Early Attentional Modulation by Working Memory Training in Young Adult ADHD Patients during a Risky Decision-Making Task
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Working Memory Task
2.3. Working Memory Training Protocol
2.4. Probability Gambling Task
2.5. EEG Recording and Analyses
3. Results
3.1. Participants’ Clinical Assessment
3.2. Working Memory Performance
3.3. Probabilistic Gambling Task
3.4. Event Related Potentials Triggered by Gambling Choice
3.5. Effect of WM Training Condition on Differential Topographic Maps
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barkley, R.A. Major life activity and health outcomes associated with Attention-Deficit/Hyperactivity Disorder. J. Clin. Psychiatry 2002, 63 (Suppl. 12), 10–15. [Google Scholar] [PubMed]
- Kessler, R.C.; Adler, L.A.; Barkley, R.; Biederman, J.; Conners, C.K.; Faraone, S.V.; Greenhill, L.L.; Jaeger, S.; Secnik, K.; Spencer, T.; et al. Patterns and predictors of Attention-Deficit/Hyperactivity Disorder persistence into adulthood: Results from the national comorbidity survey replication. Biol. Psychiatry 2005, 57, 1442–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northover, C.; Thapar, A.; Langley, K.; van Goozen, S. Emotion Regulation in Adolescent Males with Attention-Deficit Hyperactivity Disorder: Testing the Effects of Comorbid Conduct Disorder. Brain Sci. 2015, 5, 369–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhardt, P.E.; Nobes, G.; Pischedda, S. The Relationship between Adult Symptoms of Attention-Deficit/Hyperactivity Disorder and Criminogenic Cognitions. Brain Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Gazzaley, A.; Nobre, A.C. Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci. 2012, 16, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Mueller, A.; Hong, D.S.; Shepard, S.; Moore, T. Linking ADHD to the Neural Circuitry of Attention. Trends Cogn. Sci. 2017, 21, 474–488. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Fundamentals of attention-deficit/hyperactivity disorder: Circuits and pathways. J. Clin. Psychiatry 2006, 67 (Suppl. 8), 7–12. [Google Scholar]
- De La Fuente, A.; Xia, S.; Branch, C.; Li, X. A review of Attention-Deficit/Hyperactivity Disorder from the perspective of brain networks. Front. Hum. Neurosci. 2013, 7, e00192. [Google Scholar] [CrossRef] [Green Version]
- Lenartowicz, A.; Delorme, A.; Walshaw, P.D.; Cho, A.L.; Bilder, R.M.; McGough, J.J.; McCracken, J.T.; Makeig, S.; Loo, S.K. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: Vigilance, encoding, and maintenance. J. Neurosci. 2014, 34, 1171–1182. [Google Scholar] [CrossRef]
- Duffy, F.H.; Shankardass, A.; McAnulty, G.B.; Als, H. A unique pattern of cortical connectivity characterizes patients with attention deficit disorders: A large electroencephalographic coherence study. BMC Med. 2017, 15, 51. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Qi, X.L.; Riley, M.R.; Constantinidis, C. Working memory capacity is enhanced by distributed prefrontal activation and invariant temporal dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 7095–7100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingberg, T.; Forssberg, H.; Westerberg, H. Training of working memory in children with ADHD. J. Clin. Exp. Neuropsychol. 2002, 24, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.T.; Long, D.L.; Green, D.; Iosif, A.M.; Dixon, J.F.; Miller, M.R.; Fassbender, C.; Schweitzer, J.B. Will working memory training generalize to improve off-task behavior in children with attention-deficit/ hyperactivity disorder? Neurotherapeutics 2012, 9, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.X.; Lishak, V.; Tannock, R.; Woltering, S. Effects of working memory training on neural correlates of Go/Nogo response control in adults with ADHD: A randomized controlled trial. Neuropsychologia 2017, 95, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Katz, B.; Buschkuehl, M.; Jaeggi, S.M.; Shah, P. Exploring N-Back Cognitive Training for Children With ADHD. J. Atten. Disord. 2018, 1087054718779230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschkuehl, M.; Jaeggi, S.M.; Jonides, J. Neuronal effects following working memory training. Dev. Cogn. Neurosci. 2012, 2 (Suppl. 1), S167–S179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwaighofer, M.; Fischer, F.; Bühner, M. Does Working Memory Training Transfer? A Meta-Analysis Including Training Conditions as Moderators. Educ. Psychol. 2015, 50, 138–166. [Google Scholar] [CrossRef]
- Muris, P.; Roodenrijs, D.; Kelgtermans, L.; Sliwinski, S.; Berlage, U.; Baillieux, H.; Deckers, A.; Gunther, M.; Paanakker, B.; Holterman, I. No Medication for My Child! A Naturalistic Study on the Treatment Preferences for and Effects of Cogmed Working Memory Training Versus Psychostimulant Medication in Clinically Referred Youth with ADHD. Child Psychiatry Hum. Dev. 2018, 49, 974–992. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, S.; Halfon, O.; Fornari, E.; Urben, S.; Bader, M. Cognitive Working Memory Training (CWMT) in adolescents suffering from Attention-Deficit/Hyperactivity Disorder (ADHD): A controlled trial taking into account concomitant medication effects. Psychiatry Res. 2018, 269, 79–85. [Google Scholar] [CrossRef]
- Capodieci, A.; Re, A.M.; Fracca, A.; Borella, E.; Carretti, B. The efficacy of a training that combines activities on working memory and metacognition: Transfer and maintenance effects in children with ADHD and typical development. J. Clin. Exp. Neuropsychol. 2019, 41, 1074–1087. [Google Scholar] [CrossRef]
- Rapport, M.D.; Orban, S.A.; Kofler, M.J.; Friedman, L.M. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin. Psychol. Rev. 2013, 33, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.; Brandeis, D.; Holtmann, M.; Cortese, S. Computer-based cognitive training for ADHD: A review of current evidence. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 807–824. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Ferrin, M.; Brandeis, D.; Buitelaar, J.; Daley, D.; Dittmann, R.W.; Holtmann, M.; Santosh, P.; Stevenson, J.; Stringaris, A.; et al. Cognitive training for attention-deficit/hyperactivity disorder: Meta- analysis of clinical and neuropsychological outcomes from randomized controlled trials. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melby-Lervåg, M.; Redick, T.S.; Hulme, C. Working Memory Training Does Not Improve Performance on Measures of Intelligence or Other Measures of “Far Transfer”: Evidence From a Meta-Analytic Review. Perspect. Psychol. Sci. 2016, 11, 512–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechara, A.; Martin, E.M. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology 2004, 18, 152–162. [Google Scholar] [CrossRef]
- Lawrence, A.J.; Luty, J.; Bogdan, N.A.; Sahakian, B.J.; Clark, L. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction 2009, 104, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- Malloy-Diniz, L.; Fuentes, D.; Leite, W.B.; Correa, H.; Bechara, A. Impulsive behavior in adults with attention deficit/ hyperactivity disorder: Characterization of attentional, motor and cognitive impulsiveness. J. Int. Neuropsychol. Soc. 2007, 13, 693–698. [Google Scholar] [CrossRef]
- Ibanez, A.; Cetkovich, M.; Petroni, A.; Urquina, H.; Baez, S.; Gonzalez-Gadea, M.L.; Kamienkowski, J.E.; Torralva, T.; Torrente, F.; Strejilevich, S.; et al. The Neural Basis of Decision-Making and Reward Processing in Adults with Euthymic Bipolar Disorder or Attention-Deficit/Hyperactivity Disorder (ADHD). PLoS ONE 2012, 7, e37306. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, R.; Rizzo, P.; Steinhausen, H.C. Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. J. Neural Transm. (Vienna) 2008, 115, 201–209. [Google Scholar] [CrossRef]
- Duarte, N.A.; Woods, S.P.; Rooney, A.; Atkinson, J.H.; Grant, I.; Translational Methamphetamine AIDS Research Center Group. Working memory deficits affect risky decision-making in methamphetamine users with Attention-Deficit/Hyperactivity Disorder. J. Psychiatr. Res. 2012, 46, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Coghill, D.R.; Seth, S.; Matthews, K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: Advancing beyond the three-pathway models. Psychol. Med. 2014, 44, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A.; Fischer, M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 503–513. [Google Scholar] [PubMed]
- Matthies, S.; Philipsen, A.; Svaldi, J. Risky decision making in adults with ADHD. J. Behav. Ther. Exp. Psychiatry 2012, 43, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Kalbe, E.; Labudda, K.; Fujiwara, E.; Kessler, J.; Markowitsch, H.J. Decision-making impairments in patients with pathological gambling. Psychiatry Res. 2005, 133, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.N. The neurobiology of pathological gambling and drug addiction: An overview and new findings. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Fridberg, D.J.; Gerst, K.R.; Finn, P.R. Effects of working memory load, a history of conduct disorder, and sex on decision making in substance dependent individuals. Drug Alcohol. Depend. 2013, 133, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.F.; Wang, Y.; Shi, H.S.; Liu, L.L.; Chen, X.J.; Chen, Y.H. Effects of working memory load on uncertain decision-making: Evidence from the Iowa Gambling Task. Front. Psychol. 2015, 6, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehricke, J.G.; Kruggel, F.; Thampipop, T.; Alejo, S.D.; Tatos, E.; Fallon, J.; Muftuler, L.T. The brain anatomy of attention-deficit/hyperactivity disorder in young adults—A magnetic resonance imaging study. PLoS ONE 2017, 12, e0175433. [Google Scholar] [CrossRef] [Green Version]
- Lilienthal, L.; Tamez, E.; Shelton, J.T.; Myerson, J.; Hale, S. Dual n-back training increases the capacity of the focus of attention. Psychon. Bull. Rev. 2013, 20, 135–141. [Google Scholar] [CrossRef]
- Schweizer, S.; Leung, J.; Kievit, R.; Speekenbrink, M.; Trender, W.; Hampshire, A.; Blakemore, S. Protocol for an app-based affective control training for adolescents: Proof-of-principle double-blind randomized controlled trial [version 2; peer review: 4 approved]. Wellcome Open Res. 2019, 4, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesrobian, S.K.; Villa, A.E.P.; Bader, M.; Götte, L.; Lintas, A. Event-Related Potentials during a Gambling Task in Young Adults with Attention-Deficit/Hyperactivity Disorder. Front. Hum. Neurosci. 2018, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2000, 284, 3043–3045. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Conners, C.K.; Erhardt, D.; Sparrow, E. Conner’s Adult ADHD Rating Scales: Technical Manual; Multi-Health Systems Incorporated (MHS): North Tonawanda, NY, USA, 1999. [Google Scholar]
- Kessler, R.C.; Adler, L.; Ames, M.; Demler, O.; Faraone, S.; Hiripi, E.; Howes, M.J.; Jin, R.; Secnik, K.; Spencer, T.; et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychol. Med. 2005, 35, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumeaux, P.; Mercier, C.; Roche, S.; Iwaz, J.; Bader, M.; Stéphan, P.; Ecochard, R.; Revol, O. Validation of the French Version of Conners’ Parent Rating Scale Revised, Short Version: Factorial Structure and Reliability. Can. J. Psychiatry 2016, 61, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Perrig, W.J. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. USA 2008, 105, 6829–6833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesrobian, S.K. Does working memory training affect decision making? A neuroeconomic study. Ph.D. Thesis, Faculty of Medicine and Biology, University of Lausanne, Lausanne, Switzerland, 2015. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV); NCS Pearson: San Antonio, TX, USA, 2008. [Google Scholar]
- Kessels, R.P.; van Zandvoort, M.J.; Postma, A.; Kappelle, L.J.; de Haan, E.H. The Corsi Block-Tapping Task: Standardization and normative data. Appl. Neuropsychol. 2000, 7, 252–258. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Sommer, T.; Raz, A.; Posner, M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002, 14, 340–347. [Google Scholar] [CrossRef]
- Gneezy, U.; Potters, J. An experiment on risk taking and evaluation periods. Q. J. Econ. 1997, 112, 631–645. [Google Scholar] [CrossRef] [Green Version]
- Luck, S.J. An Introduction to Event-Related Potentials and Their Neural Origins; Massachusetts Institute of Technology: Cambridge, MA, USA, 2005. [Google Scholar]
- Brunet, D.; Murray, M.M.; Michel, C.M. Spatiotemporal analysis of multichannel EEG: CARTOOL. Comput. Intell. Neurosci. 2011, 2011, 813870. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Multiple significance tests: the Bonferroni method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef] [Green Version]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Statistics and Computing; Springer: New York, NY, USA, 2002; p. 498. [Google Scholar]
- Castellanos, F.X.; Tannock, R. Neuroscience of Attention-Deficit/Hyperactivity Disorder: The search for endophenotypes. Nat. Rev. Neurosci. 2002, 3, 617–628. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Vinogradov, S.; Rumsey, J.; Sherrill, J.; Wagner, A. Cognitive training in mental disorders: Update and future directions. Am. J. Psychiatry 2014, 171, 510–522. [Google Scholar] [CrossRef]
- Ansari, S. The therapeutic potential of working memory training for treating mental disorders. Front. Hum. Neurosci. 2015, 9, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomone, S.; Fleming, G.R.; Shanahan, J.M.; Castorina, M.; Bramham, J.; O’Connell, R.G.; Robertson, I.H. The effects of a Self-Alert Training (SAT) program in adults with ADHD. Front. Hum. Neurosci. 2015, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Lambez, B.; Harwood-Gross, A.; Golumbic, E.Z.; Rassovsky, Y. Non-pharmacological interventions for cognitive difficulties in ADHD: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 120, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Woltering, S.; Gu, C.; Liu, Z.X.; Tannock, R. Visuospatial Working Memory Capacity in the Brain After Working Memory Training in College Students With ADHD: A Randomized Controlled Trial. J. Atten. Disord. 2019, 1087054719879487. [Google Scholar] [CrossRef] [PubMed]
- Salminen, T.; Frensch, P.; Strobach, T.; Schubert, T. Age-specific differences of dual n-back training. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2016, 23, 18–39. [Google Scholar] [CrossRef]
- Matysiak, O.; Kroemeke, A.; Brzezicka, A. Working Memory Capacity as a Predictor of Cognitive Training Efficacy in the Elderly Population. Front. Aging Neurosci. 2019, 11, 126. [Google Scholar] [CrossRef] [Green Version]
- Au, J.; Sheehan, E.; Tsai, N.; Duncan, G.J.; Buschkuehl, M.; Jaeggi, S.M. Improving fluid intelligence with training on working memory: A meta-analysis. Psychon. Bull. Rev. 2015, 22, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Blacker, K.J.; Negoita, S.; Ewen, J.B.; Courtney, S.M. N-back versus Complex Span Working Memory Training. J. Cogn. Enhanc. 2017, 1, 434–454. [Google Scholar] [CrossRef]
- Theiling, J.; Petermann, F. Neuropsychological Profiles on the WAIS-IV of Adults With ADHD. J. Atten. Disord. 2016, 20, 913–924. [Google Scholar] [CrossRef] [PubMed]
- van Ewijk, H.; Heslenfeld, D.J.; Luman, M.; Rommelse, N.N.; Hartman, C.A.; Hoekstra, P.; Franke, B.; Buitelaar, J.K.; Oosterlaan, J. Visuospatial working memory in ADHD patients, unaffected siblings, and healthy controls. J. Atten. Disord. 2014, 18, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jing, J.; Igarashi, K.; Fan, L.; Yang, S.; Li, Y.; Jin, Y. Executive function predicts the visuospatial working memory in autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res. 2018, 11, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Kalanthroff, E. Visuospatial processing bias in ADHD: A potential artifact in the Wechsler Adult Intelligence Scale and the Rorschach Inkblots Test. Psychol. Assess. 2019, 31, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Lawlor-Savage, L.; Goghari, V.M. Dual N-Back Working Memory Training in Healthy Adults: A Randomized Comparison to Processing Speed Training. PLoS ONE 2016, 11, e0151817. [Google Scholar] [CrossRef] [PubMed]
- Minear, M.; Brasher, F.; Guerrero, C.B.; Brasher, M.; Moore, A.; Sukeena, J. A simultaneous examination of two forms of working memory training: Evidence for near transfer only. Mem. Cognit. 2016, 44, 1014–1037. [Google Scholar] [CrossRef]
- Soveri, A.; Antfolk, J.; Karlsson, L.; Salo, B.; Laine, M. Working memory training revisited: A multi-level meta-analysis of n-back training studies. Psychon. Bull. Rev. 2017, 24, 1077–1096. [Google Scholar] [CrossRef] [Green Version]
- Holmes, J.; Woolgar, F.; Hampshire, A.; Gathercole, S.E. Are Working Memory Training Effects Paradigm-Specific? Front. Psychol. 2019, 10, 1103. [Google Scholar] [CrossRef] [Green Version]
- Mesrobian, S.K.; Lintas, A.; Jacquerod, M.; Bader, M.; Götte, L.; Villa, A.E. An ERP Study Reveals How Training with Dual N-Back Task Affects Risky Decision Making in a Gambling Task in ADHD Patients. In Advances in Cognitive Neurodynamics (VI); Delgado-García, J.M., Pan, X., Sánchez-Campusano, R., Wang, R., Eds.; Springer: Singapore, 2018; chapter 34; pp. 271–277. [Google Scholar]
- Liston, C.; Malter Cohen, M.; Teslovich, T.; Levenson, D.; Casey, B.J. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol. Psychiatry 2011, 69, 1168–1177. [Google Scholar] [CrossRef]
- Helenius, P.; Laasonen, M.; Hokkanen, L.; Paetau, R.; Niemivirta, M. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia 2011, 49, 1889–1896. [Google Scholar] [CrossRef]
- Cheung, C.H.M.; Rijsdijk, F.; McLoughlin, G.; Brandeis, D.; Banaschewski, T.; Asherson, P.; Kuntsi, J. Cognitive and neurophysiological markers of ADHD persistence and remission. Br. J. Psychiatry 2016, 208, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Brydges, C.R.; Ozolnieks, K.L.; Roberts, G. Working memory—Not processing speed—Mediates fluid intelligence deficits associated with attention deficit/hyperactivity disorder symptoms. J. Neuropsychol. 2017, 11, 362–377. [Google Scholar] [CrossRef]
- Pollak, Y.; Shalit, R.; Aran, A. Risk taking and adult attention deficit/hyperactivity disorder: A gap between real life behavior and experimental decision making. Psychiatry Res. 2018, 259, 56–62. [Google Scholar] [CrossRef]
- Cui, J.F.; Chen, Y.H.; Wang, Y.; Shum, D.H.K.; Chan, R.C.K. Neural correlates of uncertain decision making: ERP evidence from the Iowa Gambling Task. Front. Hum. Neurosci. 2013, 7, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, Q. Electrophysiological correlates of decision-making in high-risk versus low-risk conditions of a gambling game. Psychophysiology 2011, 48, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- San Martín, R.; Appelbaum, L.G.; Pearson, J.M.; Huettel, S.A.; Woldorff, M.G. Rapid brain responses independently predict gain maximization and loss minimization during economic decision making. J. Neurosci. 2013, 33, 7011–7019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, K.; Kimura, M.; Iwaki, S. Temporal prediction modulates the evaluative processing of “good” action feedback: An electrophysiological study. Psychophysiology 2016, 53, 1552–1559. [Google Scholar] [CrossRef]
- West, R.; Bailey, K.; Anderson, S. Transient and sustained ERP activity related to feedback processing in the probabilistic selection task. Int. J. Psychophysiol. 2018, 126, 1–12. [Google Scholar] [CrossRef]
- Abouzari, M.; Oberg, S.; Tata, M. Theta-band oscillatory activity differs between gamblers and nongamblers comorbid with attention-deficit hyperactivity disorder in a probabilistic reward-learning task. Behav. Brain Res. 2016, 312, 195–200. [Google Scholar] [CrossRef]
- Coleman, B.; Marion, S.; Rizzo, A.; Turnbull, J.; Nolty, A. Virtual Reality Assessment of Classroom - Related Attention: An Ecologically Relevant Approach to Evaluating the Effectiveness of Working Memory Training. Front. Psychol. 2019, 10, 1851. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.d.S.; Paula, J.J.d.; Alvim-Soares Júnior, A.M.; Diniz, B.S.; Romano-Silva, M.A.; Malloy-Diniz, L.F.; Miranda, D.M.d. ADHD inattentive symptoms mediate the relationship between intelligence and academic performance in children aged 6-14. Braz. J. Psychiatry 2014, 36, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perchet, C.; Revol, O.; Fourneret, P.; Mauguière, F.; Garcia-Larrea, L. Attention shifts and anticipatory mechanisms in hyperactive children: An ERP study using the Posner paradigm. Biol. Psychiatry 2001, 50, 44–57. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J. A review of electrophysiology in Attention-Deficit/Hyperactivity Disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003, 114, 171–183. [Google Scholar] [CrossRef]
- Sable, J.J.; Knopf, K.L.; Kyle, M.R.; Schully, L.T.; Brooks, M.M.; Parry, K.H.; Thompson, I.A.; Suna, E.B.; Stowe, R.; Flink, L.A.; et al. Attention-deficit hyperactivity disorder reduces automatic attention in young adults. Psychophysiology 2013, 50, 308–313. [Google Scholar] [CrossRef]
- Baijot, S.; Cevallos, C.; Zarka, D.; Leroy, A.; Slama, H.; Colin, C.; Deconinck, N.; Dan, B.; Cheron, G. EEG Dynamics of a Go/Nogo Task in Children with ADHD. Brain Sci. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Hillyard, S.A.; Vogel, E.K.; Luck, S.J. Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 1257–1270. [Google Scholar] [CrossRef] [Green Version]
- Zani, A.; Proverbio, A.M. Is that a belt or a snake? Object attentional selection affects the early stages of visual sensory processing. Behav. Brain Funct. 2012, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Lavie, N. Distracted and confused? Selective attention under load. Trends Cogn. Sci. 2005, 9, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cosman, J.D.; Mordkoff, J.T.; Vecera, S.P. Stimulus recognition occurs under high perceptual load: Evidence from correlated flankers. J. Exp. Psychol. Hum. Percept. Perform. 2016, 42, 2077–2083. [Google Scholar] [CrossRef]
- Singhal, A. Differentiating between spatial and object-based working memory using complex stimuli: An erp study. Int. J. Neurosci. 2006, 116, 1457–1469. [Google Scholar] [CrossRef]
- Bomba, M.D.; Singhal, A. ERP evidence of early cross-modal links between auditory selective attention and visuo-spatial memory. Brain Cogn. 2010, 74, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Quak, M.; Langford, Z.D.; London, R.E.; Talsma, D. Contralateral delay activity does not reflect behavioral feature load in visual working memory. Biol. Psychol. 2018, 137, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Evoked alpha and early access to the knowledge system: The P1 inhibition timing hypothesis. Brain Res. 2011, 1408, 52–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zani, A.; Proverbio, A.M. Endogenous attention to object features modulates the ERP C1 component. Cogn. Neurosci. 2018, 9, 66–67. [Google Scholar] [CrossRef]
- Crick, F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc. Natl. Acad. Sci. USA 1984, 81, 4586–4590. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.E.P.; Tetko, I.V.; Dutoit, P.; De Ribaupierre, Y.; De Ribaupierre, F. Corticofugal modulation of functional connectivity within the auditory thalamus of rat, guinea pig and cat revealed by cooling deactivation. J. Neurosci. Methods 1999, 86, 161–178. [Google Scholar] [CrossRef]
- Lintas, A.; Schwaller, B.; Villa, A.E.P. Visual thalamocortical circuits in parvalbumin-deficient mice. Brain Res. 2013, 1536, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.; Mecklinger, A. The late posterior negativity in ERP studies of episodic memory: Action monitoring and retrieval of attribute conjunctions. Biol. Psychol. 2003, 64, 91–117. [Google Scholar] [CrossRef]
- Gui, P.; Ku, Y.; Li, L.; Li, X.; Bodner, M.; Lenz, F.A.; Wang, L.; Zhou, Y.D. Neural correlates of visuo-tactile crossmodal paired-associate learning and memory in humans. Neuroscience 2017, 362, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Paller, K.A.; Kutas, M. Brain Potentials during Memory Retrieval Provide Neurophysiological Support for the Distinction between Conscious Recollection and Priming. J. Cogn. Neurosci. 1992, 4, 375–392. [Google Scholar] [CrossRef] [Green Version]
- Ranganath, C.; Paller, K.A. Neural correlates of memory retrieval and evaluation. Brain Res. Cogn. Brain Res. 2000, 9, 209–222. [Google Scholar] [CrossRef]
- Wilding, E.L. In what way does the parietal ERP old/new effect index recollection? Int. J. Psychophysiol. 2000, 35, 81–87. [Google Scholar] [CrossRef]
- Nessler, D.; Mecklinger, A.; Penney, T.B. Event related brain potentials and illusory memories: The effects of differential encoding. Brain Res. Cogn. Brain Res. 2001, 10, 283–301. [Google Scholar] [CrossRef]
- Nikolaidis, A.; Voss, M.W.; Lee, H.; Vo, L.T.K.; Kramer, A.F. Parietal plasticity after training with a complex video game is associated with individual differences in improvements in an untrained working memory task. Front. Hum. Neurosci. 2014, 8, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeggi, S.M.; Buschkuehl, M.; Shah, P.; Jonides, J. The role of individual differences in cognitive training and transfer. Mem. Cognit. 2014, 42, 464–480. [Google Scholar] [CrossRef]
- Mawjee, K.; Woltering, S.; Tannock, R. Working Memory Training in Post-Secondary Students with ADHD: A Randomized Controlled Study. PLoS ONE 2015, 10, e0137173. [Google Scholar] [CrossRef]
- Hale, T.S.; Kane, A.M.; Tung, K.L.; Kaminsky, O.; McGough, J.J.; Hanada, G.; Loo, S.K. Abnormal Parietal Brain Function in ADHD: Replication and Extension of Previous EEG Beta Asymmetry Findings. Front. Psychiatry 2014, 5, 87. [Google Scholar] [CrossRef]


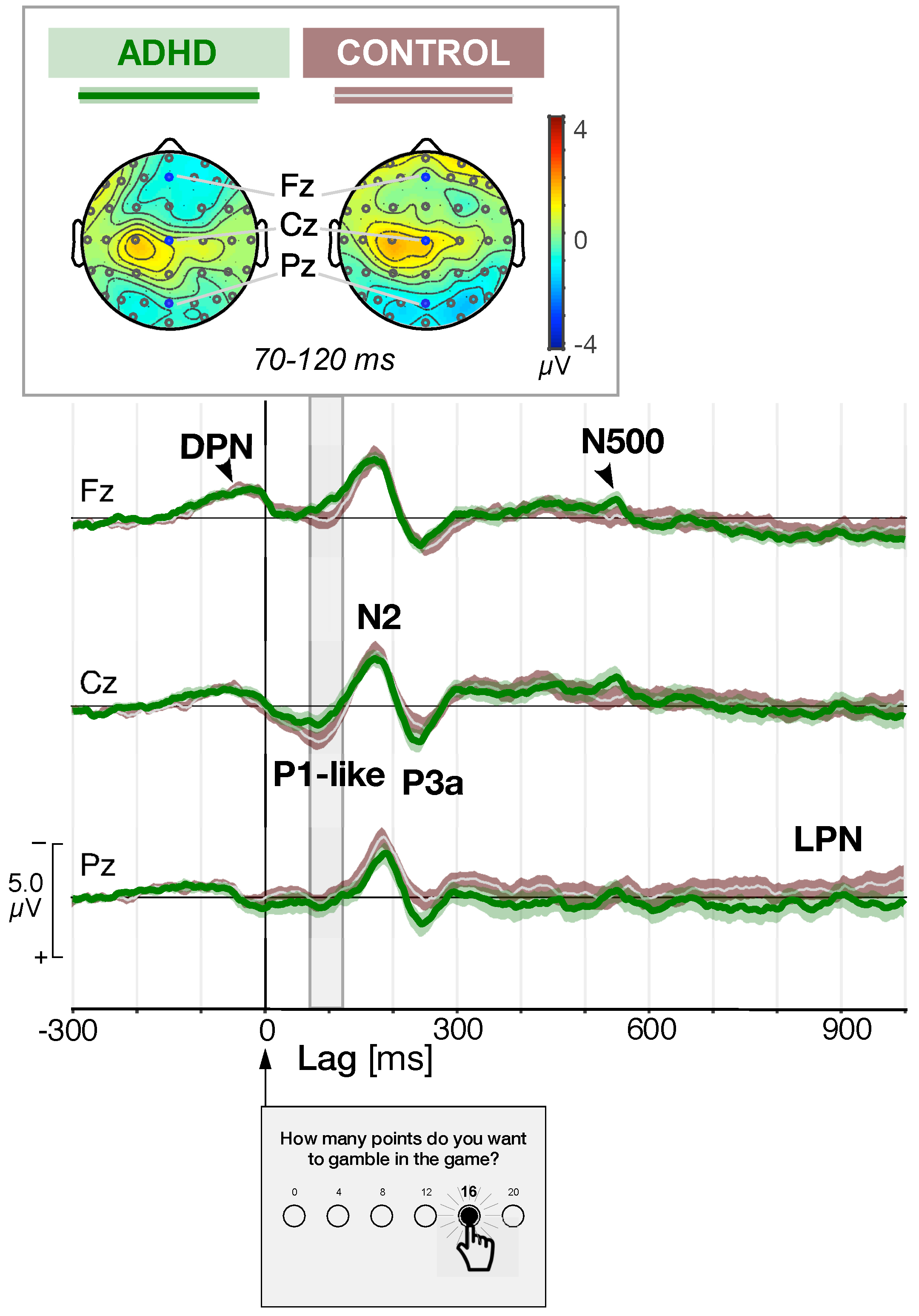

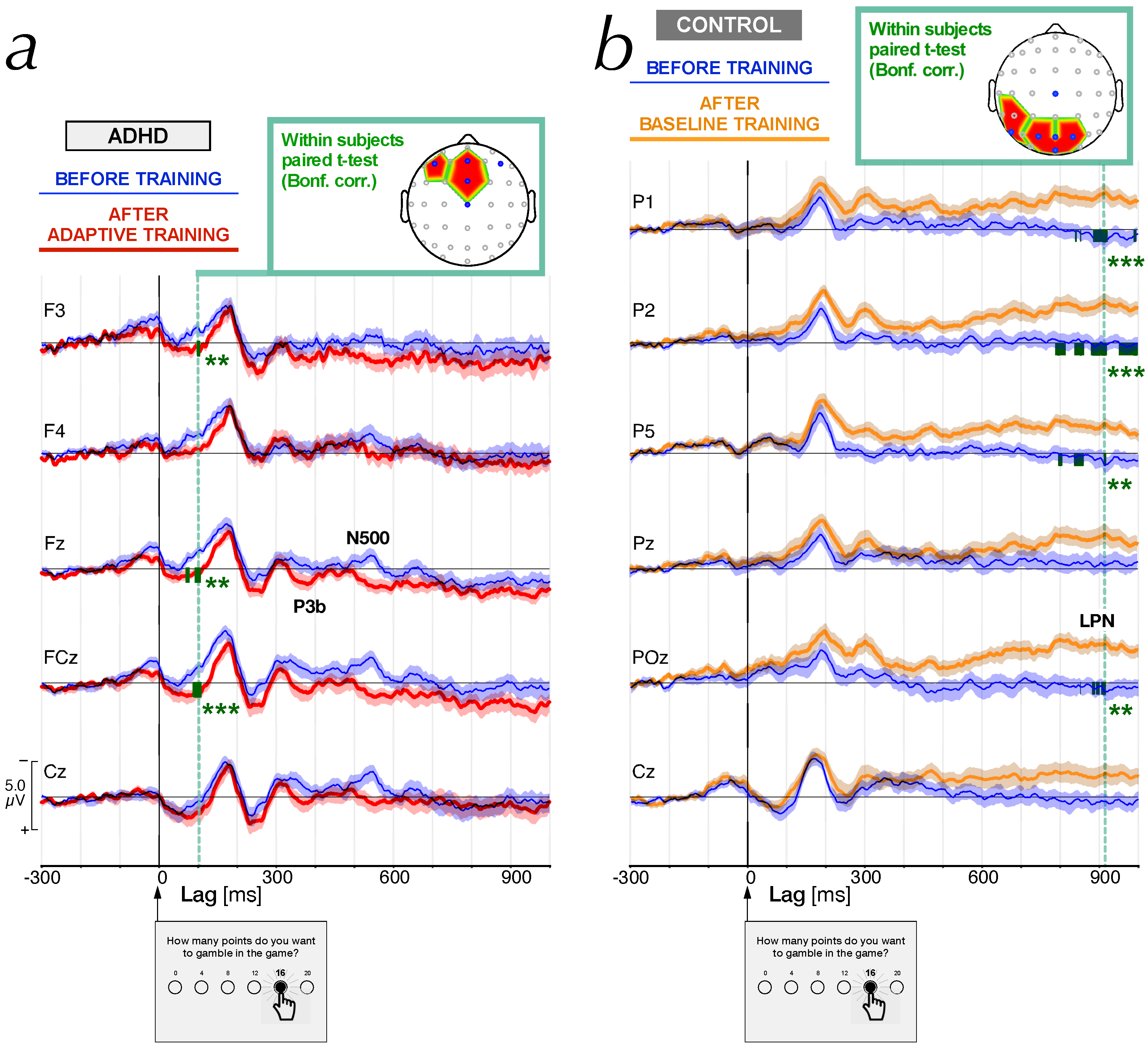
| Controls | ADHD | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Adaptive | Baseline | Adaptive | Effect | Pr() | |||||
| Sample size (N) | 18 | 19 | 14 | 14 | ||||||
| Dualn-Back level | 2.76 | |||||||||
| 7.67 | ** | |||||||||
| pre-training | 2.12 | 1.95 | 1.97 | 1.80 | 101.2 | *** | ||||
| 2.20 (0.12) | 1.90 (0.06) | 2.10 (0.13) | 1.84 (0.11) | 0.13 | ||||||
| 1.06 | ||||||||||
| post-training | 2.77 | 3.95 | 2.45 | 3.33 | 26.4 | *** | ||||
| 2.91 (0.17) | 3.80 (0.23) | 2.52 (0.16) | 3.55 (0.29) | 0.04 | ||||||
| WAIS-IV digit span (normalized score) | 11.28 | ** | ||||||||
| 14.99 | *** | |||||||||
| pre-training | 13.50 | 11.00 | 10.50 | 10.00 | 6.95 | ** | ||||
| 13.67 (0.68) | 11.11 (0.31) | 11.14 (0.94) | 9.86 (0.72) | 2.22 | ||||||
| 0.11 | ||||||||||
| post-training | 15.50 | 13.00 | 13.00 | 11.50 | 0.03 | |||||
| 14.83 (0.62) | 12.26 (0.55) | 12.43 (1.04) | 11.57 (0.84) | 0.05 | ||||||
| Corsi block-tapping task (percentiles) | 0.06 | |||||||||
| 0.00 | ||||||||||
| pre-training | 90.0 | 80.0 | 70.0 | 80.0 | 0.80 | |||||
| 78.3 (5.1) | 72.9 (4.9) | 60.7 (7.4) | 67.1 (6.8) | 0.00 | ||||||
| 3.56 | ||||||||||
| post-training | 80.0 | 80.0 | 85.0 | 80.0 | 0.00 | |||||
| 78.9 (4.6) | 69.0 (4.9) | 70.4 (8.0) | 75.4 (4.7) | 0.00 | ||||||
| Group: | Controls | ADHD | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Level: | Baseline | Adaptive | Baseline | Adaptive | Effect | Pr() | ||||
| Sample size (N) | 18 | 19 | 14 | 14 | ||||||
| Response time (ms) | 5.26 | * | ||||||||
| 1.4 | ||||||||||
| pre-training | 914 | 1289 | 1305 | 1332 | 18.65 | *** | ||||
| 1199 (155) | 1396 (203) | 1542 (192) | 1573 (233) | 0.01 | ||||||
| 1.40 | ||||||||||
| post-training | 738 | 812 | 926 | 996 | 0.01 | |||||
| 764 (73) | 826 (79) | 942 (107) | 1201 (217) | 0.61 | ||||||
| Total Gains (points) | 2.24 | |||||||||
| 1.17 | ||||||||||
| pre-training | 1886 | 1868 | 1812 | 1878 | 0.05 | |||||
| 1947 (48) | 1875 (39) | 1853 (46) | 1902 (45) | 3.79 | ||||||
| 0.55 | ||||||||||
| post-training | 1890 | 1868 | 1848 | 1806 | 0.36 | |||||
| 2010 (92) | 1850 (58) | 1809 (52) | 1857 (65) | 0.27 | ||||||
| Risk index | 1.14 | |||||||||
| 8.68 | ** | |||||||||
| pre-training | 0.18 | −0.22 | 0.15 | −0.08 | 0.29 | |||||
| 0.18 (0.12) | −0.15 (0.09) | −0.02 (0.14) | −0.09 (0.12) | 1.20 | ||||||
| 0.10 | ||||||||||
| post-training | 0.18 | 0.16 | −0.04 | 0.12 | ||||||
| 0.25 (0.14) | −0.09 (0.09) | 0.06 (0.15) | −0.14 (0.10) | 0.04 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaquerod, M.E.; Mesrobian, S.K.; Villa, A.E.P.; Bader, M.; Lintas, A. Early Attentional Modulation by Working Memory Training in Young Adult ADHD Patients during a Risky Decision-Making Task. Brain Sci. 2020, 10, 38. https://doi.org/10.3390/brainsci10010038
Jaquerod ME, Mesrobian SK, Villa AEP, Bader M, Lintas A. Early Attentional Modulation by Working Memory Training in Young Adult ADHD Patients during a Risky Decision-Making Task. Brain Sciences. 2020; 10(1):38. https://doi.org/10.3390/brainsci10010038
Chicago/Turabian StyleJaquerod, Manon E., Sarah K. Mesrobian, Alessandro E. P. Villa, Michel Bader, and Alessandra Lintas. 2020. "Early Attentional Modulation by Working Memory Training in Young Adult ADHD Patients during a Risky Decision-Making Task" Brain Sciences 10, no. 1: 38. https://doi.org/10.3390/brainsci10010038





