Enhanced Novel Object Recognition and Spatial Memory in Rats Selectively Bred for High Nicotine Preference
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Behavioral Experiments
2.3. Novel Object Recognition Test
2.4. Novel Location Recognition Test
2.5. Morris Water Maze
2.6. Statistical Analysis
3. Results
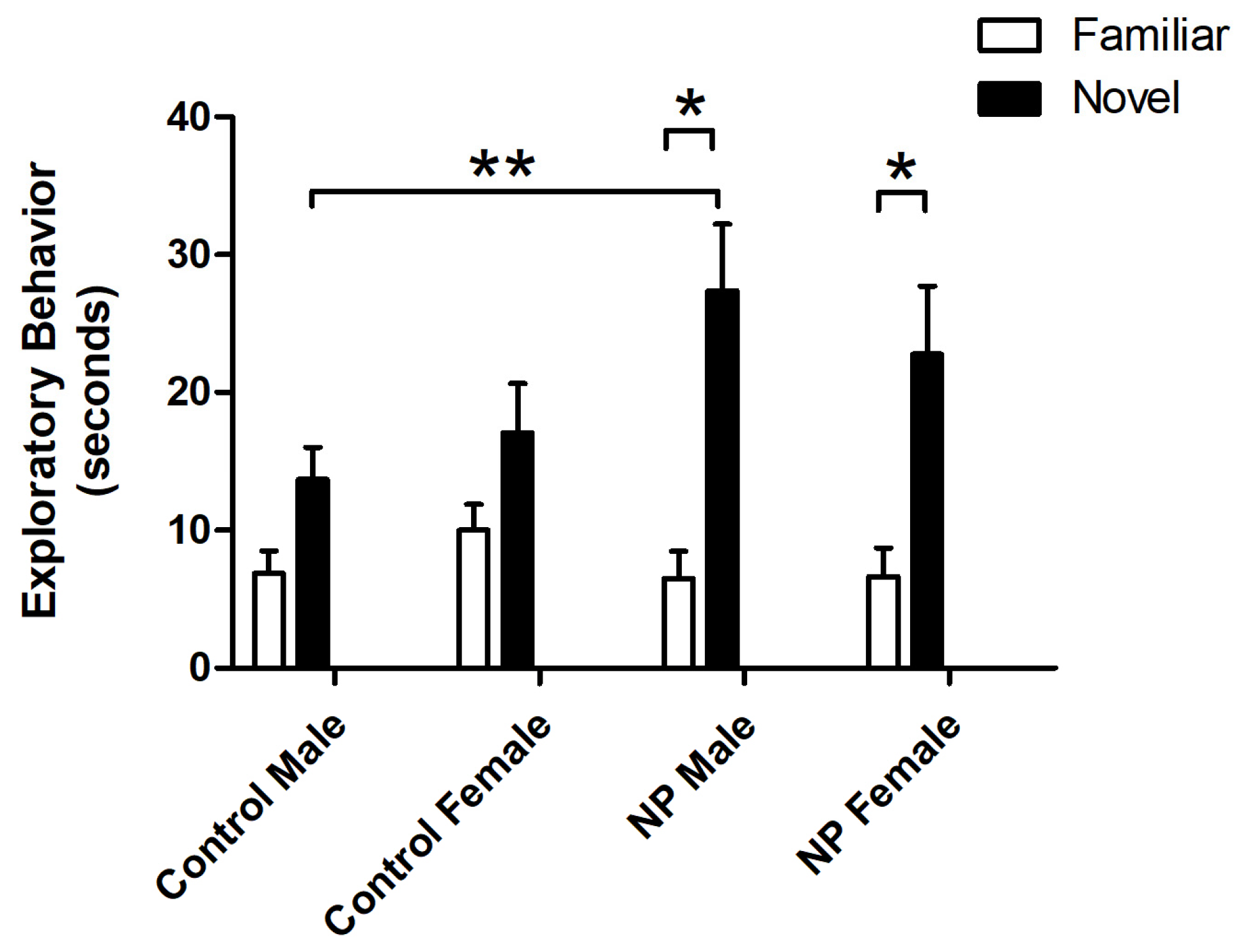
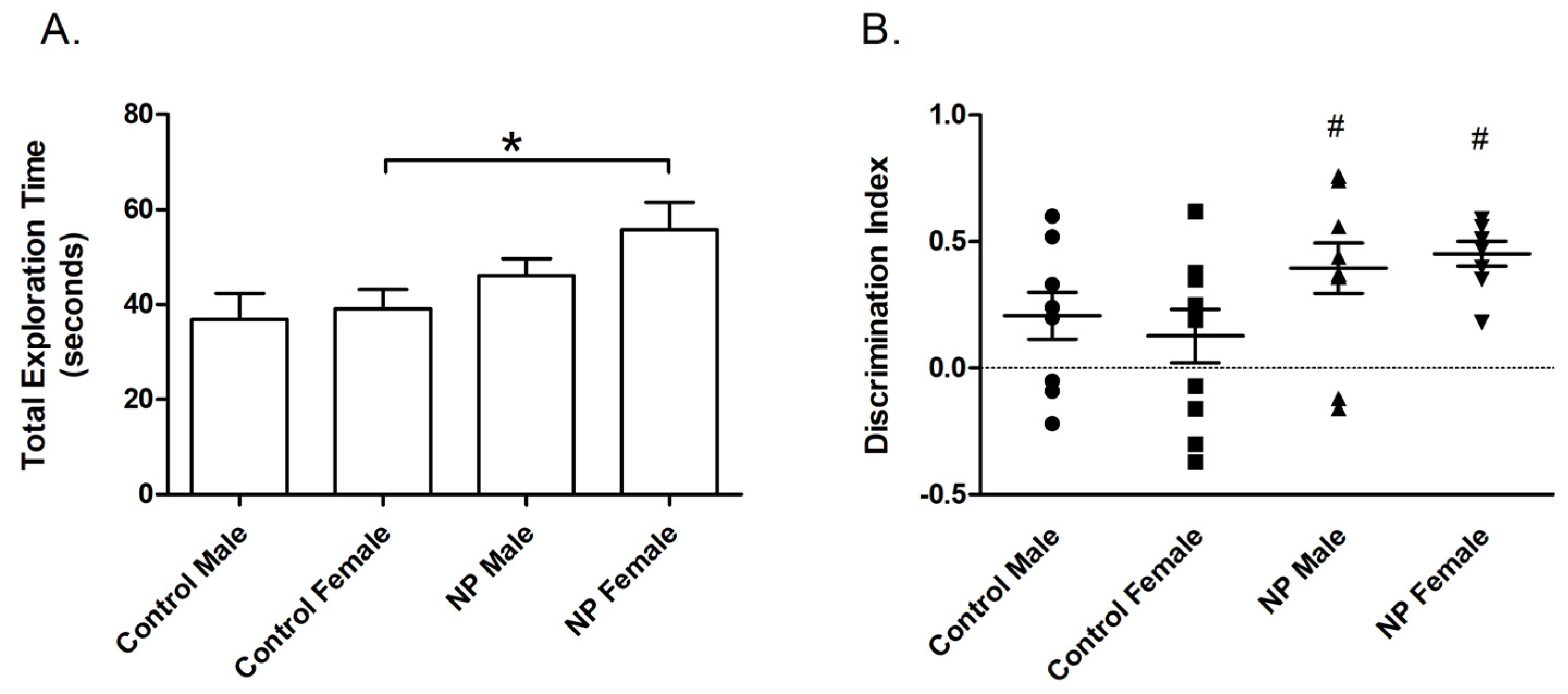
3.1. Novel Object Recognition
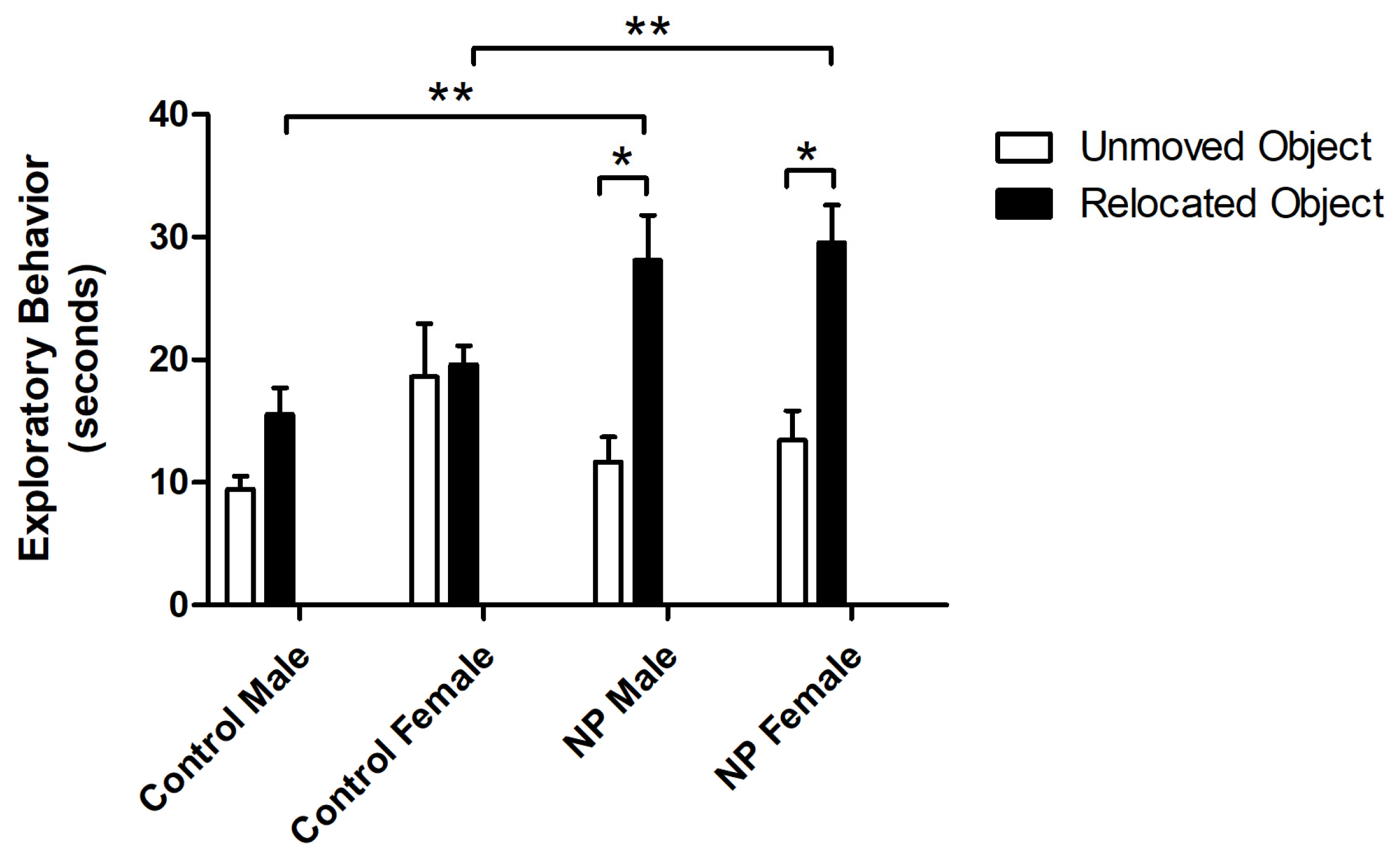
3.2. Novel Location Recognition Test
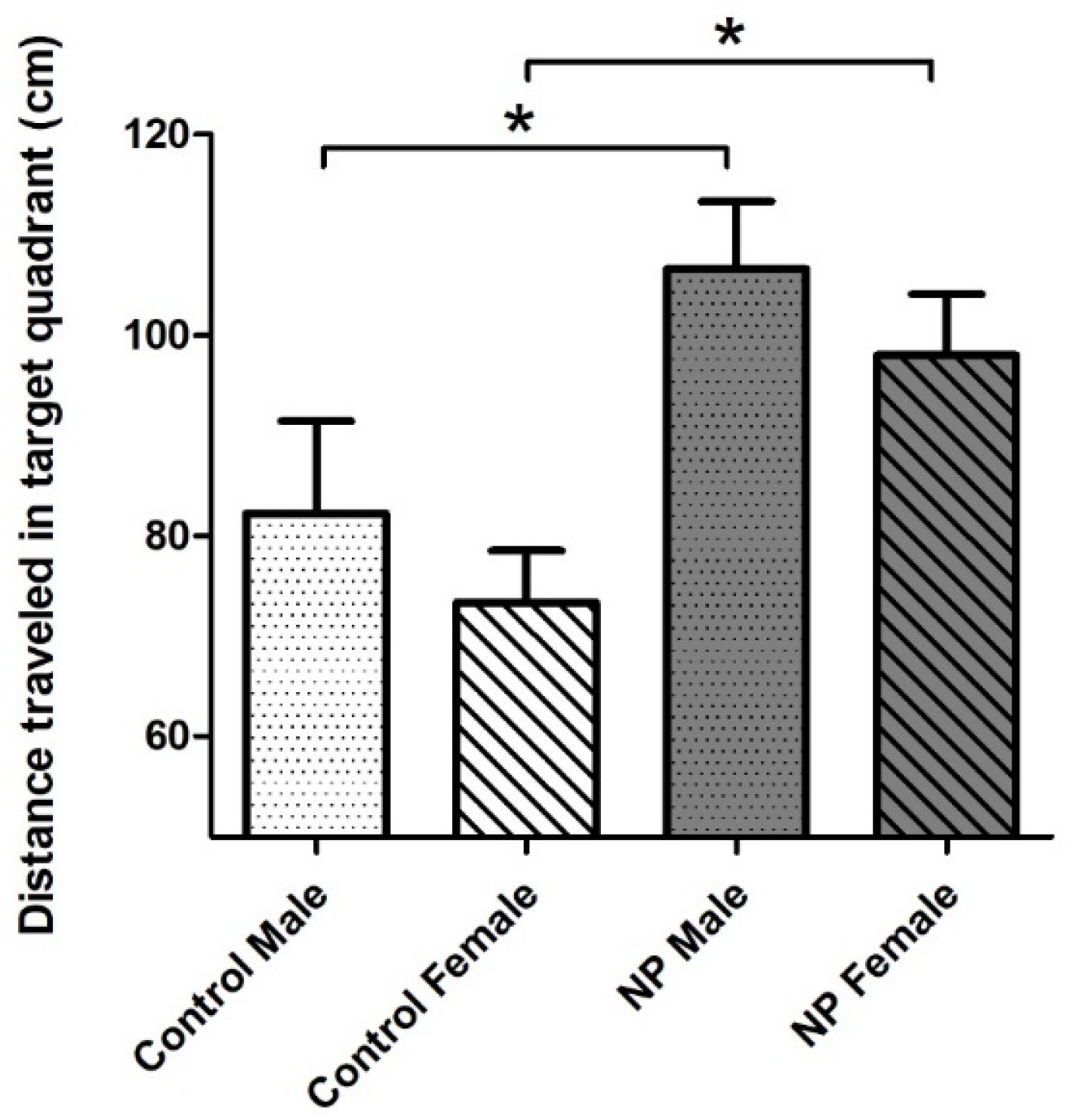
3.3. Morris Water Maze
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gould, T.J.; Leach, P.T. Cellular, Molecular, and Genetic Substrates Underlying the Impact of Nicotine on Learning. Neurobiol. Learn. Mem. 2014, 107, 108–132. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.C.; Vicens, P.; Carrasco, M.C.; Redolat, R. Effects of Nicotine on Spatial Learning in C57BL Mice. Behav. Pharmacol. 1999, 10, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, P.; Kellar, K.; Aisen, P.; White, H.; Wesnes, K.; Coderre, E.; Pfaff, A.; Wilkins, H.; Howard, D.; Levin, E.D. Nicotine Treatment of Mild Cognitive Impairment: A 6-Month Double-Blind Pilot Clinical Trial. Neurology 2012, 78, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Nesil, T.; Kanit, L.; Pogun, S. Nicotine Intake and Problem Solving Strategies Are Modified during a Cognitively Demanding Water Maze Task in Rats. Pharmacol. Biochem. Behav. 2015, 138, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Valentine, G.; Sofuoglu, M. Cognitive Effects of Nicotine: Recent Progress. Curr. Neuropharmacol. 2018, 16, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.O.; Brown, M.W.; McCabe, B.J.; Aggleton, J.P. Effects of the Novelty or Familiarity of Visual Stimuli on the Expression of the Immediate Early Gene C-Fos in Rat Brain. Neuroscience 1995, 69, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Tripathi, S.J.; Srikumar, B.N.; Raju, T.R.; Shankaranarayana Rao, B.S. Chronic Brain Stimulation Rewarding Experience Ameliorates Depression-Induced Cognitive Deficits and Restores Aberrant Plasticity in the Prefrontal Cortex. Brain Stimul. 2019, 12, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.J.; Chakraborty, S.; Srikumar, B.N.; Raju, T.R.; Shankaranarayana Rao, B.S. Inactivation of Basolateral Amygdala Prevents Stress-Induced Astroglial Loss in the Prefrontal Cortex. Mol. Neurobiol. 2019, 56, 350–366. [Google Scholar] [CrossRef]
- Barker, G.R.I.; Bird, F.; Alexander, V.; Warburton, E.C. Recognition Memory for Objects, Place, and Temporal Order: A Disconnection Analysis of the Role of the Medial Prefrontal Cortex and Perirhinal Cortex. J. Neurosci. 2007, 27, 2948–2957. [Google Scholar] [CrossRef]
- Barker, G.R.I.; Warburton, E.C. When Is the Hippocampus Involved in Recognition Memory? J. Neurosci. 2011, 31, 10721–10731. [Google Scholar] [CrossRef] [PubMed]
- Weible, A.P.; Rowland, D.C.; Pang, R.; Kentros, C. Neural Correlates of Novel Object and Novel Location Recognition Behavior in the Mouse Anterior Cingulate Cortex. J. Neurophysiol. 2009, 102, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.W.; Adoff, M.D.; Wilkinson, D.S.; Gould, T.J. The Effects of Acute, Chronic, and Withdrawal from Chronic Nicotine on Novel and Spatial Object Recognition in Male C57BL/6J Mice. Psychopharmacology 2011, 217, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Izumi, S.; Fukao, A.; Nishitani, N.; Deyama, S.; Kaneda, K. Nicotine Enhances Object Recognition Memory through Inhibition of Voltage-Dependent Potassium 7 Channels in the Medial Prefrontal Cortex of Mice. J. Pharmacol. Sci. 2021, 147, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Melichercik, A.M.; Elliott, K.S.; Bianchi, C.; Ernst, S.M.; Winters, B.D. Nicotinic Receptor Activation in Perirhinal Cortex and Hippocampus Enhances Object Memory in Rats. Neuropharmacology 2012, 62, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Hancock, D.B.; Markunas, C.A.; Bierut, L.J.; Johnson, E.O. Human Genetics of Addiction: New Insights and Future Directions. Curr. Psychiatry Rep. 2018, 20, 8. [Google Scholar] [CrossRef]
- Borrego-Soto, G.; Xitlalli Perez-Paramo, Y.; Hernández-Cabrera, F.; Miroslaba Alvarado-Monroy, F.; Borrego, G.; Robles-Zamora, A.; Lazarus, P.; Rojas-Martinez, A. Biochemical and Genetic Biomarkers Associated with Nicotine Dependence in Mexican Smokers. Pharmacol. Res. Perspect. 2023, 11, e01142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ahn, W.-Y.; Seweryn, M.; Sadee, W. Combined Genetic Influence of the Nicotinic Receptor Gene Cluster CHRNA5/A3/ B4 on Nicotine Dependence. BMC Genom. 2018, 19, 826. [Google Scholar] [CrossRef]
- Evans, D.E.; Macqueen, D.A.; Jentink, K.G.; Park, J.Y.; Lin, H.-Y.; Drobes, D.J. CHRNA5 Variants Moderate the Effect of Nicotine Deprivation on a Neural Index of Cognitive Control. Genes Brain Behav. 2014, 13, 626–632. [Google Scholar] [CrossRef]
- Kenney, J.W.; Gould, T.J. Modulation of Hippocampus-Dependent Learning and Synaptic Plasticity by Nicotine. Mol. Neurobiol. 2008, 38, 101–121. [Google Scholar] [CrossRef]
- Breckel, T.P.K.; Giessing, C.; Gieseler, A.; Querbach, S.; Reuter, M.; Thiel, C.M. Nicotinergic Modulation of Attention-Related Neural Activity Differentiates Polymorphisms of DRD2 and CHRNA4 Receptor Genes. PLoS ONE 2015, 10, e0126460. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.J.; Romm, E.; Campbell, S.M.; Collins, A.C. Variation of Nicotinic Binding Sites among Inbred Strains. Pharmacol. Biochem. Behav. 1989, 33, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Portugal, G.S.; Wilkinson, D.S.; Kenney, J.W.; Sullivan, C.; Gould, T.J. Strain-Dependent Effects of Acute, Chronic, and Withdrawal from Chronic Nicotine on Fear Conditioning. Behav. Genet. 2012, 42, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Golding, J.; Gregory, S.; Northstone, K.; Pembrey, M.; Watkins, S.; Iles-Caven, Y.; Suderman, M. Human Transgenerational Observations of Regular Smoking before Puberty on Fat Mass in Grandchildren and Great-Grandchildren. Sci. Rep. 2022, 12, 1139. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.J. Epigenetic and Long-Term Effects of Nicotine on Biology, Behavior, and Health. Pharmacol. Res. 2023, 192, 106741. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.M.; Sanders, K.N.; Wageman, C.R.; Knopik, V.S.; Stitzel, J.A.; O’Neill, H.C. Developmental Nicotine Exposure Precipitates Multigenerational Maternal Transmission of Nicotine Preference and ADHD-like Behavioral, Rhythmometric, Neuropharmacological, and Epigenetic Anomalies in Adolescent Mice. Neuropharmacology 2019, 149, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Nesil, T.; Kanit, L.; Li, M.D.; Pogun, S. Nine Generations of Selection for High and Low Nicotine Intake in Outbred Sprague-Dawley Rats. Behav. Genet. 2013, 43, 436–444. [Google Scholar] [CrossRef]
- Nesil, T.; Kanit, L.; Ugur, M.; Pogun, S. Nicotine Withdrawal in Selectively Bred High and Low Nicotine Preferring Rat Lines. Pharmacol. Biochem. Behav. 2015, 131, 91–97. [Google Scholar] [CrossRef]
- Ozturk, B.; Pogun, S.; Kanit, L. Increased Alcohol Preference and Intake in Nicotine-Preferring Rats. Am. J. Drug Alcohol. Abuse 2020, 46, 408–420. [Google Scholar] [CrossRef]
- Bayoglu, M.; Ozturk Bintepe, M.; Kanit, L.; Balkan, B.; Gozen, O.; Koylu, E.O.; Keser, A. Decreased Anxiety-like Behavior in a Selectively Bred High Nicotine-Preferring Rat Line. Int. J. Neurosci. 2023, 1–11. [Google Scholar] [CrossRef]
- Bardo, M.T.; Donohew, R.L.; Harrington, N.G. Psychobiology of Novelty Seeking and Drug Seeking Behavior. Behav. Brain Res. 1996, 77, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Bevins, R.A.; Besheer, J.; Palmatier, M.I.; Jensen, H.C.; Pickett, K.S.; Eurek, S. Novel-Object Place Conditioning: Behavioral and Dopaminergic Processes in Expression of Novelty Reward. Behav. Brain Res. 2002, 129, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Meliani, K. A New One-Trial Test for Neurobiological Studies of Memory in Rats. III. Spatial vs. Non-Spatial Working Memory. Behav. Brain Res. 1992, 51, 83–92. [Google Scholar] [CrossRef]
- Ennaceur, A.; Neave, N.; Aggleton, J.P. Spontaneous Object Recognition and Object Location Memory in Rats: The Effects of Lesions in the Cingulate Cortices, the Medial Prefrontal Cortex, the Cingulum Bundle and the Fornix. Exp. Brain Res. 1997, 113, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Facundo Morici, J.; Weisstaub, N.V.; Zold, C.L. Hippocampal-Medial Prefrontal Cortex Network Dynamics Predict Performance during Retrieval in a Context-Guided Object Memory Task. Proc. Natl. Acad. Sci. USA 2022, 119, e2203024119. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac: A Fast and Robust Software for Tracking Organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef]
- Morris, R. Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.Z.; Hassan, Z.; Has, C. Experimental Animals Morris Water Maze: A Versatile and Pertinent Tool for Assessing Spatial Learning and Memory. Exp. Anim. 2022, 71, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, F.E. Procedures for Detecting Outlying Observations in Samples. Technometrics 1969, 11, 21. [Google Scholar] [CrossRef]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 141, e58593. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A New One-Trial Test for Neurobiological Studies of Memory in Rats. 1: Behavioral Data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 2017, e55718. [Google Scholar] [CrossRef]
- Okada, K.; Hashimoto, K.; Kobayashi, K. Cholinergic Regulation of Object Recognition Memory. Front. Behav. Neurosci. 2022, 16, 996089. [Google Scholar] [CrossRef] [PubMed]
- Solari, N.; Hangya, B. Cholinergic Modulation of Spatial Learning, Memory and Navigation. Eur. J. Neurosci. 2018, 48, 2199–2230. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Dauvermann, M.R.; Lee, G.; Dawson, N. Glutamatergic Regulation of Cognition and Functional Brain Connectivity: Insights from Pharmacological, Genetic and Translational Schizophrenia Research. Br. J. Pharmacol. 2017, 174, 3136–3160. [Google Scholar] [CrossRef]
- Pistillo, F.; Clementi, F.; Zoli, M.; Gotti, C. Nicotinic, Glutamatergic and Dopaminergic Synaptic Transmission and Plasticity in the Mesocorticolimbic System: Focus on Nicotine Effects. Prog. Neurobiol. 2015, 124, 1–27. [Google Scholar] [CrossRef]
- Pons, S.; Fattore, L.; Cossu, G.; Tolu, S.; Porcu, E.; Mcintosh, J.M.; Changeux, J.P.; Maskos, U.; Fratta, W. Behavioral/Systems/Cognitive Crucial Role of 4 and 6 Nicotinic Acetylcholine Receptor Subunits from Ventral Tegmental Area in Systemic Nicotine Self-Administration. J. Neurosci. 2008, 28, 12318–12327. [Google Scholar] [CrossRef] [PubMed]
- Maskos, U.; Molles, B.E.; Pons, S.; Besson, M.; Guiard, B.P.; Guilloux, J.P.; Evrard, A.; Cazala, P.; Cormier, A.; Mameli-Engvall, M.; et al. Nicotine Reinforcement and Cognition Restored by Targeted Expression of Nicotinic Receptors. Nature 2005, 436, 103–107. [Google Scholar] [CrossRef]
- Chao, O.Y.; Nikolaus, S.; Yang, Y.-M.; Huston, J.P. Neuronal Circuitry for Recognition Memory of Object and Place in Rodent Models. Neurosci. Biobehav. Rev. 2022, 141, 104855. [Google Scholar] [CrossRef]
- Perry, D.C.; Xiao, Y.; Nguyen, H.N.; Musachio, J.L.; Dávila-García, M.I.; Kellar, K.J. Measuring Nicotinic Receptors with Characteristics of A4b2, A3b2 and A3b4 Subtypes in Rat Tissues by Autoradiography. J. Neurochem. 2002, 82, 468–481. [Google Scholar] [CrossRef]
- Wada, E.; Wada, K.; Boulter, J.; Deneris, E.; Heinemann, S.; Patrick, J.; Swanson, L.W. Distribution of Alpha 2, Alpha 3, Alpha 4, and Beta 2 Neuronal Nicotinic Receptor Subunit MRNAs in the Central Nervous System: A Hybridization Histochemical Study in the Rat. J. Comp. Neurol. 1989, 284, 314–335. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Izumi, S.; Fukao, A.; Ito, S.; Nishitani, N.; Deyama, S.; Kaneda, K. Nicotine Enhances Object Recognition Memory via Stimulating A4β2 and A7 Nicotinic Acetylcholine Receptors in the Medial Prefrontal Cortex of Mice. Biol. Pharm. Bull. 2021, 44, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Arthur, D.; Levin, E.D. Chronic Inhibition of A4β2 Nicotinic Receptors in the Ventral Hippocampus of Rats: Impacts on Memory and Nicotine Response. Psychopharmacology 2002, 160, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Petro, A.; Rezvani, A.H.; Pollard, N.; Christopher, N.C.; Strauss, M.; Avery, J.; Nicholson, J.; Rose, J.E. Nicotinic A7- or Β2-Containing Receptor Knockout: Effects on Radial-Arm Maze Learning and Long-Term Nicotine Consumption in Mice. Behav. Brain Res. 2009, 196, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, D.B.; Sandager-Nielsen, K.; Dyhring, T.; Smith, M.; Jacobsen, A.-M.; Nielsen, E.Ø.; Grunnet, M.; Christensen, J.K.; Peters, D.; Kohlhaas, K.; et al. Augmentation of Cognitive Function by NS9283, a Stoichiometry-Dependent Positive Allosteric Modulator of A2-and A4-Containing Nicotinic Acetylcholine Receptors Keywords Allosteric Modulation; Cognitive Dysfunction; Therapeutics. Br. J. Pharmacol. 2012, 167, 164–182. [Google Scholar] [CrossRef]
- Decker, M.W.; Bannon, A.W.; Curzon, P.; Gunther, K.L.; Brioni, J.D.; Holladay, M.W.; Lin, N.H.; Li, Y.; Daanen, J.F.; Buccafusco, J.J.; et al. ABT-089 [2-Methyl-3-(2-(S)-Pyrrolidinylmethoxy)Pyridine Dihydrochloride]: II. A Novel Cholinergic Channel Modulator with Effects on Cognitive Performance in Rats and Monkeys. J. Pharmacol. Exp. Ther. 1997, 283, 247–258. [Google Scholar]
- Rollema, H.; Hajós, M.; Seymour, P.A.; Kozak, R.; Majchrzak, M.J.; Guanowsky, V.; Horner, W.E.; Chapin, D.S.; Hoffmann, W.E.; Johnson, D.E.; et al. Preclinical Pharmacology of the α4β2 NAChR Partial Agonist Varenicline Related to Effects on Reward, Mood and Cognition. Biochem. Pharmacol. 2009, 78, 813–824. [Google Scholar] [CrossRef]
- Zorzo, C.; Arias, J.L.; Méndez, M. Are There Sex Differences in Spatial Reference Memory in the Morris Water Maze? A Large-Sample Experimental Study. Learn. Behav. 2023, 1, 3. [Google Scholar] [CrossRef]
- Safari, S.; Ahmadi, N.; Mohammadkhani, R.; Ghahremani, R.; Khajvand-Abedeni, M.; Shahidi, S.; Komaki, A.; Salehi, I.; Asaad Karimi, S. Sex Differences in Spatial Learning and Memory and Hippocampal Long-Term Potentiation at Perforant Pathway-Dentate Gyrus (PP-DG) Synapses in Wistar Rats. Behav. Andbrain Funct. 2021, 17, 9. [Google Scholar] [CrossRef]
- Kanit, L.; Taşkiran, D.; Furedy, J.J.; Kulali, B.; McDonald, R.; Pöğün, S. Nicotine Interacts with Sex in Affecting Rat Choice between “Look-out” and “Navigational” Cognitive Styles in the Morris Water Maze Place Learning Task. Brain Res. Bull. 1998, 46, 441–445. [Google Scholar] [CrossRef]
- Kanit, L.; Taskiran, D.; Yilmaz, O.A.; Balkan, B.; Demirgören, S.; Furedy, J.J.; Pögün, S. Sexually Dimorphic Cognitive Style in Rats Emerges after Puberty. Brain Res. Bull. 2000, 52, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Sinal, T.S.; Kostenuik, M.A.; Ossenkopp, K.P.; Kavaliers, M. Sex Differences in Performance in the Morris Water Maze and the Effects of Initial Nonstationary Hidden Platform Training. Behav. Neurosci. 1996, 110, 1309–1320. [Google Scholar] [CrossRef]
- Chesler, E.J.; Juraska, J.M. Acute Administration of Estrogen and Progesterone Impairs the Acquisition of the Spatial Morris Water Maze in Ovariectomized Rats. Horm. Behav. 2000, 38, 234–242. [Google Scholar] [CrossRef]
- Wingo, T.; Nesil, T.; Choi, J.S.; Li, M.D. Novelty Seeking and Drug Addiction in Humans and Animals: From Behavior to Molecules. J. Neuroimmune Pharmacol. 2016, 11, 456–470. [Google Scholar] [CrossRef]
- Bardo, M.T.; Neisewander, J.L.; Kelly, T.H. Individual Differences and Social Influences on the Neurobehavioral Pharmacology of Abused Drugs. Pharmacol. Rev. 2013, 65, 255–290. [Google Scholar] [CrossRef]
- Ersche, K.D.; Turton, A.J.; Pradhan, S.; Bullmore, E.T.; Robbins, T.W. Drug Addiction Endophenotypes: Impulsive versus Sensation-Seeking Personality Traits. Biol. Psychiatry 2010, 68, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Dellu, F.; Piazza, P.V.; Mayo, W.; Le Moal, M.; Simon, H. Novelty-Seeking in Rats--Biobehavioral Characteristics and Possible Relationship with the Sensation-Seeking Trait in Man. Neuropsychobiology 1996, 34, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ballaz, S.J. Differential Novelty Detection in Rats Selectively Bred for Novelty-Seeking Behavior. Neurosci. Lett. 2009, 461, 45–48. [Google Scholar] [CrossRef]
- Cain, M.E.; Saucier, D.A.; Bardo, M.T. Novelty Seeking and Drug Use: Contribution of an Animal Model. Exp. Clin. Psychopharmacol. 2005, 13, 367–375. [Google Scholar] [CrossRef]
- Yang, Z.; Nesil, T.; Wingo, T.; Chang, S.L.; Li, M.D. HIV-1 Proteins Influence Novelty-Seeking Behavior and Alter Region-Specific Transcriptional Responses to Chronic Nicotine Treatment in HIV-1Tg Rats. Nicotine Tob. Res. 2017, 19, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Thorsell, A.; Slawecki, C.J.; El Khoury, A.; Mathe, A.A.; Ehlers, C.L. The Effects of Social Isolation on Neuropeptide Y Levels, Exploratory and Anxiety-Related Behaviors in Rats. Pharmacol. Biochem. Behav. 2006, 83, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Grahame, N.J.; Gould, T.D. Affect-Related Behaviors in Mice Selectively Bred for High and Low Voluntary Alcohol Consumption. Behav. Genet. 2012, 42, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Möller, C.; Wiklund, L.; Thorsell, A.; Hyytiä, P.; Heilig, M. Decreased Measures of Experimental Anxiety in Rats Bred for High Alcohol Preference. Alcohol. Clin. Exp. Res. 1997, 21, 656–660. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekci, E.; Gokmen, R.C.; Kanit, L.; Gozen, O.; Balkan, B.; Koylu, E.O.; Keser, A. Enhanced Novel Object Recognition and Spatial Memory in Rats Selectively Bred for High Nicotine Preference. Brain Sci. 2024, 14, 427. https://doi.org/10.3390/brainsci14050427
Bekci E, Gokmen RC, Kanit L, Gozen O, Balkan B, Koylu EO, Keser A. Enhanced Novel Object Recognition and Spatial Memory in Rats Selectively Bred for High Nicotine Preference. Brain Sciences. 2024; 14(5):427. https://doi.org/10.3390/brainsci14050427
Chicago/Turabian StyleBekci, Eren, Ramazan Can Gokmen, Lutfiye Kanit, Oguz Gozen, Burcu Balkan, Ersin O. Koylu, and Aysegul Keser. 2024. "Enhanced Novel Object Recognition and Spatial Memory in Rats Selectively Bred for High Nicotine Preference" Brain Sciences 14, no. 5: 427. https://doi.org/10.3390/brainsci14050427





