study. J. Psychol. 1991, 125, 301–310. [Google Scholar] [CrossRef]
- Nambudin, D.E.; Young, R.C. A case of late-onset crack dependence and subsequent psychosis in the elderly. J. Subst. Abuse Treat. 1991, 8, 253–255. [Google Scholar] [CrossRef]
- Rosenthal, R.N.; Miner, C.R. Differential diagnosis of substance-induced psychosis and schizophrenia in patients with substance use disorders. Schizophr. Bull. 1997, 23, 187–193. [Google Scholar] [CrossRef]
- Rosse, R.B.; Collins, J.P., Jr.; Fay-McCarthy, M.; Alim, T.M.; Wyatt, R.J.; Deutsch, S.I. Phenomenologic comparison of the idiopathic psychosis of schizophrenia and drug-induced cocaine and phencyclidine psychoses: A retrospective study. Clin. Neuropharmacol. 1994, 17, 359–369. [Google Scholar] [CrossRef]
- Satel, S.L.; Edell, W.S. Cocaine-induced paranoia and psychosis proneness. Am. J. Psychiatry 1991, 148, 1708–1711. [Google Scholar]
- Serper, M.R.; Chou, J.C.; Allen, M.H.; Czobor, P.; Cancro, R. Symptomatic overlap of cocaine intoxication and acute schizophrenia at emergency presentation. Schizophr. Bull. 1999, 25, 387–394. [Google Scholar] [CrossRef]
- Sherer, M.A.; Kumor, K.M.; Cone, E.J.; Jaffe, J.H. Suspiciousness induced by four-hour intravenous infusions of cocaine. Preliminary findings. Arch. Gen. Psychiatry 1998, 45, 673–677. [Google Scholar]
- Taylor, W.A.; Staby, A.E. Acute treatment of alcohol and cocaine emergencies. Recent Dev. Alcohol. 1992, 10, 179–191. [Google Scholar]
- Tueth, M.J. High incidence of psychosis in cocaine intoxication and preventing violence. Am. J. Emerg. Med. 1993, 11, 676. [Google Scholar] [CrossRef]
- Cocaine and related disorders. Available online: http://www.minddisorders.com/Br-Del/Cocaine-and-related-disorders.html (accessed on 28 December 2012).
- Nunes, J.V.; Broderick, P.A. Novel research translates to clinical cases of schizophrenic and cocaine psychosis. Neuropsychiatr. Dis. Treat. 2007, 3, 475–485. [Google Scholar]
- Waters, F. Auditory Hallucinations in Psychiatric Illness. Psychiatr. Times 2010, 27, 3. [Google Scholar]
- Satel, S.L.; Seibyl, J.P.; Charney, D.S. Prolonged cocaine psychosis implies underlying major psychopathology. J. Clin. Psychiatry 1991, 52, 349–350. [Google Scholar]
- Lieberman, J.A.; Kinon, B.I.; Loebel, A.D. Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr. Bull. 1990, 16, 97–110. [Google Scholar] [CrossRef]
- Buckley, P.F. Substance abuse in schizophrenia: A review. J. Clin. Psychiatry 1998, 59, S26–S30. [Google Scholar]
- Muesser, K.T.; Nishith, P.; Tracy, H.; de Girolamo, J.; Molinaro, M. Expectations and motives for substance use in schizophrenia. Schizophr. Bull. 1995, 21, 367–378. [Google Scholar] [CrossRef]
- Caton, C.L.; Samet, S.; Hasin, D.S. When acute-stage psychosis and substance use co-occur: Differentiating substance-induced and primary psychotic disorders. J. Psychiatr. Pract. 2000, 6, 256–266. [Google Scholar] [CrossRef]
- Batki, S.L.; Harris, D.S. Quantitative drug levels in stimulant psychosis: Relationship to symptom severity, catecholamines and hyperkinesia. Am. J. Addict. 2004, 13, 461–470. [Google Scholar] [CrossRef]
- Rosse, R.; Deutsch, S.; Chilton, M. Cocaine addicts prone to cocaine-induced psychosis have lower body mass index than cocaine addicts resistant to cocaine-induced psychosis—Implications for the cocaine model of psychosis proneness. Isr. J. Psychiatry Relat. Sci. 2005, 42, 45–50. [Google Scholar]
- Boutros, N.N.; Gooding, D.; Sundaresan, K.; Burroughs, S.; Johanson, C.E. Cocaine-dependence and cocaine-induced paranoia and mid-latency auditory evoked responses and sensory gating. Psychiatry Res. 2006, 145, 147–154. [Google Scholar] [CrossRef]
- Tang, Y.L.; Kranzler, H.R.; Gelernter, J.; Farrer, L.A.; Cubells, J.F. Comorbid psychiatric diagnoses and their association with cocaine-induced psychosis in cocaine-dependent subjects. Am. J. Addict. 2007, 16, 343–351. [Google Scholar] [CrossRef]
- Merlo, L.J.; Carnes, P.J.; Gold, M.S. Response to “Comorbid psychiatric diagnoses and their association with cocaine-induced psychosis in cocaine-dependent subjects”. Am. J. Addict. 2008, 17, 247–248. [Google Scholar] [CrossRef]
- Tang, Y.L.; Kranzler, H.R.; Gelernter, J.; Farrer, L.A.; Pearson, D.; Cubells, J.F. Transient Cocaine-Associated Behavioral Symptoms Rated with a New Instrument, the Scale for Assessment of Positive Symptoms for Cocaine-Induced Psychosis (SAPS-CIP). Am. J. Addict. 2009, 18, 339–345. [Google Scholar]
- Roncero, C.; Daigre, C.; Gonzalvo, B.; Valero, S.; Castells, X.; Grau-López, L.; Eiroa-Orosa, F.J.; Casas, M. Risk factors for cocaine-induced psychosis in cocaine-dependent patients. Eur. Psychiatry 2013, 28, 141–146. [Google Scholar] [CrossRef]
- Vorspan, F.; Bloch, V.; Brousse, G.; Bellais, L.; Gascon, J.; Lépine, J.P. Prospective assessment of transient cocaine-induced psychotic symptoms in a clinical setting. Am. J. Addict. 2011, 20, 535–537. [Google Scholar] [CrossRef]
- Fiorentini, A.; Volonteri, L.S.; Dragogna, F.; Rovera, C.; Maffini, M.; Mauri, M.C.; Altamura, C.A. Substance-Induced Psychoses: A Critical Review of the Literature. Curr. Drug Abuse Rev. 2011, 4, 228–240. [Google Scholar] [CrossRef]
- Vorspan, F.; Brousse, G.; Bloch, V.; Bellais, L.; Romo, L.; Guillem, E.; Coeuru, P.; Lépine, J.P. Cocaine-induced psychotic symptoms in French cocaine addicts. Psychiatry Res. 2013, 200, 1074–1076. [Google Scholar]
- Delavenne, H.; Garcia, F.D.; Lacoste, J.; Cortese, S.; Charles-Nicolas, A.; Ballon, N. Psychosis in a cocaine-dependent patient with ADHD during treatment with methylphenidate. Gen. Hosp. Psychiatry 2012. [Google Scholar] [CrossRef]
- Davis, M. The mammalian startle response. In Neural Mechanisms of Startle Behavior; Plenum Press: New York, NY, USA, 1984; pp. 287–342. [Google Scholar]
- Valasamis, B.; Schmid, S. Habituation and Prepulse Inhibition of Acoustic Startle in Rodents. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Lang, P.J.; Davis, M. Emotion, motivation, and the brain: Reflex foundations in animal and human research. Prog. Brain Res. 2006, 156, 3–29. [Google Scholar] [CrossRef]
- Ilango, A.; Shumake, J.; Wetzel, W.; Scheich, H.; Ohl, F.W. The role of dopamine in the context of aversive stimuli with particular reference to acoustically signaled avoidance learning. Front. Neurosci. 2012, 6, 132. [Google Scholar]
- Parker, K.J.; Hyde, S.A.; Buckmaster, C.L.; Tanaka, S.M.; Brewster, K.K.; Schatzberg, A.F.; Lyons, D.M.; Woodward, S.H. Somatic and neuroendocrine responses to standard and biologically salient acoustic startle stimuli in monkeys. Psychoneuroendocrinology 2011, 36, 547–556. [Google Scholar] [CrossRef]
- Hoffman, H.S.; Ison, J.R. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol. Rev. 1980, 87, 175–189. [Google Scholar] [CrossRef]
- Peak, H. Time order error in successive judgments and in reflexes. I. Inhibition of the judgment and the reflex. J. Exp. Psychol. 1939, 25, 535–565. [Google Scholar] [CrossRef]
- Hoffman, H.S.; Fleshler, M. Startle reaction: Modification by background acoustic stimulation. Science 1963, 141, 928–930. [Google Scholar]
- Hoffman, H.S.; Searle, J.L. Acoustic variables in the modification of startle reaction in the rat. J. Comp. Physiol. Psychol. 1965, 60, 53–58. [Google Scholar] [CrossRef]
- Pickney, L.A. Inhibition of the startle reflex in the rat by prior tactile stimulation. Anim. Learn. Behav. 1976, 4, 467–472. [Google Scholar] [CrossRef]
- Ison, J.R.; Hammond, G.R. Modification of the startle reflex in the rat by changes in the auditory and visual environments. J. Comp. Physiol. Psychol. 1971, 75, 435–452. [Google Scholar] [CrossRef]
- Ray, W.J.; Molnar, C.; Aikins, D.; Yamasaki, A.; Newman, M.G.; Castonguay, L.; Borkovec, T.D. Startle response in generalized anxiety disorder. Depress Anxiety 2009, 26, 147–154. [Google Scholar] [CrossRef]
- Conzelmann, A.; Mucha, R.F.; Jacob, C.P.; Weyers, P.; Romanos, J.; Gerdes, A.B.; Baehne, C.G.; Boreatti-Hümmer, A.; Heine, M.; Alpers, G.W.; et al. Abnormal affective responsiveness in attention-deficit/hyperactivity disorder: Subtype differences. Biol. Psychiatry 2009, 65, 578–585. [Google Scholar] [CrossRef]
- Geyer, M.A.; Braff, D.L. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr. Bull. 1987, 13, 643–668. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Caine, S.B.; Braff, D.L.; Geyer, M.A. The neural substrates of sensorimotor gating of the startle reflex: A review of recent findings and their implications. J. Psychopharmacol. 1992, 6, 176–190. [Google Scholar] [CrossRef]
- Ralph, R.J.; Paulus, M.P.; Fumagalli, F.; Caron, M.G.; Geyer, M.A. Prepulse inihibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: Differential effects of D1 and D2 receptor antagonists. J. Neurosci. 2001, 21, 305–313. [Google Scholar]
- Faraday, M.M.; Grunberg, N.E. The importance of acclimation in acoustic startle amplitude and pre-pulse inhibition testing of male and female rats. Pharmacol. Biochem. Behav. 2000, 66, 375–381. [Google Scholar] [CrossRef]
- Doherty, J.M.; Masten, V.L.; Powell, S.B.; Ralph, R.J.; Klamer, D.; Low, M.J.; Geyer, M.A. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology 2008, 33, 2648–2656. [Google Scholar] [CrossRef]
- Meincke, U.; Light, G.A.; Geyer, M.A.; Braff, D.L.; Gouzoulis-Mayfrank, E. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry Res. 2004, 126, 51–61. [Google Scholar] [CrossRef]
- Quiñones-Jenab, V.; Ho, A.; Schlussman, S.D.; Franck, J.; Kreek, M.J. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav. Brain Res. 1999, 101, 15–20. [Google Scholar] [CrossRef]
- Quiñones-Jenab, V.; Perrotti, L.I.; Fabian, S.J.; Chin, J.; Russo, S.J.; Jenab, S. Endocrinological basis of sex differences in cocaine-induced behavioral responses. Ann. N. Y. Acad. Sci. 2001, 937, 140–171. [Google Scholar]
- Broderick, P.A.; Ho, H.; Huang, J.; Wat, K.; Shahidullah, J.; Murthy, V.; Wenning, L.; Buchheim, L. Caffeine may neuroprotect against cocaine-induced dopamine release in accumbens of female rats. Soc. Neurosci. Abstr. 2007, 33. Program Poster 767.7. [Google Scholar]
- Nesbitt, J.; Raju, C.; Vidal, L.; Alimova, A.; Steiner, J.; Broderick, P.A.; Friedman, E. Sexual dimorphism studies of cocaine and caffeine in conditioned place preference: Estrous cycle stage and open-field behaviors. Soc. Neurosci. Abstr. 2008, 34. Program Poster 561.23/FF33. [Google Scholar]
- Broderick, P.A. Studies of oxidative stress mechanisms using a morphine/ascorbate animal model and novel N-stearoyl cerebroside and laurate sensors. J. Neural Transm. 2008, 115, 7–17. [Google Scholar] [CrossRef]
- Lynch, W.J.; Roth, M.E.; Mickelberg, J.L.; Carroll, M.E. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol. Biochem. Behav. 2001, 68, 641–646. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Becker, J.B.; Molenda, H.; Hummer, D.L. Gender Differences in the Behavioral Responses to Cocaine and Amphetamine. Ann. N. Y. Acad. Sci. 2001, 937, 172–187. [Google Scholar] [CrossRef]
- Becker, J.B.; Hu, M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008, 29, 36–47. [Google Scholar] [CrossRef]
- Wissman, A.M.; McCollum, A.F.; Huang, G.-Z.; Nikrodhanond, A.A.; Woolley, C.S. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 2011, 61, 217–227. [Google Scholar] [CrossRef]
- Jackson, L.; Robinson, T.; Becker, J. Sex Differences and Hormonal Influences on Acquisition of Cocaine Self-Administration in Rats. Neuropsychopharmacology 2006, 31, 129–138. [Google Scholar]
- Lehmann, J.; Pryce, C.; Feldon, J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav. Brain Res. 1999, 104, 113–117. [Google Scholar] [CrossRef]
- Błaszczyk, J.; Tajchert, K. Sex and strain differences of acoustic startle reaction development in adolescent albino Wistar and hooded rats. Acta Neurobiol. Exp. (Wars.) 1996, 56, 919–925. [Google Scholar]
- Plappert, C.F.; Rodenbücher, A.M.; Pilz, P.K. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiol. Behav. 2005, 84, 585–594. [Google Scholar] [CrossRef]
- Fletcher, P.J.; Selhi, Z.F.; Azampanah, A.; Sills, T.L. Reduced brain serotonin activity disrupts prepulse inhibition of the acoustic startle reflex. Effects of 5,7-dihydroxytryptamine and p-chlorophenylalanine. Neuropsychopharmacology 2001, 24, 399–409. [Google Scholar] [CrossRef]
- Prinssen, E.P.; Assié, M.B.; Koek, W.; Kleven, M.S. Depletion of 5-HT disrupts prepulse inhibition in rats: Dependence on the magnitude of depletion, and reversal by a 5-HT precursor. Neuropsychopharmacology 2002, 26, 340–347. [Google Scholar] [CrossRef]
- Semenova, S.; Hoyer, D.; Geyer, M.A.; Markou, A. Somatostatin-28 modulates prepulse inhibition of the acoustic startle response, reward processes and spontaneous locomotor activity in rats. Neuropeptides 2010, 44, 421–429. [Google Scholar] [CrossRef]
- Kinkead, B.; Yan, F.; Owens, M.J.; Nemeroff, C.B. Endogenous neurotensin is involved in estrous cycle related alterations in prepulse inhibition of the acoustic startle reflex in female rats. Psychoneuroendocrinology 2008, 33, 178–187. [Google Scholar] [CrossRef]
- Metherate, R.; Intskirveli, I.; Kawai, H.D. Nicotinic filtering of sensory processing in auditory cortex. Front. Behav. Neurosci. 2012, 6, 1–8. [Google Scholar]
- Rudomin, P. Central control of information transmission through the intraspinal arborizations of sensory fibers examined 100 years after Ramón y Cajal. Prog. Brain Res. 2002, 136, 409–421. [Google Scholar] [CrossRef]
- Rose, P.K.; Scott, S.H. Sensory-motor control: A long-awaited behavioral correlate of presynaptic inhibition. Nat. Neurosci. 2003, 6, 1243–1245. [Google Scholar] [CrossRef]
- Seki, K.; Perlmutter, S.I.; Fetz, E.E. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat. Neurosci. 2003, 6, 1309–1316. [Google Scholar] [CrossRef]
- Koch, M. The neurobiology of startle. Prog. Neurobiol. 1999, 59, 107–128. [Google Scholar] [CrossRef]
- Koch, M.; Friauf, E. Glycine receptors in the caudal pontine reticular formation: Are they important for the inhibition of the acoustic startle response? Brain Res. 1995, 671, 63–72. [Google Scholar] [CrossRef]
- Koch, M.; Schnitzler, H.U. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 1997, 89, 35–49. [Google Scholar] [CrossRef]
- Koch, M.; Kungel, M.; Herbert, H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp. Brain Res. 1993, 97, 71–82. [Google Scholar]
- Koch, M.; Fendt, M.; Kretschmer, B.D. Role of the substantia nigra pars reticulata in sensorimotor gating, measured by prepulse inhibition of startle in rats. Behav. Brain Res. 2000, 117, 153–162. [Google Scholar] [CrossRef]
- Fendt, M.; Li, L.; Yeomans, J.S. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl.) 2001, 156, 216–224. [Google Scholar] [CrossRef]
- Leumann, L.; Sterchi, D.; Vollenweider, F.; Ludewig, K.; Früh, H. A neural network approach to the acoustic startle reflex and prepulse inhibition. Brain Res. Bull. 2001, 56, 101–110. [Google Scholar] [CrossRef]
- Leitner, D.S.; Cohen, M.E. Role of the inferior colliculus in the inhibition of acoustic startle in the rat. Physiol. Behav. 1985, 34, 65–70. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Broderick, P.A.; Rosenbaum, T. Sex-Specific Brain Deficits in Auditory Processing in an Animal Model of Cocaine-Related Schizophrenic Disorders. Brain Sci. 2013, 3, 504-520. https://doi.org/10.3390/brainsci3020504
Broderick PA, Rosenbaum T. Sex-Specific Brain Deficits in Auditory Processing in an Animal Model of Cocaine-Related Schizophrenic Disorders. Brain Sciences. 2013; 3(2):504-520. https://doi.org/10.3390/brainsci3020504
Chicago/Turabian StyleBroderick, Patricia A., and Taylor Rosenbaum. 2013. "Sex-Specific Brain Deficits in Auditory Processing in an Animal Model of Cocaine-Related Schizophrenic Disorders" Brain Sciences 3, no. 2: 504-520. https://doi.org/10.3390/brainsci3020504