Role for Astrocytes in mGluR-Dependent LTD in the Neocortex and Hippocampus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Slice and Cell Preparation
2.2. Electrophysiological Recordings
2.3. Multi-Photon Fluorescent Ca2+-Imaging in Astrocytes
2.4. Measurement of Extracellular ATP Concentration in Brain Tissue
2.5. Data Analysis
3. Results
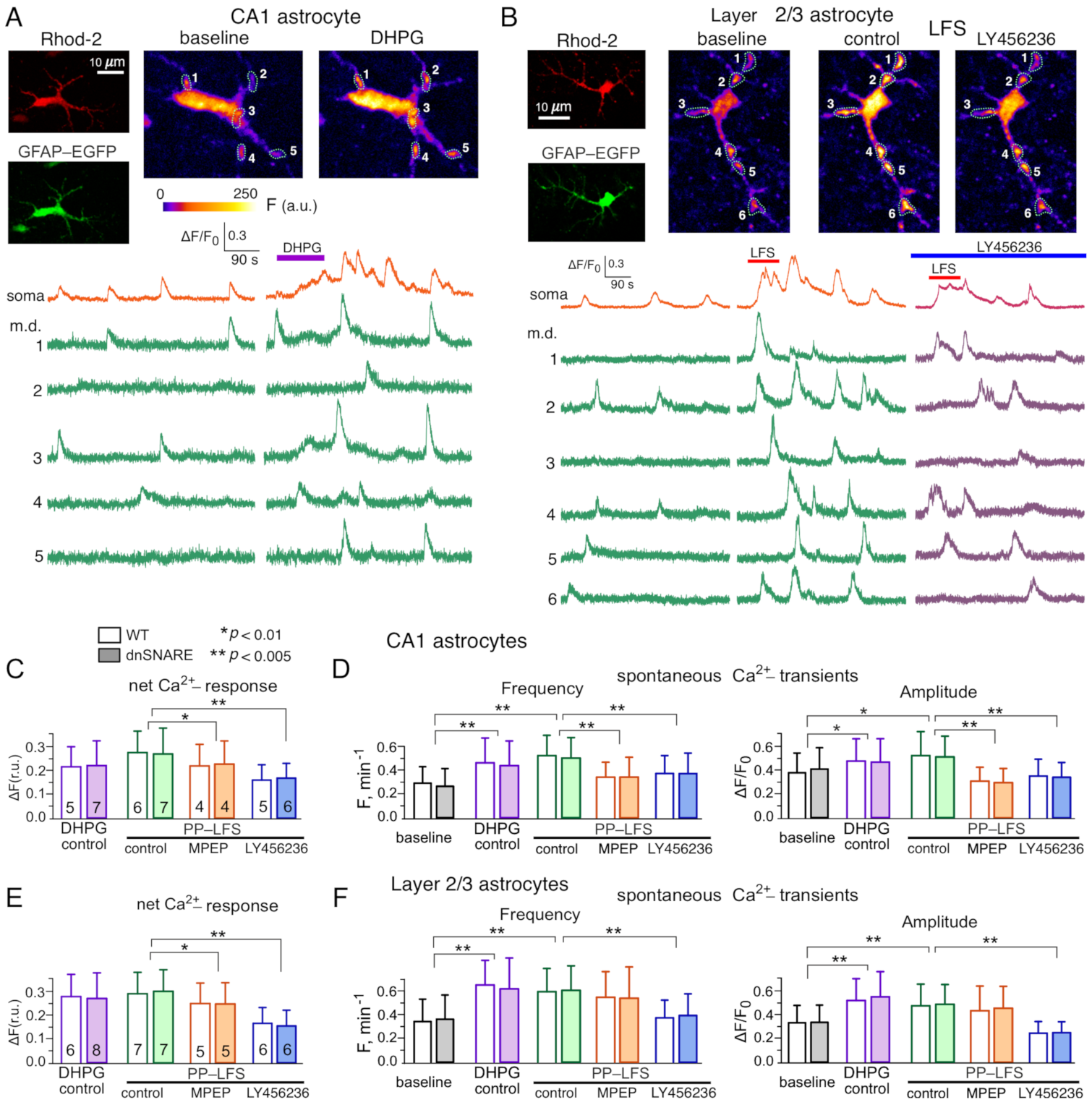
3.1. Impact of mGluRs on Intracellular Ca2+-Signaling and Release of Gliotransmitters in Neocortical and Hippocampal Astrocytes
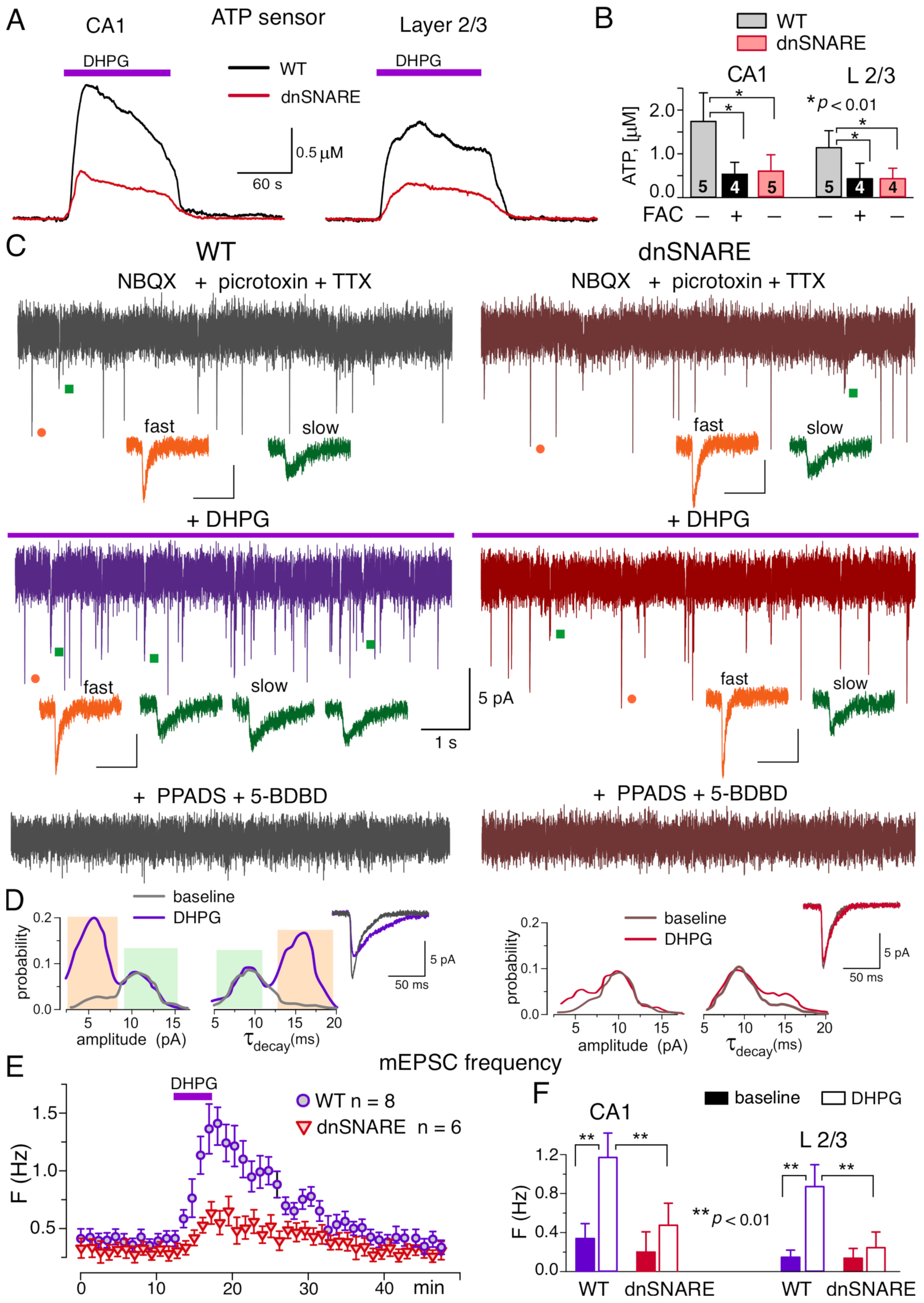
3.2. mGluR Receptors Induce the Release of ATP from Hippocampal and Neocortical Astrocytes
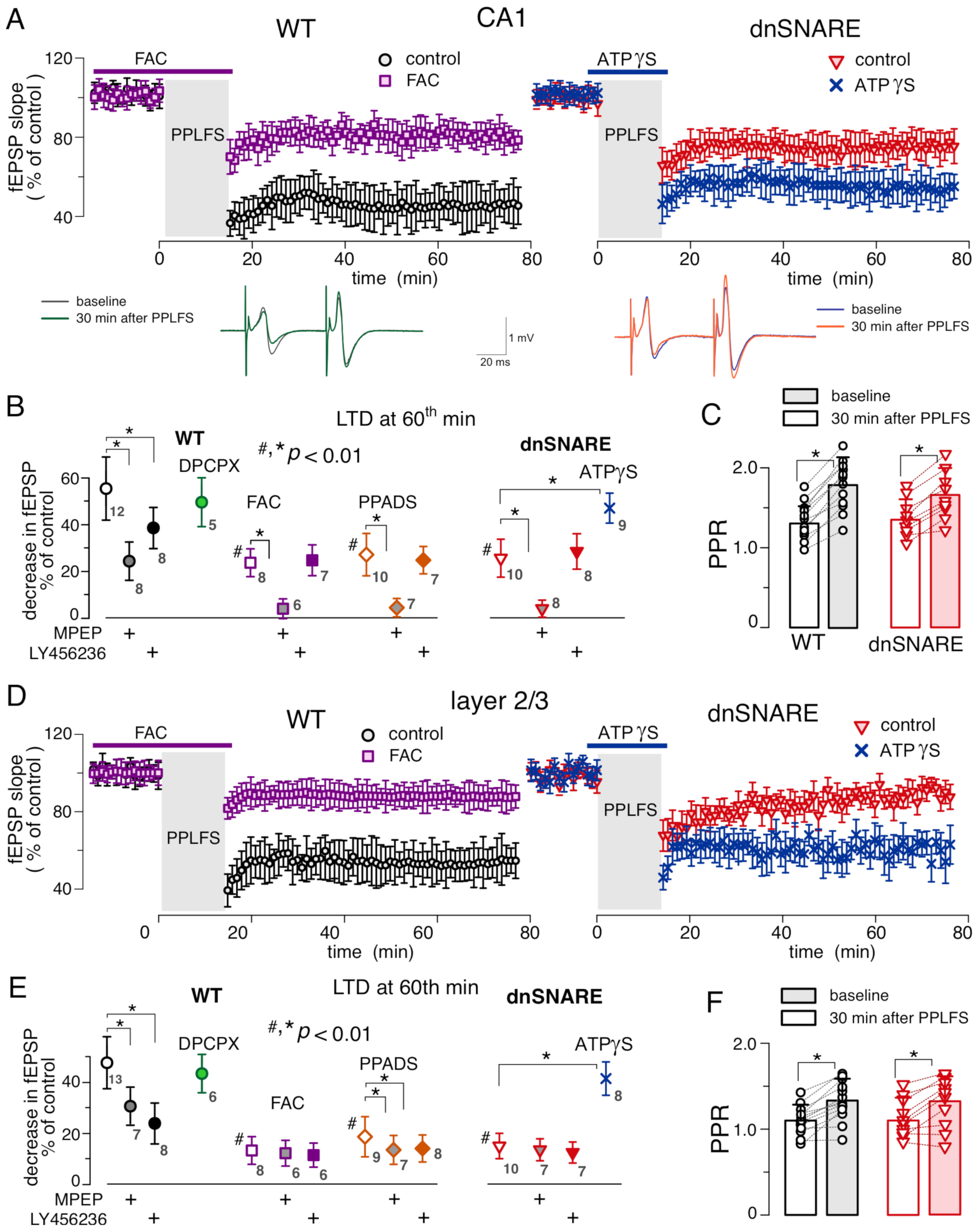
3.3. Impact of Impaired Gliotransmission on mGluR-Dependent LTD in Hippocampus and Neocortex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collingridge, G.L.; Peineau, S.; Howland, J.G.; Wang, Y.T. Long-term depression in the CNS. Nat. Rev. Neurosci. 2010, 11, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Luscher, C.; Huber, K.M. Group 1 mGluR-dependent synaptic long-term depression: Mechanisms and implications for circuitry and disease. Neuron 2010, 65, 445–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond Black-and-White. J. Neurosci. 2018, 38, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Abraham, W.C. Astrocytes and synaptic plasticity in health and disease. Exp. Brain Res. 2017, 235, 1645–1655. [Google Scholar] [CrossRef]
- Halassa, M.M.; Haydon, P.G. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef] [Green Version]
- Henneberger, C.; Papouin, T.; Oliet, S.H.; Rusakov, D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The tripartite glutamatergic synapse. Neuropharmacology 2021, 199, 108758. [Google Scholar] [CrossRef]
- Pascual, O.; Casper, K.B.; Kubera, C.; Zhang, J.; Revilla-Sanchez, R.; Sul, J.Y.; Takano, H.; Moss, S.J.; McCarthy, K.; Haydon, P.G. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005, 310, 113–116. [Google Scholar] [CrossRef]
- Han, J.; Kesner, P.; Metna-Laurent, M.; Duan, T.; Xu, L.; Georges, F.; Koehl, M.; Abrous, D.N.; Mendizabal-Zubiaga, J.; Grandes, P.; et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 2012, 148, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Cavaccini, A.; Durkee, C.; Kofuji, P.; Tonini, R.; Araque, A. Astrocyte Signaling Gates Long-Term Depression at Corticostriatal Synapses of the Direct Pathway. J. Neurosci. 2020, 40, 5757–5768. [Google Scholar] [CrossRef]
- Min, R.; Nevian, T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci. 2012, 15, 746–753. [Google Scholar] [CrossRef]
- Pougnet, J.T.; Compans, B.; Martinez, A.; Choquet, D.; Hosy, E.; Boue-Grabot, E. P2X-mediated AMPA receptor internalization and synaptic depression is controlled by two CaMKII phosphorylation sites on GluA1 in hippocampal neurons. Sci. Rep. 2016, 6, 31836. [Google Scholar] [CrossRef]
- Boue-Grabot, E.; Pankratov, Y. Modulation of Central Synapses by Astrocyte-Released ATP and Postsynaptic P2X Receptors. Neural. Plast. 2017, 2017, 9454275. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tan, Z.; Zeng, L.; Zhang, X.; He, Y.; Gao, W.; Wu, X.; Li, Y.; Bu, B.; Wang, W.; et al. Heterosynaptic long-term depression mediated by ATP released from astrocytes. Glia 2013, 61, 178–191. [Google Scholar] [CrossRef]
- Hulme, S.R.; Jones, O.D.; Raymond, C.R.; Sah, P.; Abraham, W.C. Mechanisms of heterosynaptic metaplasticity. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130148. [Google Scholar] [CrossRef] [Green Version]
- Gordon, G.R.; Iremonger, K.J.; Kantevari, S.; Ellis-Davies, G.C.; MacVicar, B.A.; Bains, J.S. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 2009, 64, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Pougnet, J.T.; Toulme, E.; Martinez, A.; Choquet, D.; Hosy, E.; Boue-Grabot, E. ATP P2X receptors downregulate AMPA receptor trafficking and postsynaptic efficacy in hippocampal neurons. Neuron 2014, 83, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Rasooli-Nejad, S.; Palygin, O.; Lalo, U.; Pankratov, Y. Cannabinoid receptors contribute to astroglial Ca(2)(+)-signalling and control of synaptic plasticity in the neocortex. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20140077. [Google Scholar] [CrossRef]
- Lalo, U.; Palygin, O.; Rasooli-Nejad, S.; Andrew, J.; Haydon, P.G.; Pankratov, Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 2014, 12, e1001747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halassa, M.M.; Florian, C.; Fellin, T.; Munoz, J.R.; Lee, S.Y.; Abel, T.; Haydon, P.G.; Frank, M.G. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 2009, 61, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankratov, Y.; Lalo, U. Role for astroglial alpha1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front. Cell. Neurosci. 2015, 9, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalo, U.; Palygin, O.; Verkhratsky, A.; Grant, S.G.; Pankratov, Y. ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD-95 multi-protein complex. Sci. Rep. 2016, 6, 33609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankratov, Y.; Lalo, U.; Verkhratsky, A.; North, R.A. Quantal release of ATP in mouse cortex. J. Gen. Physiol. 2007, 129, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Eales, K.L.; Palygin, O.; O’Loughlin, T.; Rasooli-Nejad, S.; Gaestel, M.; Muller, J.; Collins, D.R.; Pankratov, Y.; Correa, S.A. The MK2/3 cascade regulates AMPAR trafficking and cognitive flexibility. Nat. Commun. 2014, 5, 4701. [Google Scholar] [CrossRef] [Green Version]
- Frenguelli, B.G.; Wigmore, G.; Llaudet, E.; Dale, N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J. Neurochem. 2007, 101, 1400–1413. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, E.; Bushong, E.A.; Haustein, M.D.; Tong, X.; Jackson-Weaver, O.; Kracun, S.; Xu, J.; Sofroniew, M.V.; Ellisman, M.H.; Khakh, B.S. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 2013, 141, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Gourine, A.V.; Kasymov, V.; Marina, N.; Tang, F.; Figueiredo, M.F.; Lane, S.; Teschemacher, A.G.; Spyer, K.M.; Deisseroth, K.; Kasparov, S. Astrocytes control breathing through pH-dependent release of ATP. Science 2010, 329, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Pankratov, Y.; Castro, E.; Miras-Portugal, M.T.; Krishtal, O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur. J. Neurosci. 1998, 10, 3898–3902. [Google Scholar] [CrossRef]
- Tang, W.; Szokol, K.; Jensen, V.; Enger, R.; Trivedi, C.A.; Hvalby, O.; Helm, P.J.; Looger, L.L.; Sprengel, R.; Nagelhus, E.A. Stimulation-evoked Ca2+ signals in astrocytic processes at hippocampal CA3-CA1 synapses of adult mice are modulated by glutamate and ATP. J. Neurosci. 2015, 35, 3016–3021. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lou, N.; Xu, Q.; Tian, G.F.; Peng, W.G.; Han, X.; Kang, J.; Takano, T.; Nedergaard, M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006, 9, 816–823. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalo, U.; Pankratov, Y. Role for Astrocytes in mGluR-Dependent LTD in the Neocortex and Hippocampus. Brain Sci. 2022, 12, 1718. https://doi.org/10.3390/brainsci12121718
Lalo U, Pankratov Y. Role for Astrocytes in mGluR-Dependent LTD in the Neocortex and Hippocampus. Brain Sciences. 2022; 12(12):1718. https://doi.org/10.3390/brainsci12121718
Chicago/Turabian StyleLalo, Ulyana, and Yuriy Pankratov. 2022. "Role for Astrocytes in mGluR-Dependent LTD in the Neocortex and Hippocampus" Brain Sciences 12, no. 12: 1718. https://doi.org/10.3390/brainsci12121718
APA StyleLalo, U., & Pankratov, Y. (2022). Role for Astrocytes in mGluR-Dependent LTD in the Neocortex and Hippocampus. Brain Sciences, 12(12), 1718. https://doi.org/10.3390/brainsci12121718




