Attention-Modulated Cortical Responses as a Biomarker for Tinnitus †
Abstract
:1. Introduction
2. Methods
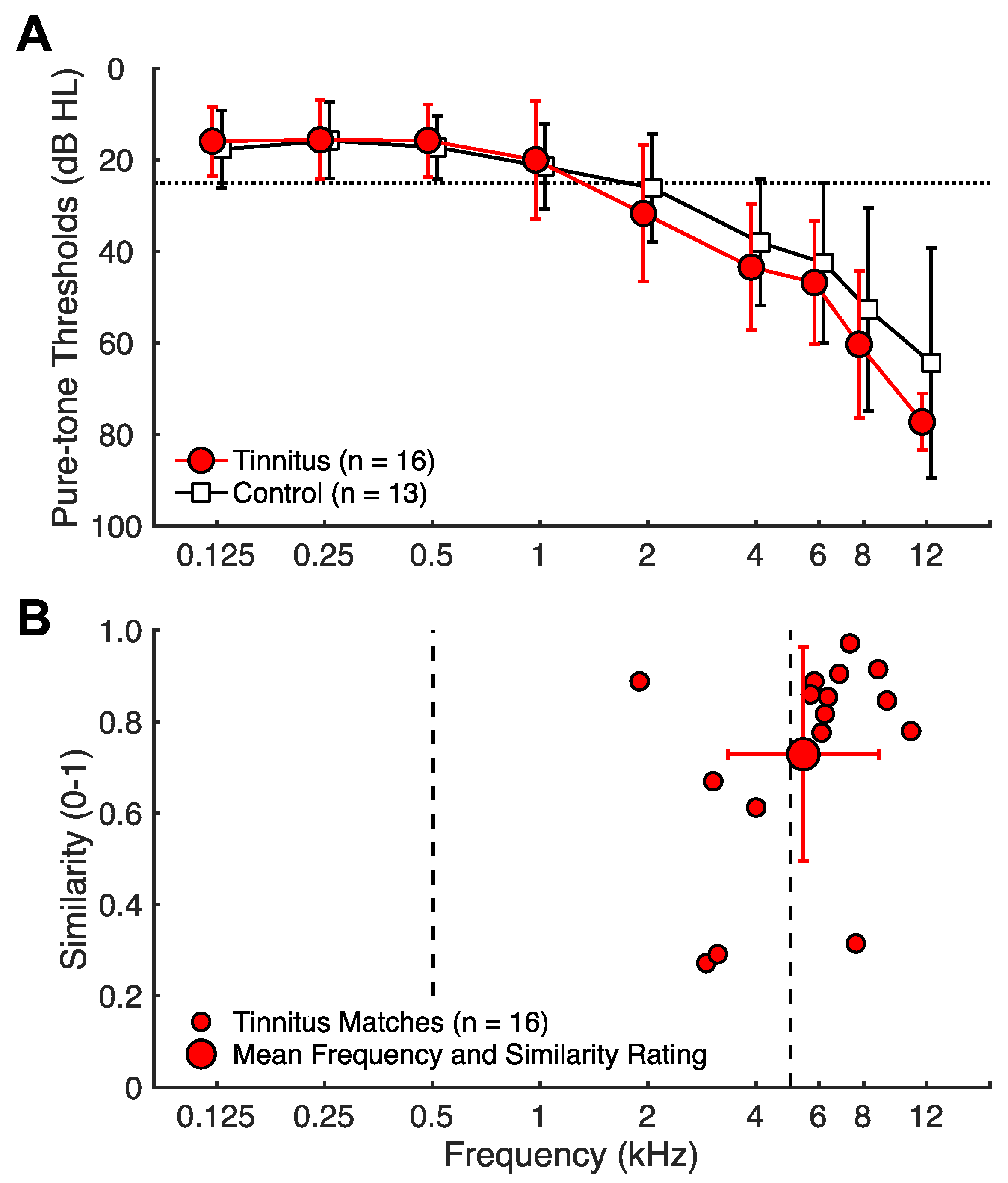
2.1. Subjects
2.2. Stimuli
2.3. Procedures
2.4. Data Acquisition and Analysis
2.5. Statistical Analysis
3. Results
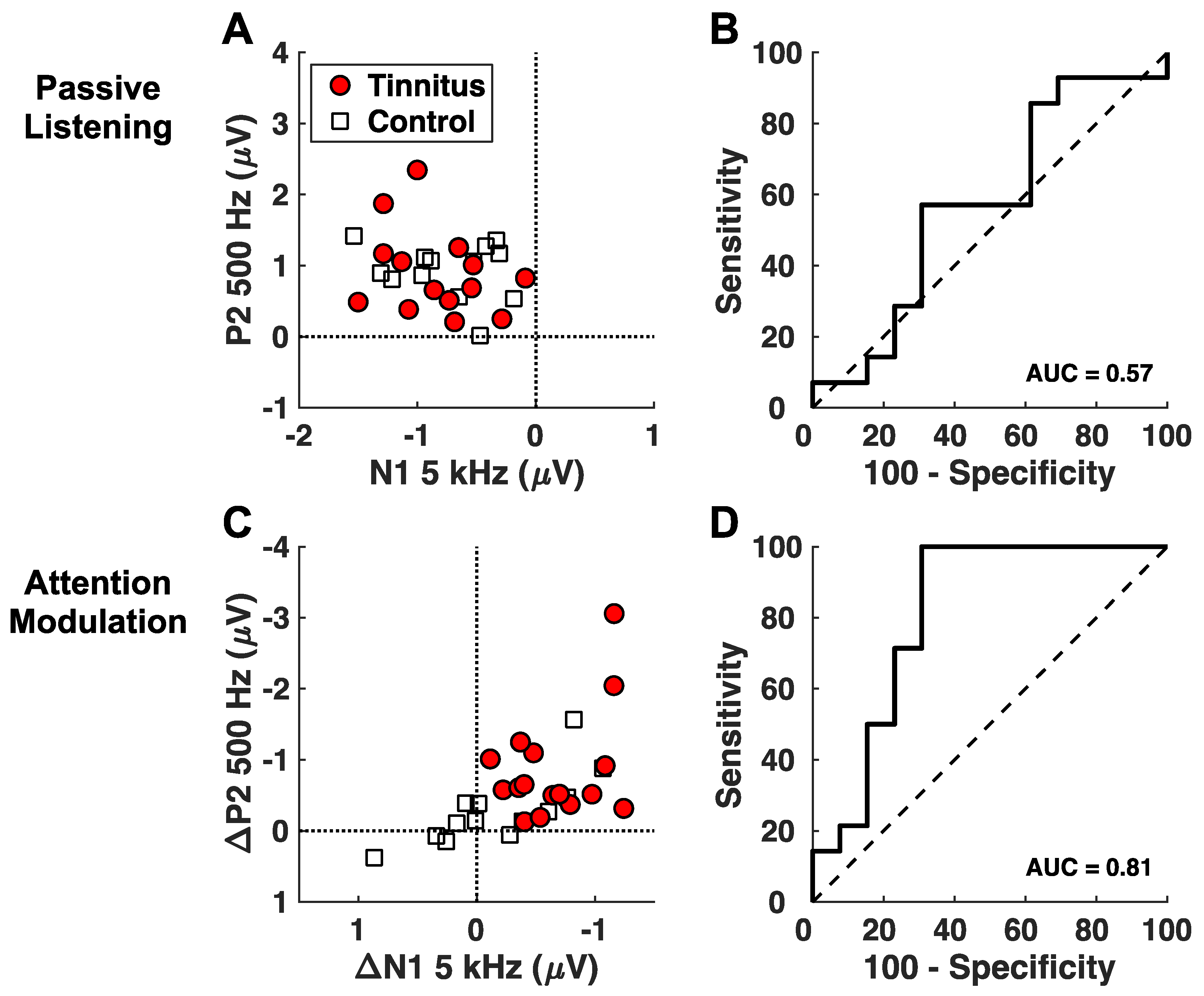
3.1. Cortical Responses in Passive Listening
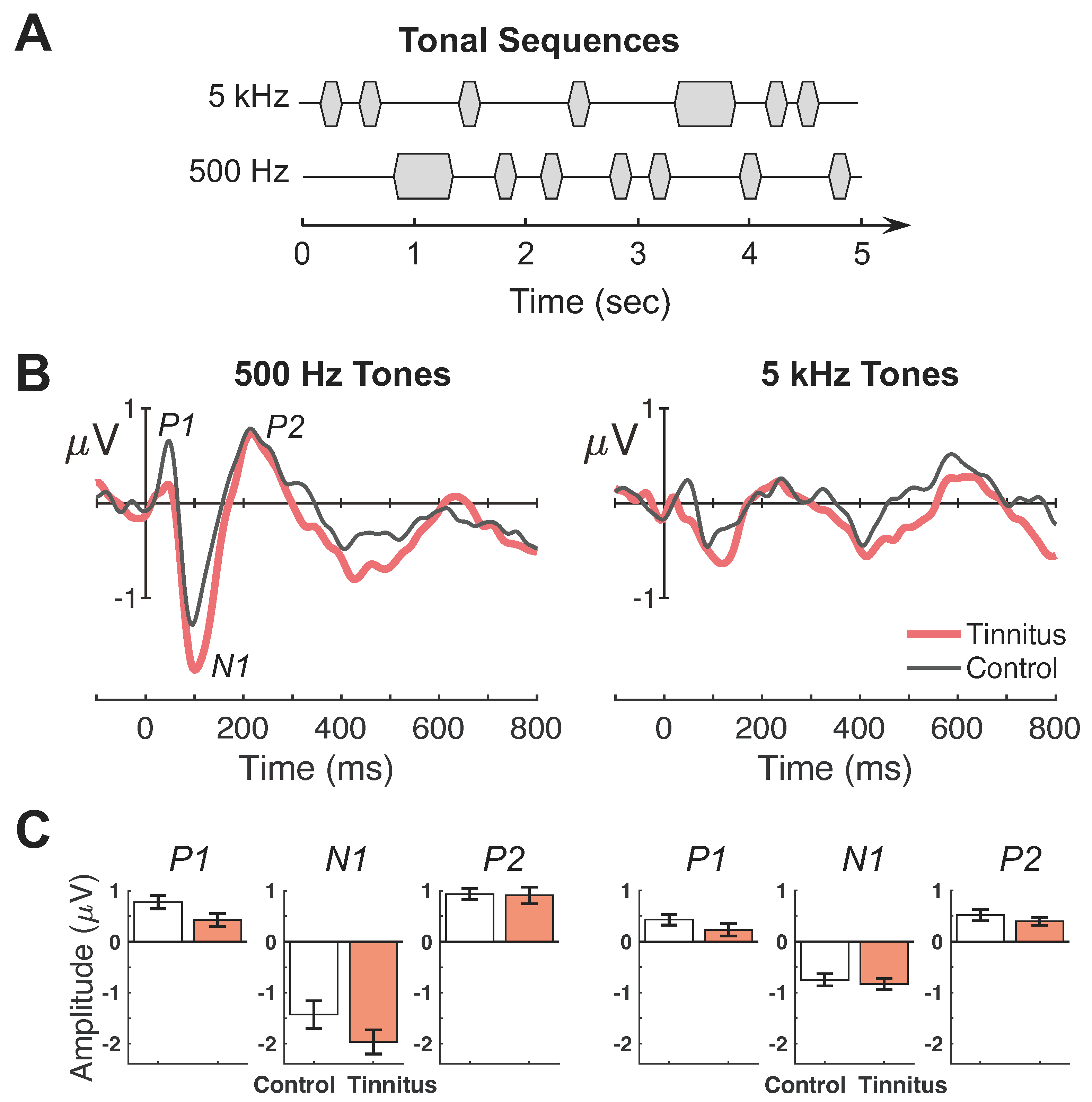
3.2. Cortical Responses in Attentive Listening
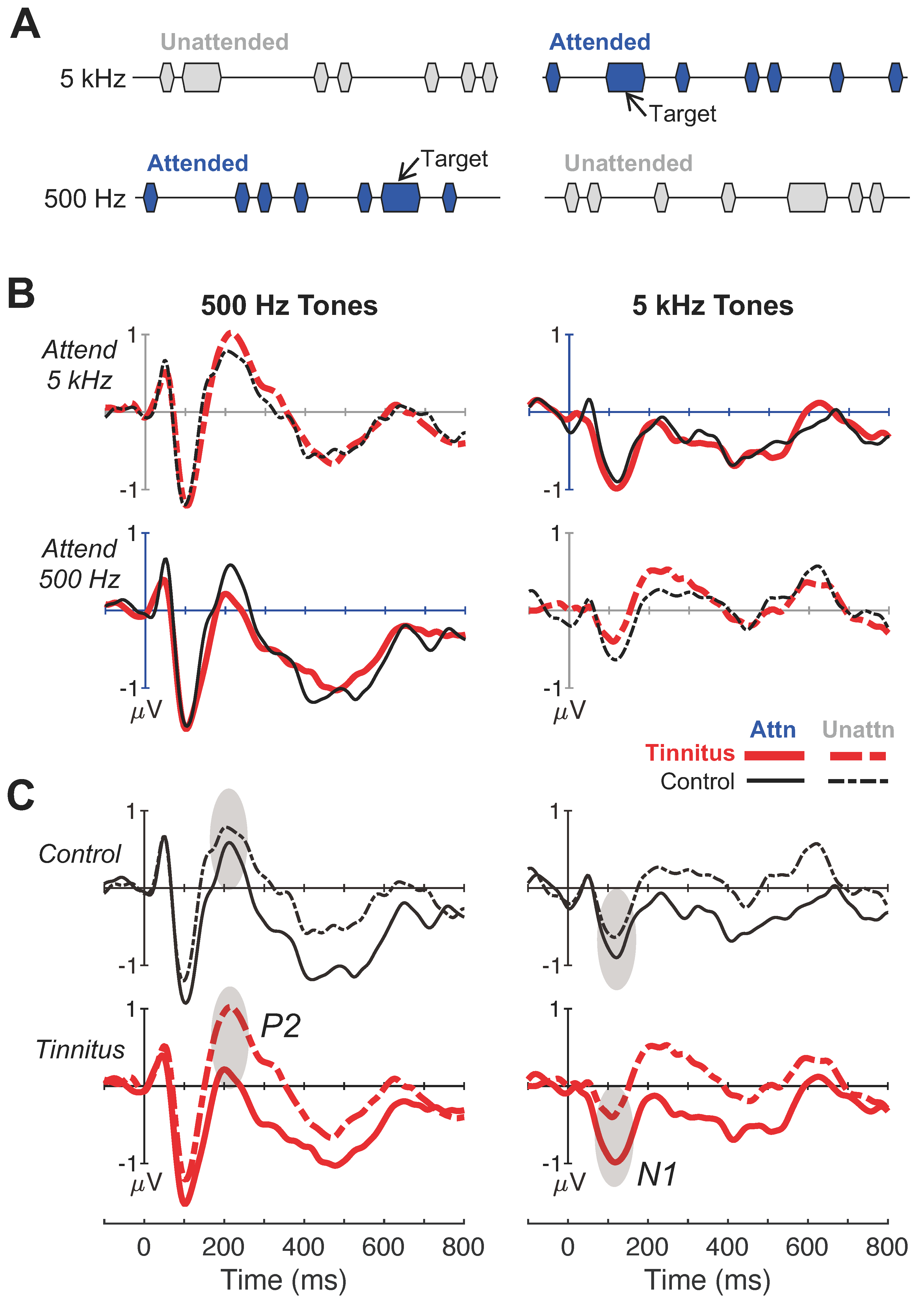
4. Discussion
4.1. Comparison with Previous Studies
4.2. Mechanisms of Attention Modulation in Tinnitus
4.3. Clinical Implications
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shargorodsky, J.; Curhan, G.C.; Farwell, W.R. Prevalence and Characteristics of Tinnitus among US Adults. Am. J. Med. 2010, 123, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Roberts, L.E. The Neuroscience of Tinnitus. Trends Neurosci. 2004, 27, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Roberts, L.E. The Neuroscience of Tinnitus: Understanding Abnormal and Normal Auditory Perception. Front. Syst. Neurosci. 2012, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Elgoyhen, A.B.; Romo, R.; Langguth, B. Phantom Percepts: Tinnitus and Pain as Persisting Aversive Memory Networks. Proc. Natl. Acad. Sci. USA 2011, 108, 8075–8080. [Google Scholar] [CrossRef] [PubMed]
- Knipper, M.; van Dijk, P.; Schulze, H.; Mazurek, B.; Krauss, P.; Scheper, V.; Warnecke, A.; Schlee, W.; Schwabe, K.; Singer, W.; et al. The Neural Bases of Tinnitus: Lessons from Deafness and Cochlear Implants. J. Neurosci. 2020, 40, 7190–7202. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Herrmann, B.S.; Levine, R.A.; Melcher, J.R. Brainstem Auditory Evoked Potentials Suggest a Role for the Ventral Cochlear Nucleus in Tinnitus. JARO J. Assoc. Res. Otolaryngol. 2012, 13, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Schaette, R.; McAlpine, D. Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. J. Neurosci. 2011, 31, 13452–13457. [Google Scholar] [CrossRef]
- Zeng, F.-G. An Active Loudness Model Suggesting Tinnitus as Increased Central Noise and Hyperacusis as Increased Nonlinear Gain. Hear. Res. 2013, 295, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.G. Tinnitus and Hyperacusis: Central Noise, Gain and Variance. Curr. Opin. Physiol. 2020, 18, 123–129. [Google Scholar] [CrossRef]
- Schilling, A.; Sedley, W.; Gerum, R.; Metzner, C.; Tziridis, K.; Maier, A.; Schulze, H.; Zeng, F.-G.; Friston, K.J.; Krauss, P. Predictive Coding and Stochastic Resonance as Fundamental Principles of Auditory Phantom Perception. Brain 2023, 146, 4809–4825. [Google Scholar] [CrossRef]
- Johannesen, P.T.; Lopez-Poveda, E.A. Age-Related Central Gain Compensation for Reduced Auditory Nerve Output for People with Normal Audiograms, with and without Tinnitus. iScience 2021, 24, 102658. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-G.; Turner, K.E. Tinnitus as Central Noise Revealed by Increased Loudness at Thresholds. Front. Audiol. Otol. 2023, 1, 1272880. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; Leaver, A.M.; Mühlau, M. Tuning Out the Noise: Limbic-Auditory Interactions in Tinnitus. Neuron 2010, 66, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rauschecker, J.P.; May, E.S.; Maudoux, A.; Ploner, M. Frontostriatal Gating of Tinnitus and Chronic Pain. Trends Cogn. Sci. 2015, 19, 567–578. [Google Scholar] [CrossRef]
- Roberts, L.E.; Husain, F.T.; Eggermont, J.J. Role of Attention in the Generation and Modulation of Tinnitus. Neurosci. Biobehav. Rev. 2013, 37, 1754–1773. [Google Scholar] [CrossRef]
- Vanneste, S.; De Ridder, D. The Auditory and Non-Auditory Brain Areas Involved in Tinnitus. An Emergent Property of Multiple Parallel Overlapping Subnetworks. Front. Syst. Neurosci. 2012, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Sedley, W.; Friston, K.J.; Gander, P.E.; Kumar, S.; Griffiths, T.D. An Integrative Tinnitus Model Based on Sensory Precision. Trends Neurosci. 2016, 39, 799–812. [Google Scholar] [CrossRef]
- Rossiter, S.; Stevens, C.; Walker, G. Tinnitus and Its Effect on Working Memory and Attention. J. Speech Lang. Hear. Res. 2006, 49, 150–160. [Google Scholar] [CrossRef]
- Stevens, C.; Walker, G.; Boyer, M.; Gallagher, M. Severe Tinnitus and Its Effect on Selective and Divided Attention. Int. J. Audiol. 2007, 46, 208–216. [Google Scholar] [CrossRef]
- Hallam, R.S.; McKenna, L.; Shurlock, L. Tinnitus Impairs Cognitive Efficiency. Int. J. Audiol. 2004, 43, 218–226. [Google Scholar] [CrossRef]
- Desimone, R.; Duncan, J. Neural Mechanisms of Selective Visual Attention. Annu. Rev. Neurosci. 1995, 18, 193–222. [Google Scholar] [CrossRef]
- Koops, E.A.; Renken, R.J.; Lanting, C.P.; van Dijk, P. Cortical Tonotopic Map Changes in Humans Are Larger in Hearing Loss than in Additional Tinnitus. J. Neurosci. 2020, 40, 3178–3185. [Google Scholar] [CrossRef]
- Hamza, Y.; Zeng, F.G. Tinnitus Is Associated With Improved Cognitive Performance in Non-Hispanic Elderly With Hearing Loss. Front. Neurosci. 2021, 15, 735950. [Google Scholar] [CrossRef]
- Diesch, E.; Andermann, M.; Rupp, A. Is the Effect of Tinnitus on Auditory Steady-State Response Amplitude Mediated by Attention? Front. Syst. Neurosci. 2012, 6, 38. [Google Scholar] [CrossRef]
- Dietrich, V.; Nieschalk, M.; Stoll, W.; Rajan, R.; Pantev, C. Cortical Reorganization in Patients with High Frequency Cochlear Hearing Loss. Hear. Res. 2001, 158, 95–101. [Google Scholar] [CrossRef]
- Hoke, M.I.; Feldmann, H.; Pantev, C.I.; Liitkenhiiner, B.; Lehnertz, K. Objective Evidence of Tinnitus in Auditor Evoked Magnetic Fields. Hear. Res. 1989, 37, 281–286. [Google Scholar] [CrossRef]
- Kadner, A.; Viirre, E.; Wester, D.C.; Walsh, S.F.; Hestenes, J.; Vankov, A.; Pineda, J.A. Lateral Inhibition in the Auditory Cortex-An EEG Index of Tinnitus. Neuroreport 2002, 13, 443–446. [Google Scholar] [CrossRef]
- Sereda, M.; Adjamian, P.; Edmondson-Jones, M.; Palmer, A.R.; Hall, D.A. Auditory Evoked Magnetic Fields in Individuals with Tinnitus. Hear. Res. 2013, 302, 50–59. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Ahmad, B.K.; Moran, J.; Newman, C.W.; Tepley, N.; Wharton, J. Auditory Evoked Cortical Magnetic Field (M100-M200) Measurements in Tinnitus and Normal Groups. Hear. Res. 1991, 56, 44–52. [Google Scholar] [CrossRef]
- Jacobson, G.P.; McCaslin, D.L. A Reexamination of the Long Latency N1 Response in Patients with Tinnitus. J. Am. Acad. Audiol. 2003, 14, 393–400. [Google Scholar] [CrossRef]
- Weisz, N.; Wienbruch, C.; Dohrmann, K.; Elbert, T. Neuromagnetic Indicators of Auditory Cortical Reorganization of Tinnitus. Brain 2005, 128, 2722–2731. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Calder, J.A.; Newman, C.W.; Peterson, E.L.; Wharton, J.A.; Ahmad, B.K. Electrophysiological Indices of Selective Auditory Attention in Subjects with and without Tinnitus. Hear. Res. 1996, 97, 66–74. [Google Scholar] [CrossRef]
- Delb, W.; Strauss, D.J.; Low, Y.F.; Seidler, H.; Rheinschmitt, A.; Wobrock, T.; D’Amelio, R. Alterations in Event Related Potentials (ERP) Associated with Tinnitus Distress and Attention. Appl. Psychophysiol. Biofeedback 2008, 33, 211–221. [Google Scholar] [CrossRef]
- Paul, B.T.; Bruce, I.C.; Bosnyak, D.J.; Thompson, D.C.; Roberts, L.E. Modulation of Electrocortical Brain Activity by Attention in Individuals with and without Tinnitus. Neural Plast. 2014, 2014, 127824. [Google Scholar] [CrossRef]
- Reavis, K.M.; Rothholtz, V.S.; Tang, Q.; Carroll, J.A.; Djalilian, H.; Zeng, F.-G. Temporary Suppression of Tinnitus by Modulated Sounds. JARO J. Assoc. Res. Otolaryngol. 2012, 13, 561–571. [Google Scholar] [CrossRef]
- Roberts, L.E.; Bosnyak, D.J.; Thompson, D.C. Neural Plasticity Expressed in Central Auditory Structures with and without Tinnitus. Front. Syst. Neurosci. 2012, 6, 40. [Google Scholar] [CrossRef]
- Roberts, L.E.; Moffat, G.; Bosnyak, D.J. Residual Inhibition Functions in Relation to Tinnitus Spectra and Auditory Threshold Shift. In Proceedings of the Acta Oto-Laryngologica, Pau, France, 1 November 2006; Volume 126, pp. 27–33. [Google Scholar]
- Norena, A.; Micheyl, C.; Chéry-Croze, S.; Collet, L. Psychoacoustic Characterization of the Tinnitus Spectrum: Implications for the Underlying Mechanisms of Tinnitus. Audiol. Neurootol. 2002, 7, 358–369. [Google Scholar] [CrossRef]
- Hansen, J.C.; Hillyard, S.A. Effects of Stimulation Rate and Attribute Cuing on Event-Related Potentials During Selective Auditory Attention. Psychophysiology 1984, 21, 394–405. [Google Scholar] [CrossRef]
- Teder, W.; Alho, K.; Reinikainen, K.; Näätänen, R. Interstimulus Interval and the Selective-Attention Effect on Auditory ERPs: “N1 Enhancement” versus Processing Negativity. Psychophysiology 1993, 30, 71–81. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zeng, F.-G.; Richardson, M.; Turner, K. Tinnitus Does Not Interfere with Auditory and Speech Perception. J. Neurosci. 2020, 40, 6007–6017. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Roberts, L.E.; Bosnyak, D.J.; Bruce, I.C.; Gander, P.E.; Paul, B.T. Evidence for Differential Modulation of Primary and Nonprimary Auditory Cortex by Forward Masking in Tinnitus. Hear. Res. 2015, 327, 9–27. [Google Scholar] [CrossRef]
- Flor, H.; Diesch, E.; Struve, M.; Rupp, A.; Ritter, S.; Hu, M. Enhancement of Steady-State Auditory Evoked Magnetic Fields in Tinnitus. Neuroscience 2004, 19, 1093–1104. [Google Scholar] [CrossRef]
- Wienbruch, C.; Paul, I.; Weisz, N.; Elbert, T.; Roberts, L.E. Frequency Organization of the 40-Hz Auditory Steady-State Response in Normal Hearing and in Tinnitus. Neuroimage 2006, 33, 180–194. [Google Scholar] [CrossRef]
- Neelon, M.F.; Williams, J.; Garell, P.C. The Effects of Attentional Load on Auditory ERPs Recorded from Human Cortex. Brain Res. 2006, 1118, 94–105. [Google Scholar] [CrossRef]
- Alcaini, M.; Giard, M.-H.M.; Echallier, J.-F.J.; Pernier, J. Selective Auditory Attention Effects in Tonotopically Organized Cortical Areas: A Topographic ERP Study. Hum. Brain Mapp. 1995, 2, 159–169. [Google Scholar] [CrossRef]
- Okamoto, H.; Stracke, H.; Wolters, C.H.; Schmael, F.; Pantev, C. Attention Improves Population-Level Frequency Tuning in Human Auditory Cortex. J. Neurosci. 2007, 27, 10383–10390. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nagamine, T.; Imai, M.; Tanaka, T.; Shibasaki, H. Role of the Primary Auditory Cortex in Auditory Selective Attention Studied by Whole-Head Neuromagnerometer. Cogn. Brain Res. 1998, 7, 99–109. [Google Scholar] [CrossRef]
- Woldorff, M.G.; Gallen, C.C.; Hampson, S.A.; Hillyard, S.A.; Pantev, C.; Sobel, D.; Bloom, F.E. Modulation of Early Sensory Processing in Human Auditory Cortex during Auditory Selective Attention. Proc. Natl. Acad. Sci. USA 1993, 90, 8722–8726. [Google Scholar] [CrossRef]
- Crowley, K.E.; Colrain, I.M. A Review of the Evidence for P2 Being an Independent Component Process: Age, Sleep and Modality. Clin. Neurophysiol. 2004, 115, 732–744. [Google Scholar] [CrossRef]
- Shahin, A.; Roberts, L.E.; Pantev, C.; Trainor, L.J.; Ross, B. Modulation of P2 Auditory-Evoked Responses by the Spectral Complexity of Musical Sounds. NeuroReport 2005, 16, 1781–1785. [Google Scholar] [CrossRef]
- Alho, K.; Teder, W.; Lavikainen, J.; Naatanen, R. Strongly Focused Attention and Auditory Event-Related Potentials. Biol. Psychol. 1994, 38, 73–90. [Google Scholar] [CrossRef]
- Degerman, A.; Rinne, T.; Särkkä, A.K.; Salmi, J.; Alho, K. Selective Attention to Sound Location or Pitch Studied with Event-Related Brain Potentials and Magnetic Fields. Eur. J. Neurosci. 2008, 27, 3329–3341. [Google Scholar] [CrossRef]
- Giard, M.-H. Neurophysiological Mechanisms of Auditory Selective Attention in Humans. Front. Biosci. 2000, 5, d84. [Google Scholar] [CrossRef]
- Hansen, J.C.; Hillyard, S.A. Endogenous Brain Potentials Associated with Selective Auditory Attention. Electroencephalogr. Clin. Neurophysiol. 1980, 49, 277–290. [Google Scholar] [CrossRef]
- Michie, P.T.; Solowij, N.; Crawford, J.M.; Glue, L.C. The Effects of Between-Source Discriminability on Attended and Unattended Auditory ERPs. Psychophysiology 1993, 30, 205–220. [Google Scholar] [CrossRef]
- Näätänen, R. Attention and Brain Function; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1992. [Google Scholar]
- Eccleston, C.; Crombez, G. Pain Demands Attention: A Cognitive-Affective Model of the Interruptive Function of Pain. Psychol. Bull. 1999, 125, 356–366. [Google Scholar] [CrossRef]
- Mannarelli, D.; Pauletti, C.; Mancini, P.; Fioretti, A.; Greco, A.; De Vincentiis, M.; Fattapposta, F. Selective Attentional Impairment in Chronic Tinnitus: Evidence from an Event-Related Potentials Study. Clin. Neurophysiol. 2017, 128, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Gabr, T.A.; El-Hay, M.A.; Badawy, A. Electrophysiological and Psychological Studies in Tinnitus. Auris Nasus Larynx 2011, 38, 678–683. [Google Scholar] [CrossRef]
- Brozoski, T.; Wisner, K.; Randall, M.; Caspary, D. Chronic Sound-Induced Tinnitus and Auditory Attention in Animals. Neuroscience 2019, 407, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Adjamian, P.; Sereda, M.; Hall, D.A. The Mechanisms of Tinnitus: Perspectives from Human Functional Neuroimaging. Hear. Res. 2009, 253, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Lanting, C.P.; de Kleine, E.; van Dijk, P. Neural Activity Underlying Tinnitus Generation: Results from PET and FMRI. Hear. Res. 2009, 255, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adjamian, P.; Sereda, M.; Zobay, O.; Hall, D.A.; Palmer, A.R. Neuromagnetic Indicators of Tinnitus and Tinnitus Masking in Patients with and without Hearing Loss. JARO J. Assoc. Res. Otolaryngol. 2012, 13, 715–731. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, L.L.; Xing, C.; Xu, X.; Feng, Y.; Lv, H.; Zhao, F.; Chen, Y.C.; Cai, Y. Tinnitus Classification Based on Resting-State Functional Connectivity Using a Convolutional Neural Network Architecture. Neuroimage 2024, 290, 120566. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.E.; Huang, T.C.; Nagarajan, S.; Cheung, S.W. Tinnitus Neuroimaging. Otolaryngol. Clin. N. Am. 2020, 53, 583–603. [Google Scholar] [CrossRef]
- Turner, K.; Moshtaghi, O.; Saez, N.; Richardson, M.; Djalilian, H.; Zeng, F.-G.; Lin, H. Auditory Brainstem Response Wave I Amplitude Has Limited Clinical Utility in Diagnosing Tinnitus in Humans. Brain Sci. 2022, 12, 142. [Google Scholar] [CrossRef]
- Cima, R.F.F.; Andersson, G.; Schmidt, C.J.; Henry, J.A. Cognitive-Behavioral Treatments for Tinnitus: A Review of the Literature. J. Am. Acad. Audiol. 2014, 25, 29–61. [Google Scholar] [CrossRef]
- Tass, P.A.; Adamchic, I.; Freund, H.J.; Von Stackelberg, T.; Hauptmann, C. Counteracting Tinnitus by Acoustic Coordinated Reset Neuromodulation. Restor. Neurol. Neurosci. 2012, 30, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Hoffmann, D.; Struve, M.; Diesch, E. Auditory Discrimination Training for the Treatment of Tinnitus. Appl. Psychophysiol. Biofeedback 2004, 29, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Searchfield, G.D.; Morrison-Low, J.; Wise, K. Object Identification and Attention Training for Treating Tinnitus. Prog. Brain Res. 2007, 166, 441–460. [Google Scholar] [CrossRef]
- Krick, C.M.; Argstatter, H.; Grapp, M.; Plinkert, P.K.; Reith, W. Heidelberg Neuro-Music Therapy Restores Attention-Related Activity in the Angular Gyrus in Chronic Tinnitus Patients. Front. Neurosci. 2017, 11, 418. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, M.L.; Luo, J.; Zeng, F.-G. Attention-Modulated Cortical Responses as a Biomarker for Tinnitus. Brain Sci. 2024, 14, 421. https://doi.org/10.3390/brainsci14050421
Richardson ML, Luo J, Zeng F-G. Attention-Modulated Cortical Responses as a Biomarker for Tinnitus. Brain Sciences. 2024; 14(5):421. https://doi.org/10.3390/brainsci14050421
Chicago/Turabian StyleRichardson, Matthew L., Jiaxin Luo, and Fan-Gang Zeng. 2024. "Attention-Modulated Cortical Responses as a Biomarker for Tinnitus" Brain Sciences 14, no. 5: 421. https://doi.org/10.3390/brainsci14050421



