Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Pore Solution Analysis
2.2.1. Extraction of Pore Solution
2.2.2. Chemistry Analysis of Leaching Solution
2.2.3. Chemistry of Pore Solution
2.2.4. Characterization of Hydration Products
2.3. Corrosion Potential in Simulated Pore Solutions
3. Results and Discussion
3.1. Pore Solution Analysis
3.1.1. Pore Solution Chemistry
3.1.2. Determination of Aluminate Ion
3.1.3. Comparison with PWE
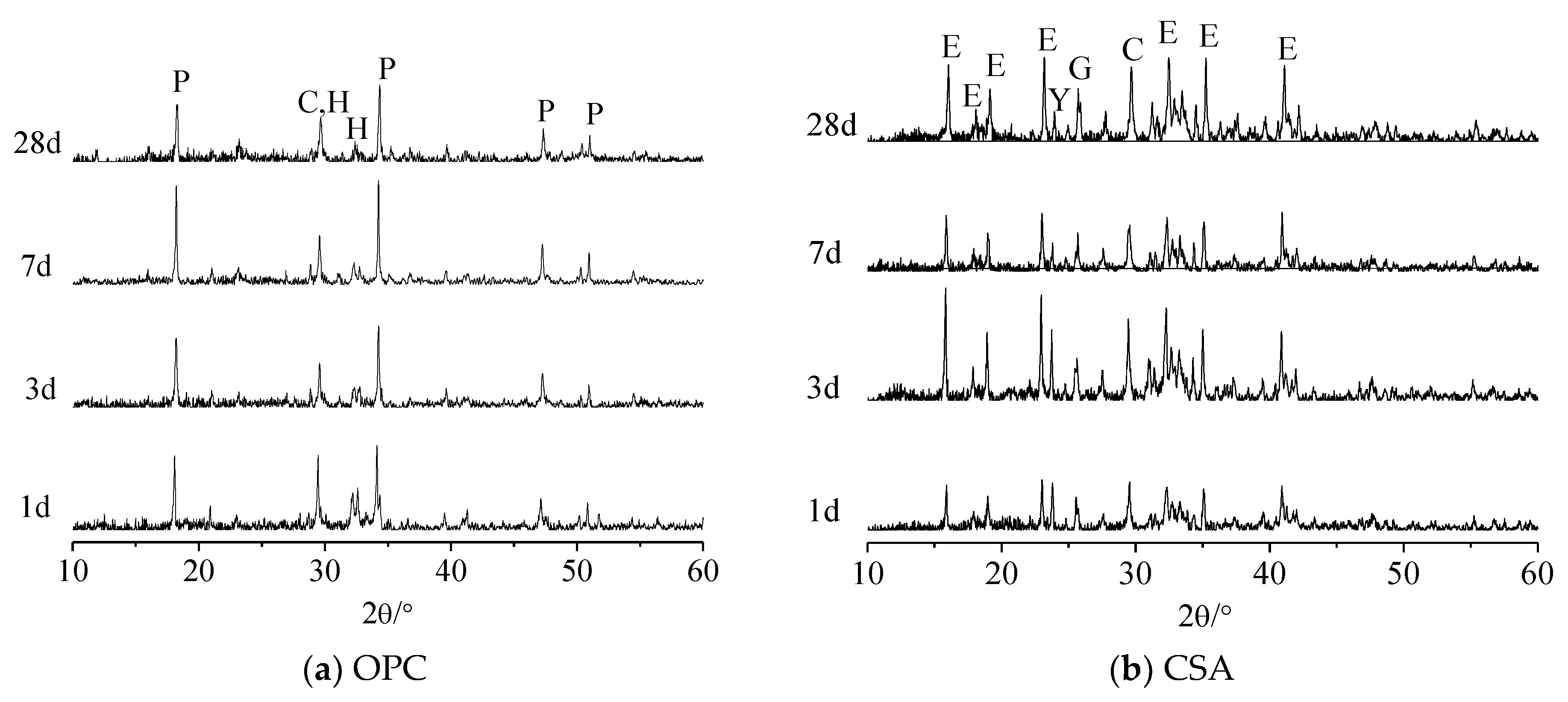
3.2. Hydration Products
3.3. Factors Influencing Steel Passivation
3.3.1. Simulated Pore Solution System
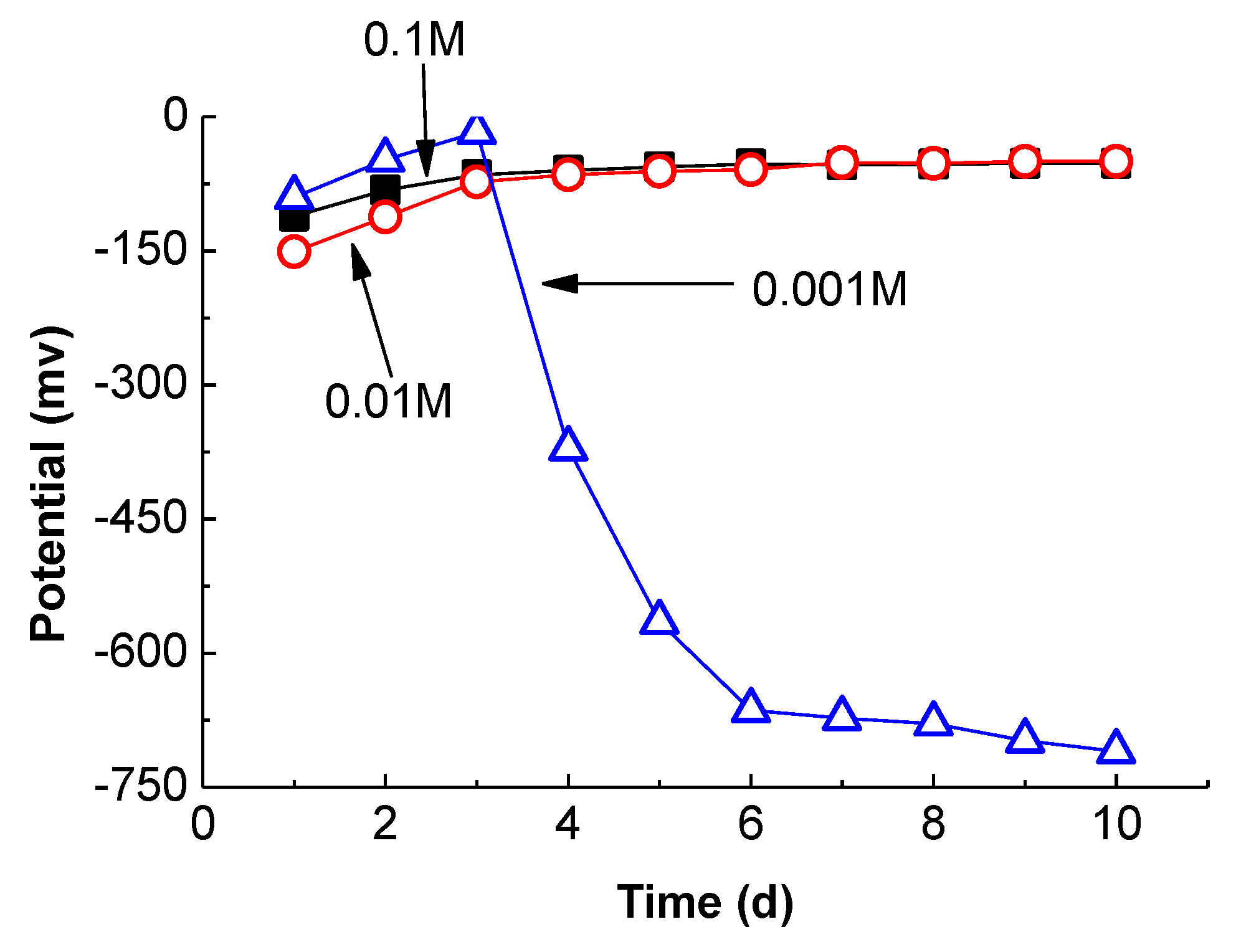
3.3.2. Effect of Alkalinity
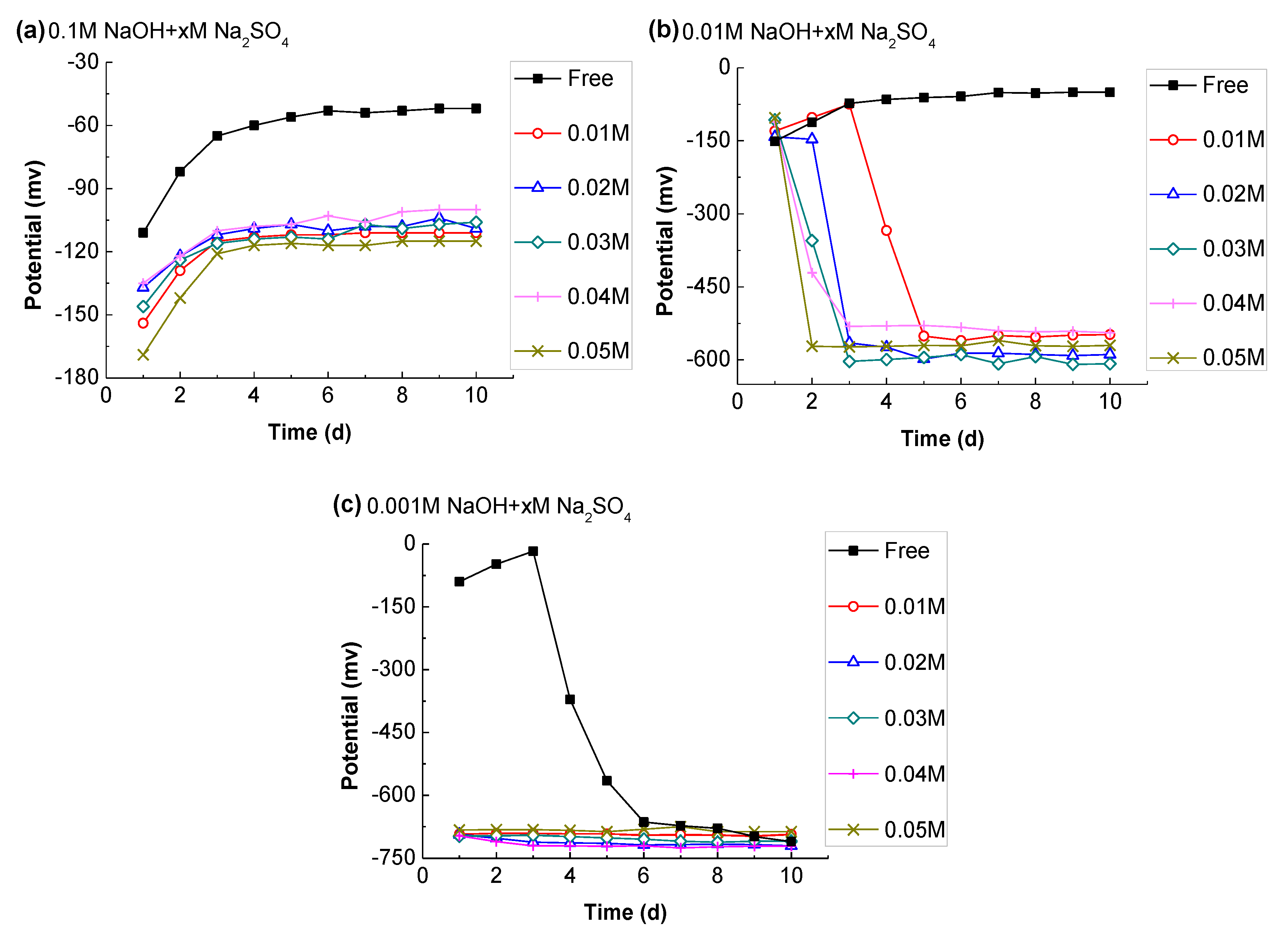
3.3.3. Effect of Sulfate Ion
3.3.4. Effect of Aluminate Ion
3.4. Corrosion Behavior of Steel in CSA Based Materials
4. Conclusions
- The cations in CSA and OPC are similar, both mainly containing Na+, K+, and little Ca2+. However, the composition of anions varies greatly.
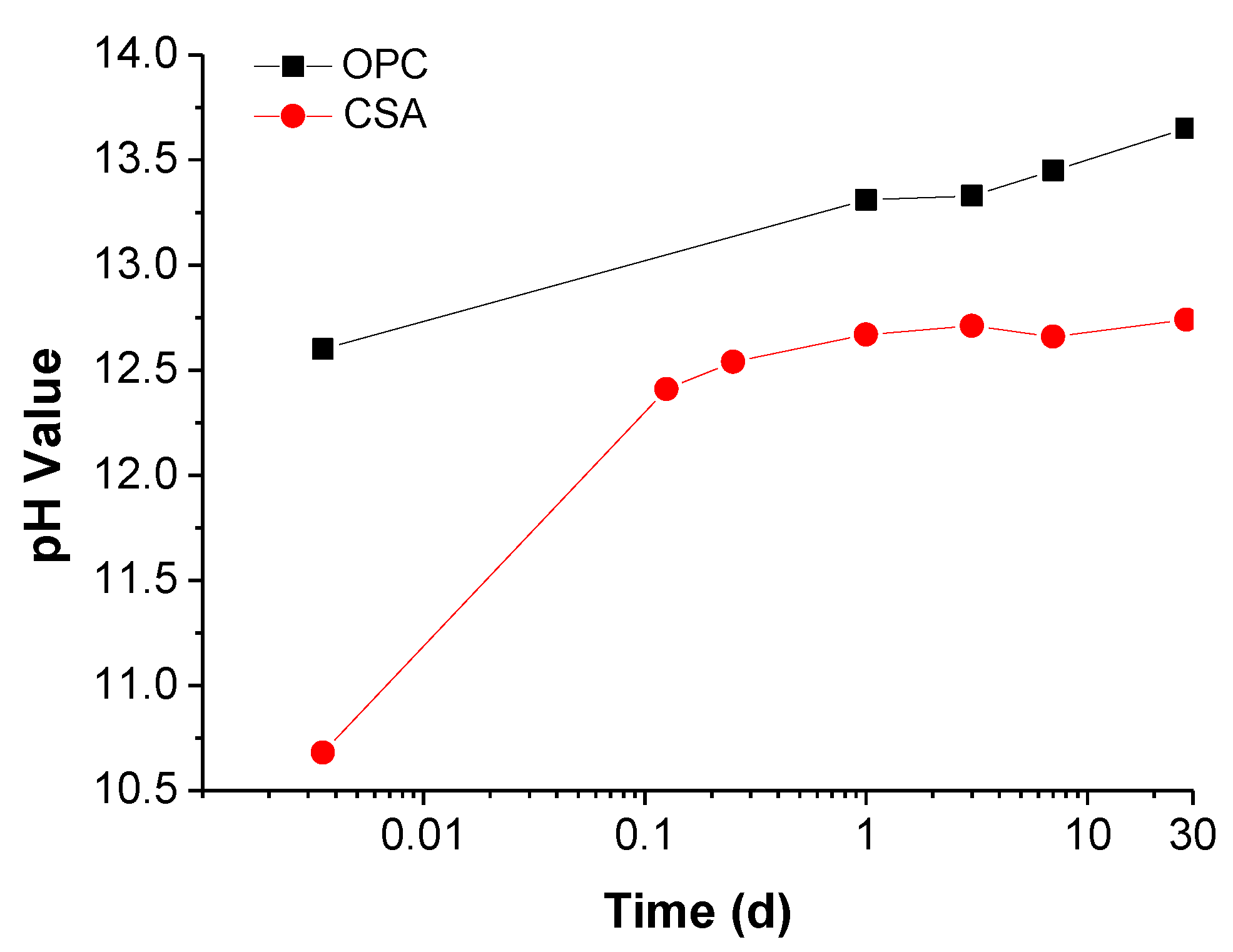
- The concentration of OH− in the CSA pore solution is only 55.34 mmol/L at 28 days, while the value of OPC is 451.3 mmol/L. The corrosion potential development in SPSs indicates that alkalinity is the most significant factor for steel passivation. When the OH− is below 0.001 M, the steel cannot passivate in any situations. Thus, the steel passive film formed in the environment of CSA is less stable than that in OPC.
- A high concentration of Al(OH)4− was detected in CSA pore solution, and its effect on steel passivation was studied. High content of Al(OH)4− can release enough OH− to offer a buffering capacity for the solution alkalinity, hence improving the stability of the passive film.
- The sulfate ions in CSA can remain at a level of about 50 mmol/L, while their concentration in the OPC was negligible after one day of hydration. According to its potential evolution, it was concluded that SO42− is adverse to the stability of passive film, but the effect was only obvious in the case of low alkalinity (C(OH) ≤ 0.01 M), and the negative impact can be reduced with Al(OH)4−.
Author Contributions
Funding
Conflicts of Interest
References
- Montemor, M.F.; Simões, A.M.P.; Ferreira, M.G.S. Chloride-Induced Corrosion on Reinforcing Steel: From the Fundamentals to the Monitoring Techniques. Cem. Concr. Compos. 2003, 25, 491–502. [Google Scholar] [CrossRef]
- Scott, A.; Alexander, M.G. Effect of Supplementary Cementitious Materials (Binder Type) on the Pore Solution Chemistry and the Corrosion of Steel in Alkaline Environments. Cem. Concr. Res. 2016, 89, 45–55. [Google Scholar] [CrossRef]
- Janotka, I.; Krajči, L. An Experimental Study on the Upgrade of Sulfoaluminate—Belite Cement Systems by Blending with Portland Cement. Adv. Cem. Res. 1999, 11, 35–41. [Google Scholar] [CrossRef]
- Kalogridis, D.; Kostogloudis, G.C.; Ftikos, C.; Malami, C. Quantitative Study of the Influence of Non-Expansive Sulfoaluminate Cement on the Corrosion of Steel Reinforcement. Cem. Concr. Res. 2000, 30, 1731–1740. [Google Scholar] [CrossRef]
- Poursaee, A.; Hansson, C.M. Reinforcing Steel Passivation in Mortar and Pore Solution. Cem. Concr. Res. 2007, 37, 1127–1133. [Google Scholar] [CrossRef]
- Cai, G.; Zhao, J. Application of Sulphoaluminate Cement to Repair Deteriorated Concrete Members in Chloride Ion Rich Environment—A Basic Experimental Investigation of Durability Properties. KSCE J. Civ. Eng. 2016, 20, 2832–2841. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; McRae, G.; Miller, T. The Effect of Concrete Pore Solution Composition on the Quality of Passive Oxide Films on Black Steel Reinforcement. Cem. Concr. Compos. 2009, 31, 2–11. [Google Scholar] [CrossRef]
- Page, C.L. Initiation of Chloride-Induced Corrosion of Steel in Concrete: Role of the Interfacial Zone. Mater. Corros. 2009, 60, 586–592. [Google Scholar] [CrossRef]
- Alhozaimy, A.; Hussain, R.R.; Al-Negheimish, A.; Al-Zaid, R.; Singh, D.D.N. Effect of Simulated Concrete Pore Solution Chemistry, Chloride Ions, and Temperature on Passive Layer Formed on Steel Reinforcement. ACI Mater. J. 2014, 111, 411–421. [Google Scholar] [CrossRef]
- Abd El Haleem, S.M.; Abd El Wanees, S.; Bahgat, A. Environmental Factors Affecting the Corrosion Behaviour of Reinforcing Steel. V. Role of Chloride and Sulphate Ions in the Corrosion of Reinforcing Steel in Saturated Ca(OH)2 solutions. Corros. Sci. 2013, 75, 1–15. [Google Scholar] [CrossRef]
- Premlall, K.; Potgieter, J.H.; Potgieter-Vermaak, S. Laser Surface Treatment to Inhibit Observed Corrosion of Reinforcing Steel in Sulphate: Alkaline Media. Anti-Corrosion Methods Mater. 2011, 58, 267–284. [Google Scholar] [CrossRef]
- Williamson, J.; Isgor, O.B. The Effect of Simulated Concrete Pore Solution Composition and Chlorides on the Electronic Properties of Passive Films on Carbon Steel Rebar. Corros. Sci. 2016, 106, 82–95. [Google Scholar]
- Shaheen, F.; Pradhan, B. Influence of Sulfate Ion and Associated Cation Type on Steel Reinforcement Corrosion in Concrete Powder Aqueous Solution in the Presence of Chloride Ions. Cem. Concr. Res. 2017, 91, 73–86. [Google Scholar] [CrossRef]
- Mammoliti, L.; Hansson, C.M. Influence of Cation on Corrosion Behavior of Reinforcing Steel in High-PH Sulfate Solutions. ACI Mater. J. 2005, 102, 279–285. [Google Scholar]
- Shang, B.; Ma, Y.; Meng, M.; Li, Y. The Roles Carbonate and Bicarbonate Ions Play on Pre-Passive HRB400 Rebars in Simulated Pore Solutions of Deteriorating Concrete. Mater. Corros. 2018, 29, 1800–1810. [Google Scholar] [CrossRef]
- Kasselouri, V.; Tsakiridis, P.; Malami, C.; Georgali, B.; Alexandridou, C. A Study on the Hydration Products of a Non-Expansive Sulfoaluminate Cement. Cem. Concr. Res. 1995, 25, 1726–1736. [Google Scholar]
- Winnefeld, F.; Lothenbach, B. Hydration of Calcium Sulfoaluminate Cements—Experimental Findings and Thermodynamic Modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A Hydration Study of Various Calcium Sulfoaluminate Cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- He, Z.; Yang, H.; Liu, M. Hydration Mechanism of Sulphoaluminate Cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 70–74. [Google Scholar] [CrossRef]
- Andersson, K.; Allard, B.; Bengtsson, M.; Magnusson, B. Chemical Composition of Cement Pore Solutions. Cem. Concr. Res. 1989, 19, 327–332. [Google Scholar] [CrossRef]
- Moragues, A.; Macias, A.; Andrade, C.; Poursaee, A.; Hansson, C.M.; Moragues, A.; Macias, A.; Andrade, C.; Blanco, G.; Bautista, A.; et al. Equilibria of the Chemical Composition of the Concrete Pore. Cem. Concr. Res. 1987, 17, 173–182. [Google Scholar] [CrossRef]
- Plusquellec, G.; Geiker, M.R.; Lindgård, J.; Duchesne, J.; Fournier, B.; De Weerdt, K. Determination of the PH and the Free Alkali Metal Content in the Pore Solution of Concrete: Review and Experimental Comparison. Cem. Concr. Res. 2017, 96, 13–26. [Google Scholar] [CrossRef]
- Andac, M.; Glasser, F.P. Pore Solution Composition of Calcium Sulfoaluminate Cement. Adv. Cem. Res. 1999, 11, 23–26. [Google Scholar] [CrossRef]
- Barneyback, R.S.; Diamond, S. Expression and Analysis of Pore Fluids from Hardened Cement Pastes and Mortars. Cem. Concr. Res. 1981, 11, 279–285. [Google Scholar] [CrossRef]
- Pavlík, V. Water Extraction of Chloride, Hydroxide and Other Ions from Hardened Cement Pastes. Cem. Concr. Res. 2000, 30, 895–906. [Google Scholar] [CrossRef]
- Li, L.; Nam, J.; Hartt, W.H. Ex Situ Leaching Measurement of Concrete Alkalinity. Cem. Concr. Res. 2005, 35, 277–283. [Google Scholar] [CrossRef]
- Brown, P.W.; Bothe, J.V. The Stability of Ettringite. Adv. Cem. Res. 1993, 5, 47–63. [Google Scholar] [CrossRef]
- Berger, S.; Aouad, G.; Cau Dit Coumes, C.; Le Bescop, P.; Damidot, D. Leaching of Calcium Sulfoaluminate Cement Pastes by Water at Regulated PH and Temperature: Experimental Investigation and Modeling. Cem. Concr. Res. 2013, 53, 211–220. [Google Scholar] [CrossRef]
- Sipos, P. The Structure of Al(III) in Strongly Alkaline Aluminate Solutions—A Review. J. Mol. Liq. 2009, 146, 1–14. [Google Scholar] [CrossRef]
- Watling, H. Spectroscopy of Concentrated Sodium Aluminate Solutions. Appl. Spectrosc. 1998, 52, 250–258. [Google Scholar] [CrossRef]
- Johnston, C.T.; Agnew, S.F.; Schoonover, J.R.; Kenney, J.W.; Page, B.; Osborn, J.; Corbin, R. Raman Study of Aluminum Speciation in Simulated Alkaline Nuclear Waste. Environ. Sci. Technol. 2002, 36, 2451–2458. [Google Scholar] [CrossRef]
- Sipos, P.; Hefter, G.; May, P.M. 27Al NMR and Raman Spectroscopic Studies of Alkaline Aluminate Solutions with Extremely High Caustic Content—Does the Octahedral Species Al(OH) 63—Exist in Solution? Talanta 2006, 70, 761–765. [Google Scholar] [CrossRef]
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The Pore Solution of Blended Cements: A Review. Mater. Struct. Constr. 2016, 49, 3341–3367. [Google Scholar] [CrossRef]
- Diamond, S. Effects of Microsilica (Silica Fume) on Pore-Solution Chemistry of Cement Pastes. J. Am. Ceram. Soc. 1983, 66, C82–C84. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-Performance Cement Matrices Based on Calcium Sulfoaluminate-Belite Compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Abd El Haleem, S.M.; Abd El Wanees, S.; Abd El Aal, E.E.; Diab, A. Environmental Factors Affecting the Corrosion Behavior of Reinforcing Steel II. Role of Some Anions in the Initiation and Inhibition of Pitting Corrosion of Steel in Ca(OH)2 solutions. Corros. Sci. 2010, 52, 292–302. [Google Scholar] [CrossRef]
- Al-Tayyib, A.J.; Somuah, S.K.; Boah, J.K.; Leblanc, P.; Al-Mana, A.I. Laboratory Study on the Effect of Sulfate Ions on Rebar Corrosion. Cem. Concr. Res. 1988, 18, 774–782. [Google Scholar] [CrossRef]
- Huet, B.; L’Hostis, V.; Tricheux, L.; Idrissi, H. Influence of Alkali, Silicate, and Sulfate Content of Carbonated Concrete Pore Solution on Mild Steel Corrosion Behavior. Mater. Corros. 2010, 61, 111–124. [Google Scholar] [CrossRef]
- Soltis, J. Passivity Breakdown, Pit Initiation and Propagation of Pits in Metallic Materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. Chloride-Induced Corrosion of Steel in Concrete. Prog. Struct. Engng. Mater. 2000, 2, 448–458. [Google Scholar] [CrossRef]


| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | Loss |
|---|---|---|---|---|---|---|---|---|---|
| CSA | 9.90 | 20.50 | 2.62 | 43.77 | 3.00 | 13.36 | 0.54 | 0.19 | 5.98 |
| OPC | 22.30 | 4.62 | 2.44 | 58.42 | 1.92 | 2.20 | 0.35 | 0.28 | 3.62 |
| Variables | Composition | Concentration of Compounds (mol/L) | ||
|---|---|---|---|---|
| NaOH | Na2SO4 | NaAlO2 | ||
| Alkalinity | M NaOH | 0.1, 0.01, 0.001 | - | - |
| Sulfate | 0.1 M NaOH + M Na2SO4 | - | 0.01, 0.02, 0.03, 0.04, 0.05 | - |
| 0.01 M NaOH + M Na2SO4 | ||||
| 0.001 M NaOH + M Na2SO4 | ||||
| Aluminate | 0.1 M NaOH + 0.05 M Na2SO4 + M NaAlO2 | - | - | 0, 0.05, 0.1, 0.15, 0.2 |
| 0.01 M NaOH + 0.05 M Na2SO4 + M NaAlO2 | - | - | 0, 0.005, 0.01, 0.015, 0.02 | |
| 0.001 M NaOH + 0.05 M Na2SO4 + M NaAlO2 | - | - | 0, 0.0005, 0.001, 0.0015, 0.002 | |
| Sample | Method | ESL (mmol/L) | |||||
|---|---|---|---|---|---|---|---|
| Time (1) | Na+ | K+ | Ca2+ | Al(OH)4− (2) | SO42− | OH− | |
| CSA | 5 min | 55.7 | 106.4 | 29.9 | 18.0 | 38.8 | 0.5 |
| 3 h | 23.7 | 48.9 | 2.4 | 30.5 | 4.3 | 5.5 | |
| 6 h | 20.1 | 36.9 | 1.9 | 32.1 | 3.9 | 5.6 | |
| 1 d | 26.1 | 37.8 | 0.7 | 33.1 | 3.2 | 6.0 | |
| 3 d | 26.1 | 35.6 | bld (3) | 32.6 | 5.9 | 6.3 | |
| 7 d | 23.9 | 35.4 | 0.5 | 28.7 | 7.5 | 5.5 | |
| 28 d | 22.4 | 36.3 | 0.4 | 25.8 | 8.1 | 6.5 | |
| OPC | 5 min | 24.1 | 108.5 | 58.9 | - | 46.2 | 39.8 |
| 1 d | 18.3 | 35.1 | 6.4 | - | bld | 56.4 | |
| 3 d | 18.4 | 33.8 | 7.4 | - | 1.7 | 57.5 | |
| 7 d | 24.0 | 42.6 | 7.1 | - | bld | 61.3 | |
| 28 d | 28.9 | 48.5 | 6.2 | - | 0.4 | 71.9 | |
| Material | Time | |||||
|---|---|---|---|---|---|---|
| 3 h | 6 h | 1 d | 3 d | 7 d | 28 d | |
| CSA | 21.0 | 16.8 | 13.9 | 13.3 | 13.0 | 12.8 |
| OPC | - | - | 23.1 | 22.4 | 20.5 | 17.9 |
| Sample | Method | Calculation (mmol/L) | |||||
|---|---|---|---|---|---|---|---|
| Time | Na+ | K+ | Ca2+ | Al(OH)4− | SO42− | OH− | |
| CSA | 5 min | 55.7 | 106.4 | 29.9 | 18.0 | 38.76 | 0.48 |
| 3 h | 89.80 | 185.80 | 9.19 | 115.70 | 16.36 | 25.74 | |
| 6 h | 100.60 | 184.80 | 7.56 | 160.70 | 19.49 | 34.70 | |
| 1 d | 163.50 | 236.30 | 4.19 | 207.10 | 20.13 | 46.60 | |
| 3 d | 171.70 | 233.50 | bld | 214.50 | 38.20 | 51.83 | |
| 7 d | 161.50 | 239.50 | 3.58 | 193.80 | 50.92 | 46.11 | |
| 28 d | 154.50 | 250.60 | 2.62 | 178.10 | 55.79 | 55.34 | |
| OPC | 5 min | 24.09 | 108.49 | 58.93 | - | 46.16 | 39.81 |
| 1 d | 61.00 | 117.30 | 1.90 | - | bld | 202.40 | |
| 3 d | 77.00 | 117.50 | 1.90 | - | 5.80 | 215.40 | |
| 7 d | 93.12 | 165.70 | 1.30 | - | bld | 279.50 | |
| 28 d | 135.80 | 221.00 | 0.8 | - | 1.80 | 451.30 | |
| Age | Species Distribution (mmol/L) | ||||
|---|---|---|---|---|---|
| Al | Al(OH)4− | Al(OH)3 | Al(OH)2+ | Al3+ | |
| 1 d | 207.1 | 207.1 | 2.3 × 10−5 | 8.0 × 10−11 | 8.3 × 10−25 |
| 3 d | 214.5 | 214.5 | 2.1 × 10−5 | 6.5 × 10−18 | 5.2 × 10−25 |
| 7 d | 193.7 | 193.7 | 2.1 × 10−5 | 7.5 × 10−18 | 7.6 × 10−25 |
| 28 d | 178.1 | 178.1 | 1.6 × 10−5 | 4.8 × 10−18 | 3.4 × 10−25 |
| Cement Composition | Concentration (mmol/L) (1) | ||||
|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | SO42− | OH− | |
| This study 28 days, w/b 0.5 Na2O% = 0.28% K2O% = 0.35% | 135.8 | 221.0 | 0.8 | 1.8 | 451.3 |
| Diamond [34] 28 days, w/b 0.5 Na2O% = 0.32% K2O% = 0.60% | - | 320.0 | - | - | 510.0 |
| Scott [2] 28 days, w/b 0.58 Na2O% = 0.19% K2O% = 0.54% | 89.4 | 216.6 | 1.0 | - | 320.0 |
| Cement Composition | Concentration (mmol/L) | |||||
|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Al(OH)4− | SO42− | OH− | |
| This study 28 day, w/b 0.5 Na2O% = 0.19% K2O% = 0.54% Al2O3% = 20.5% SO3% = 13.4% | 154.5 | 250.6 | 2.6 | 178.1 | 55.8 | 55.3 |
| Winnefeld [17] 28 day, w/b 0.72 Na2O% = 0.05% K2O% = 0.21% Al2O3% = 35.5% SO3% = 16.8% | 21.0 | 120.0 | 0.31 | 100 | 3.4 | 50.0 |
| 28 day, w/b 0.8 Na2O% = 0.13% K2O% = 0.40% Al2O3% = 26.8% SO3% = 19.5% | 34 | 82 | 0.23 | 26 | 9 | 70.0 |
| Andac [23] 30 day, w/b 0.8 Na2O% = 0.095% K2O% = 0.295% Al2O3% = 37.4% SO3% = 9.5% | 14.5 | 52 | 0.41 | 14.7 | 0.71 | 66.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhan, S.; Tang, X.; Xu, Q.; Qian, K. Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation. Appl. Sci. 2019, 9, 1092. https://doi.org/10.3390/app9061092
Wang L, Zhan S, Tang X, Xu Q, Qian K. Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation. Applied Sciences. 2019; 9(6):1092. https://doi.org/10.3390/app9061092
Chicago/Turabian StyleWang, Lingbo, Shulin Zhan, Xudong Tang, Qiang Xu, and Kuangliang Qian. 2019. "Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation" Applied Sciences 9, no. 6: 1092. https://doi.org/10.3390/app9061092





