Volumetric Variability of the Ventromedial Prefrontal Cortex Reflects the Propensity for Engaging in High-Stakes Gambling Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
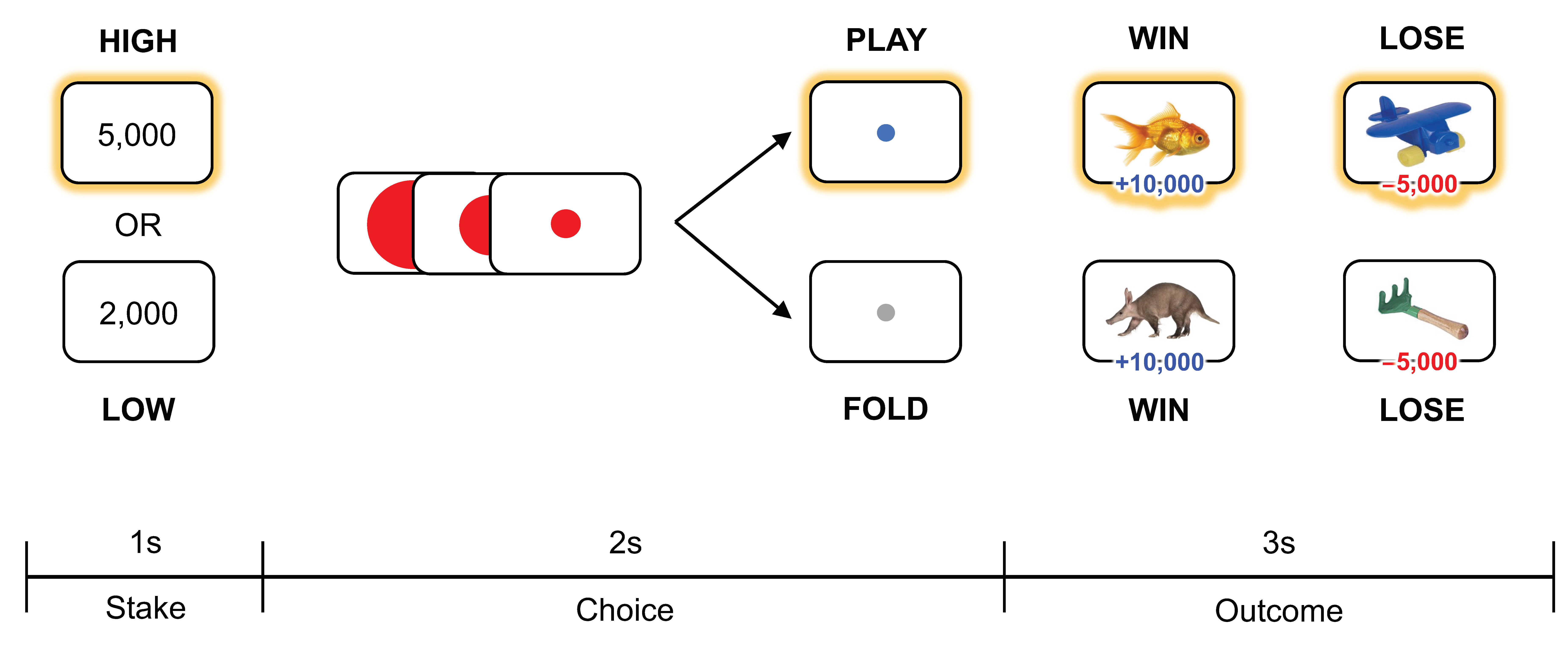
2.2. Experimental Task
2.3. Behavioral Index
2.4. Image Acquisition
2.5. Voxel-Based Morphometry
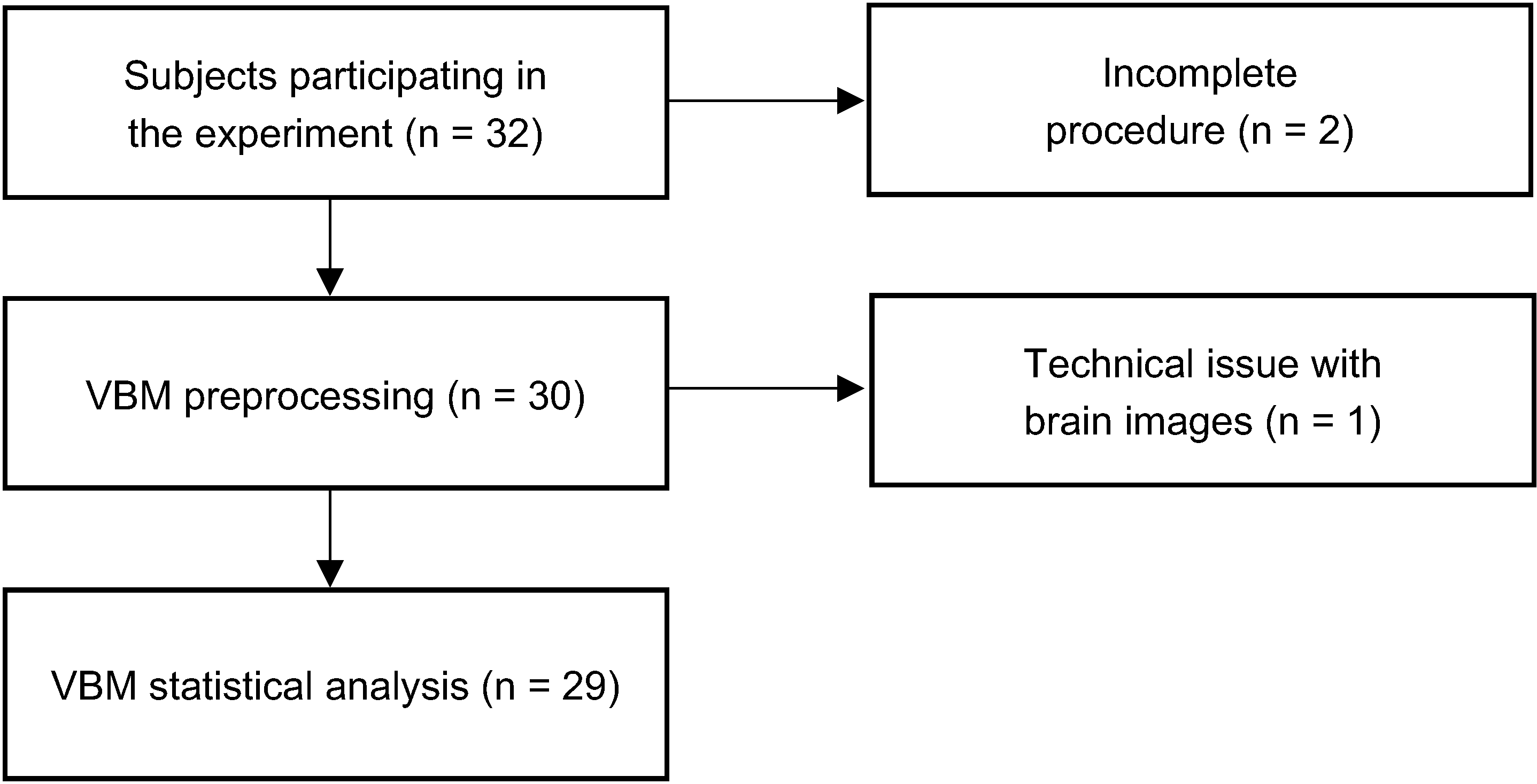
2.5.1. Preprocessing
2.5.2. Statistical Analysis
3. Results
3.1. Gambling Behavior
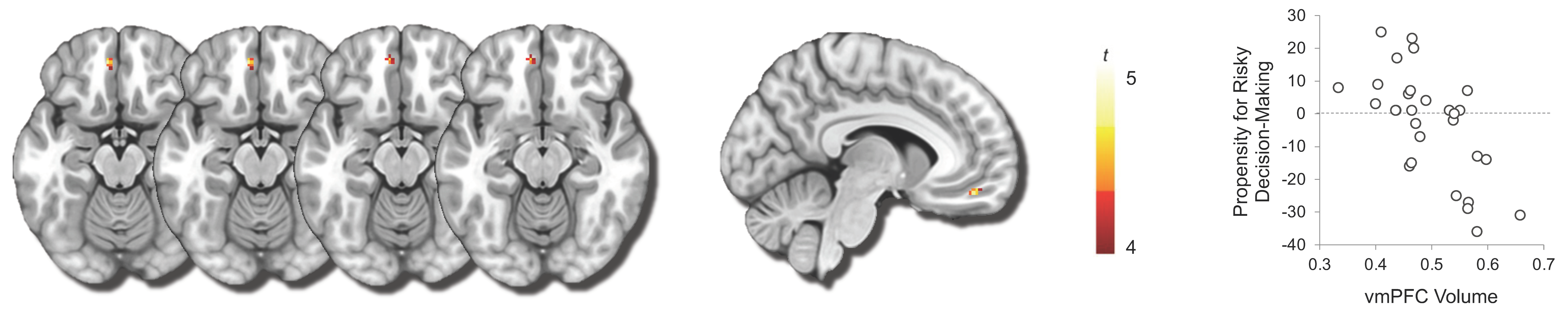
3.2. Propensity for Engaging in High-Stakes Gambling and vmPFC Volume
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levy, D.J.; Glimcher, P.W. The root of all value: A neural common currency for choice. Curr. Opin. Neurobiol. 2012, 22, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Clark, L. Decision-making during gambling: An integration of cognitive and psychobiological approaches. Phil. Trans. R. Soc. B 2010, 365, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Tranel, D.; Damasio, H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000, 123, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Rogers, R.D.; Everitt, B.J.; Baldacchino, A.; Blackshaw, A.J.; Swainson, R.; Wynne, K.; Baker, N.B.; Hunter, J.; Carthy, T.; Booker, E.; et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology 1999, 20, 322–339. [Google Scholar] [CrossRef]
- Levin, I.P.; Hart, S.S. Risk preferences in young children: Early evidence of individual differences in reaction to potential gains and losses. J. Behav. Decis. Mak 2003, 16, 397–413. [Google Scholar] [CrossRef]
- Clark, L.; Manes, F.; Antoun, N.; Sahakian, B.J.; Robbins, T.W. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia 2003, 41, 1474–1483. [Google Scholar] [CrossRef]
- Weller, J.A.; Levin, I.P.; Shiv, B.; Bechara, A. Neural correlates of adaptive decision making for risky gains and losses. Psychol. Sci. 2007, 18, 958–964. [Google Scholar] [CrossRef]
- Rogalsky, C.; Vidal, C.; Li, X.; Damasio, H. Risky decision-making in older adults without cognitive deficits: An fMRI study of VMPFC using the Iowa Gambling Task. Soc. Neurosci. 2012, 7, 178–190. [Google Scholar] [CrossRef][Green Version]
- Van Leijenhorst, L.; Moor, B.G.; de Macks, Z.A.O.; Rombouts, S.A.R.B.; Westenberg, P.M.; Crone, E.A. Adolescent risky decision-making: Neurocognitive development of reward and control regions. Neuroimage 2010, 51, 345–355. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Boileau, I.; Dreher, J.C.; Gelskov, S.; Genauck, A.; Joutsa, J.; Kaasinen, V.; Perales, J.C.; Romanczuk-Seiferth, N.; et al. Altered orbitofrontal sulcogyral patterns in gambling disorder: A multicenter study. Transl. Psychiatry 2019, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Tsurumi, K.; Murao, T.; Takemura, A.; Kawada, R.; Urayama, S.I.; Aso, T.; Sugilhara, G.I.; Miyata, J.; Murai, T.; et al. Common and differential brain abnormalities in gambling disorder subtypes based on risk attitude. Addict. Behav. 2017, 69, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Hammer, A.; Miedl, S.F.; Wiswede, D.; Marco-Pallarés, J.; Herrmann, M.; Münte, T.F. Intertemporal choice behavior is constrained by brain structure in healthy participants and pathological gamblers. Brain Struct. Funct. 2016, 221, 3157–3170. [Google Scholar] [CrossRef] [PubMed]
- Zois, E.; Kiefer, F.; Lemenager, T.; Vollstädt-Klein, S.; Mann, K.; Fauth-Bühler, M. Frontal cortex gray matter volume alterations in pathological gambling occur independently from substance use disorder. Addict. Biol. 2017, 22, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.L.; Knodt, A.R.; Ireland, D.; Morris, M.L.; Poulton, R.; Ramrakha, S.; Sison, M.L.; Moffitt, T.E.; Caspi, A.; Hariri, A.R. What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol. Sci. 2020, 31, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Benney, K.S.; Henkel, L.A. The role of free choice in memory for past decisions. Memory 2006, 14, 1001–1011. [Google Scholar] [CrossRef]
- Murty, V.P.; DuBrow, S.; Davachi, L. The simple act of choosing influences declarative memory. J. Neurosci. 2015, 35, 6255–6264. [Google Scholar] [CrossRef]
- Brady, T.F.; Konkle, T.; Alvarez, G.A.; Oliva, A. Visual long-term memory has a massive storage capacity for object details. Proc. Natl. Acad. Sci. USA 2008, 105, 14325–14329. [Google Scholar] [CrossRef]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 2001, 14, 21–36. [Google Scholar] [CrossRef]
- Douaud, G.; Smith, S.; Jenkinson, M.; Behrens, T.; Johansen-Berg, H.; Vickers, J.; James, S.; Voets, N.; Watkins, K.; Matthews, P.S.; et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007, 130, 2375–2386. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Jenkinson, M.; Smith, S. Non-linear registration, aka Spatial normalization. Available online: https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf (accessed on 13 August 2021).
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation inference for the general linear model. NeuroImage 2014, 92, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Hampton, A.N.; O’Doherty, J.P. Decoding the neural substrates of reward-related decision making with functional MRI. Proc. Natl. Acad. Sci. USA 2007, 104, 1377–1382. [Google Scholar] [CrossRef]
- Fellows, L.K.; Farah, M.J. The role of ventromedial prefrontal cortex in decision making: Judgment under uncertainty or judgment per se? Cereb. Cortex 2007, 17, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Kelley, W.M.; Macrae, C.N.; Wyland, C.L.; Caglar, S.; Inati, S.; Heatherton, T.F. Finding the self? An event-related fMRI study. J. Cogn. Neurosci 2002, 14, 785–794. [Google Scholar] [CrossRef]
- Koenigs, M.; Young, L.; Adolphs, R.; Tranel, D.; Cushman, F.; Hauser, M.; Damasio, A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 2007, 446, 908–911. [Google Scholar] [CrossRef]
- Elliott, R.; Friston, K.J.; Dolan, R.J. Dissociable neural responses in human reward systems. J. Neurosci. 2000, 20, 6159–6165. [Google Scholar] [CrossRef]
- Knutson, B.; Fong, G.W.; Bennett, S.M.; Adams, C.M.; Hommer, D.A. Region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. Neuroimage 2003, 18, 163–272. [Google Scholar] [CrossRef]
- Tom, S.M.; Fox, C.R.; Trepel, C.; Poldrack, R.A. The neural basis of loss aversion in decision-making under risk. Science 2007, 315, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Levens, S.M.; Larsen, J.T.; Bruss, J.; Tranel, D.; Bechara, A.; Mellers, B.A. What might have been? The role of the ventromedial prefrontal cortex and lateral orbitofrontal cortex in counterfactual emotions and choice. Neuropsychologia 2014, 54, 77–86. [Google Scholar] [CrossRef]
- Manohar, S.; Lockwood, P.; Drew, D.; Fallon, S.J.; Chong, T.T.; Jeyaretna, D.S.; Baker, I.; Husain, M. Reduced decision bias and more rational decision making following ventromedial prefrontal cortex damage. Cortex 2021, 138, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Coricelli, G.; Dolan, R.J.; Sirigu, A. Brain, emotion, and decision making: The paradigmatic example of regret. Trends. Cogn. Sci. 2007, 11, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Camille, N.; Coricelli, G.; Sallet, J.; Pradat-Diehl, P.; Duhamel, J.-R.; Sirigu, A. The involvement of the orbitofrontal cortex in the experience of regret. Science 2004, 304, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Masouleh, S.K.; Eickhoff, S.B.; Hoffstaedter, F.; Genon, S.; Alzheimer’s Disease Neuroimaging Initiative. Empirical examination of the replicability of associations between brain structure and psychological variables. eLife 2019, 8, e43464. [Google Scholar] [CrossRef]
- Rangel, A.; Camerer, C.; Montague, P.R. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008, 9, 545–556. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Kim, N.; Jeong, E.-J.; Kang, M.-S.; Kim, M.J. Volumetric Variability of the Ventromedial Prefrontal Cortex Reflects the Propensity for Engaging in High-Stakes Gambling Behavior. Brain Sci. 2022, 12, 1460. https://doi.org/10.3390/brainsci12111460
Lee K, Kim N, Jeong E-J, Kang M-S, Kim MJ. Volumetric Variability of the Ventromedial Prefrontal Cortex Reflects the Propensity for Engaging in High-Stakes Gambling Behavior. Brain Sciences. 2022; 12(11):1460. https://doi.org/10.3390/brainsci12111460
Chicago/Turabian StyleLee, Kyuli, Nayoung Kim, Eun-Joo Jeong, Min-Suk Kang, and M. Justin Kim. 2022. "Volumetric Variability of the Ventromedial Prefrontal Cortex Reflects the Propensity for Engaging in High-Stakes Gambling Behavior" Brain Sciences 12, no. 11: 1460. https://doi.org/10.3390/brainsci12111460
APA StyleLee, K., Kim, N., Jeong, E.-J., Kang, M.-S., & Kim, M. J. (2022). Volumetric Variability of the Ventromedial Prefrontal Cortex Reflects the Propensity for Engaging in High-Stakes Gambling Behavior. Brain Sciences, 12(11), 1460. https://doi.org/10.3390/brainsci12111460





