Modulation of Synaptic Plasticity by Vibratory Training in Young and Old Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
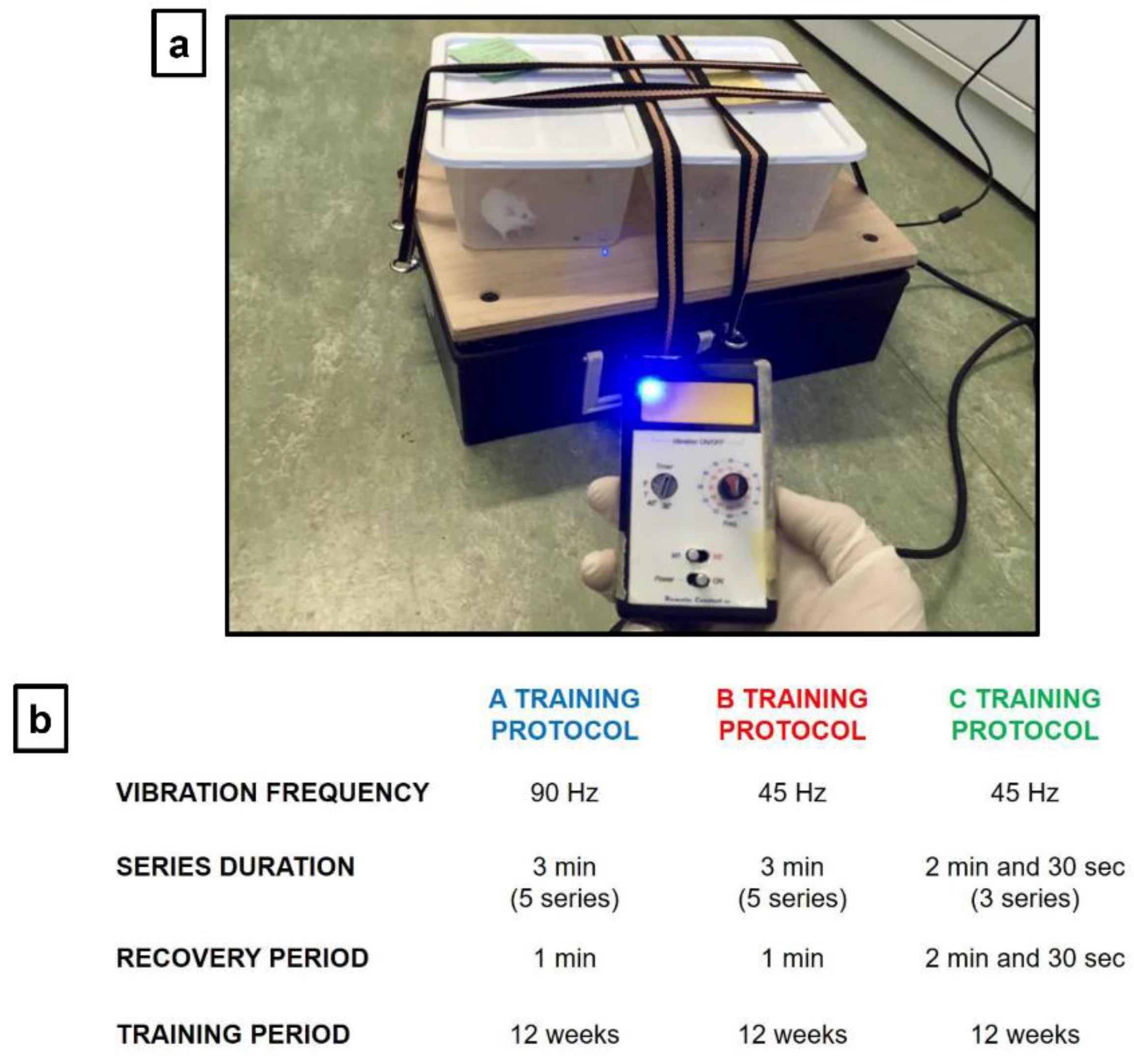
2.2. WBV Training Protocol
2.3. Extracellular Recordings in Mouse Hippocampal Slices
2.4. Statistical Analysis
3. Results
3.1. Effects of Vibratory Training on Body Weight in Young and Old Mice
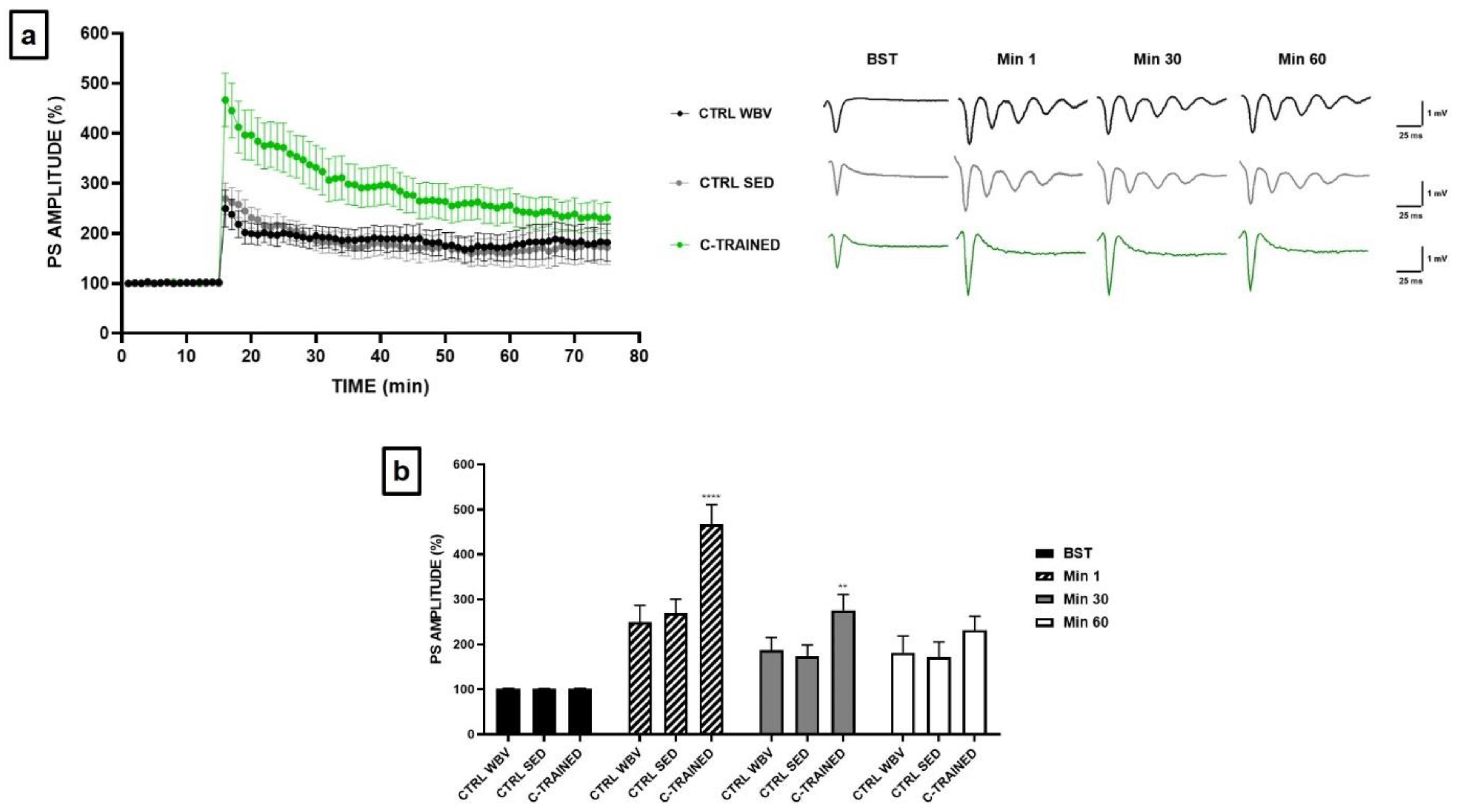
3.2. Synaptic Plasticity Following Vibratory Training
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neil, J. Mansfield Human Response to Vibration; CRC PRESS: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2005; ISBN 041528239X. [Google Scholar]
- Hulshof, C.; van Zanten, B.V. Whole-body vibration and low-back pain. A review of epidemiologic studies. Int. Arch. Occup. Environ. Health 1987, 59, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.; Heide, R. Long-term effects of whole-body vibration: A critical survey of the literature. Int. Arch. Occup. Environ. Health 1986, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Prioreschi, A.; Oosthuyse, T.; Avidon, I.; McVeigh, J. Whole body vibration increases hip bone mineral density in road cyclists. Int. J. Sports Med. 2012, 33, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Slatkovska, L.; Alibhai, S.M.H.; Beyene, J.; Cheung, A.M. Effect of whole-body vibration on BMD: A systematic review and meta-analysis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2010, 21, 1969–1980. [Google Scholar] [CrossRef]
- Figueroa, A.; Gil, R.; Wong, A.; Hooshmand, S.; Park, S.Y.; Vicil, F.; Sanchez-Gonzalez, M.A. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens. Res. 2012, 35, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Annino, G.; Padua, E.; Castagna, C.; Di Salvo, V.; Minichella, S.; Tsarpela, O.; Manzi, V.; D’Ottavio, S. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J. Strength Cond. Res. 2007, 21, 1072–1076. [Google Scholar] [CrossRef]
- Annino, G.; Iellamo, F.; Palazzo, F.; Fusco, A.; Lombardo, M.; Campoli, F.; Padua, E. Acute changes in neuromuscular activity in vertical jump and flexibility after exposure to whole body vibration. Medicine 2017, 96, e7629. [Google Scholar] [CrossRef]
- Ebid, A.A.; Ahmed, M.T.; Mahmoud Eid, M.; Mohamed, M.S.E. Effect of whole body vibration on leg muscle strength after healed burns: A randomized controlled trial. Burns 2012, 38, 1019–1026. [Google Scholar] [CrossRef]
- Salmon, J.R.; Roper, J.A.; Tillman, M.D. Does acute whole-body vibration training improve the physical performance of people with knee osteoarthritis? J. Strength Cond. Res. 2012, 26, 2983–2989. [Google Scholar] [CrossRef] [Green Version]
- Runge, M.; Rehfeld, G.; Resnicek, E. Balance training and exercise in geriatric patients. J. Musculoskelet. Neuronal Interact. 2000, 1, 61–65. [Google Scholar]
- Turbanski, S.; Haas, C.T.; Schmidtbleicher, D.; Friedrich, A.; Duisberg, P. Effects of random whole-body vibration on postural control in Parkinson’s disease. Res. Sports Med. 2005, 13, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Weng, C.; Liu, M.; Wang, Q.; Liu, L.; He, Y. Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: A pilot randomized controlled trial. Clin. Rehabil. 2014, 28, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bellia, A.; Sallì, M.; Lombardo, M.; D’Adamo, M.; Guglielmi, V.; Tirabasso, C.; Giordani, L.; Federici, M.; Lauro, D.; Foti, C.; et al. Effects of whole body vibration plus diet on insulin-resistance in middle-aged obese subjects. Int. J. Sports Med. 2014, 35, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; Iacovelli, M.; Tsarpela, O.; Cardinale, M.; Bonifazi, M.; Tihanyi, J.; Viru, M.; De Lorenzo, A.; Viru, A. Hormonal responses to whole-body vibration in men. Eur. J. Appl. Physiol. 2000, 81, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alashram, A.R.; Padua, E.; Annino, G. Effects of Whole-Body Vibration on Motor Impairments in Patients With Neurological Disorders: A Systematic Review. Am. J. Phys. Med. Rehabil. 2019, 98, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Schiller, H.H. Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J. Neurol. Neurosurg. Psychiatry 1976, 39, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Sitjà Rabert, M.; Rigau Comas, D.; Fort Vanmeerhaeghe, A.; Santoyo Medina, C.; Roqué i Figuls, M.; Romero-Rodríguez, D.; Bonfill Cosp, X. Whole-body vibration training for patients with neurodegenerative disease. Cochrane Database Syst. Rev. 2012, CD009097. [Google Scholar] [CrossRef]
- Cardinale, M.; Wakeling, J. Whole body vibration exercise: Are vibrations good for you? Br. J. Sports Med. 2005, 39, 585–589; discussion 589. [Google Scholar] [CrossRef]
- Bosco, C.; Colli, R.; Introini, E.; Cardinale, M.; Tsarpela, O.; Madella, A.; Tihanyi, J.; Viru, A. Adaptive responses of human skeletal muscle to vibration exposure. Clin. Physiol. 1999, 19, 183–187. [Google Scholar] [CrossRef]
- Hagbarth, K.E.; Eklund, G. Motor effects of muscle vibration in spasticity, rigidity and cerebellar disorders. Electroencephalogr. Clin. Neurophysiol. 1968, 25, 407. [Google Scholar] [PubMed]
- Hagbarth, K.E.; Eklund, G. The muscle vibrator—A useful tool in neurological therapeutic work. Scand. J. Rehabil. Med. 1969, 1, 26–34. [Google Scholar] [PubMed]
- Hazell, T.J.; Lemon, P.W.R. Synchronous whole-body vibration increases VO2 during and following acute exercise. Eur. J. Appl. Physiol. 2012, 112, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, H.; Al Khalili, Y. Book Physiology, Vibratory Sense; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- De Domenico, E.; D’Arcangelo, G.; Faraoni, I.; Palmieri, M.; Tancredi, V.; Graziani, G.; Grimaldi, P.; Tentori, L. Modulation of GDF11 expression and synaptic plasticity by age and training. Oncotarget 2017, 8, 57991–58002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.; Wu, T.; Hanson, J.E.; Alam, N.M.; Solanoy, H.; Ngu, H.; Lauffer, B.E.; Lin, H.H.; Dominguez, S.L.; Reeder, J.; et al. Cognitive Deficits, Changes in Synaptic Function, and Brain Pathology in a Mouse Model of Normal Aging(1,2,3). eNeuro 2015, 2. [Google Scholar] [CrossRef]
- Palmieri, M.; Cariati, I.; Scimeca, M.; Pallone, G.; Bonanno, E.; Tancredi, V.; D’Arcangelo, G.; Frank, C. Effects of short-term aerobic exercise in a mouse model of Niemann-Pick type C disease on synaptic and muscle plasticity. Ann. Ist. Super. Sanita 2019, 55. [Google Scholar] [CrossRef]
- Cristi-Montero, C.; Cuevas, M.J.; Collado, P.S. Whole-body vibration training as complement to programs aimed at weight loss. Nutr. Hosp. 2013, 28, 1365–1371. [Google Scholar] [CrossRef]
- McMullan, R.C.; Kelly, S.A.; Hua, K.; Buckley, B.K.; Faber, J.E.; Pardo-Manuel de Villena, F.; Pomp, D. Long-term exercise in mice has sex-dependent benefits on body composition and metabolism during aging. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Ang, L.-H.; Chen, W.; Yao, Y.; Ozawa, R.; Tao, E.; Yonekura, J.; Uemura, T.; Keshishian, H.; Hing, H. Lim kinase regulates the development of olfactory and neuromuscular synapses. Dev. Biol. 2006, 293, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, J.S.; Garland, T.J.; Gammie, S.C. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav. Neurosci. 2003, 117, 1243–1256. [Google Scholar] [CrossRef] [Green Version]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcangelo, G.; Triossi, T.; Buglione, A.; Melchiorri, G.; Tancredi, V. Modulation of synaptic plasticity by short-term aerobic exercise in adult mice. Behav. Brain Res. 2017, 332, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Roo, M.; Klauser, P.; Garcia, P.M.; Poglia, L.; Muller, D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog. Brain Res. 2008, 169, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.F.; Bliss, T.V.P. Plasticity in the human central nervous system. Brain 2006, 129, 1659–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, W.A.; Kurland, L.T. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia 1975, 16, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.A. Seizure disorders: The changes with age. Epilepsia 1992, 33 (Suppl. S4), S6–S14. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cariati, I.; Bonanni, R.; Pallone, G.; Annino, G.; Tancredi, V.; D’Arcangelo, G. Modulation of Synaptic Plasticity by Vibratory Training in Young and Old Mice. Brain Sci. 2021, 11, 82. https://doi.org/10.3390/brainsci11010082
Cariati I, Bonanni R, Pallone G, Annino G, Tancredi V, D’Arcangelo G. Modulation of Synaptic Plasticity by Vibratory Training in Young and Old Mice. Brain Sciences. 2021; 11(1):82. https://doi.org/10.3390/brainsci11010082
Chicago/Turabian StyleCariati, Ida, Roberto Bonanni, Gabriele Pallone, Giuseppe Annino, Virginia Tancredi, and Giovanna D’Arcangelo. 2021. "Modulation of Synaptic Plasticity by Vibratory Training in Young and Old Mice" Brain Sciences 11, no. 1: 82. https://doi.org/10.3390/brainsci11010082




