Fabrication of ZrO2(MgO)/CaAl2O4+CaAl4O7 Bilayer Structure Used for Sulfur Sensor by Laser Cladding
Abstract
:1. Introduction
2. Experimental
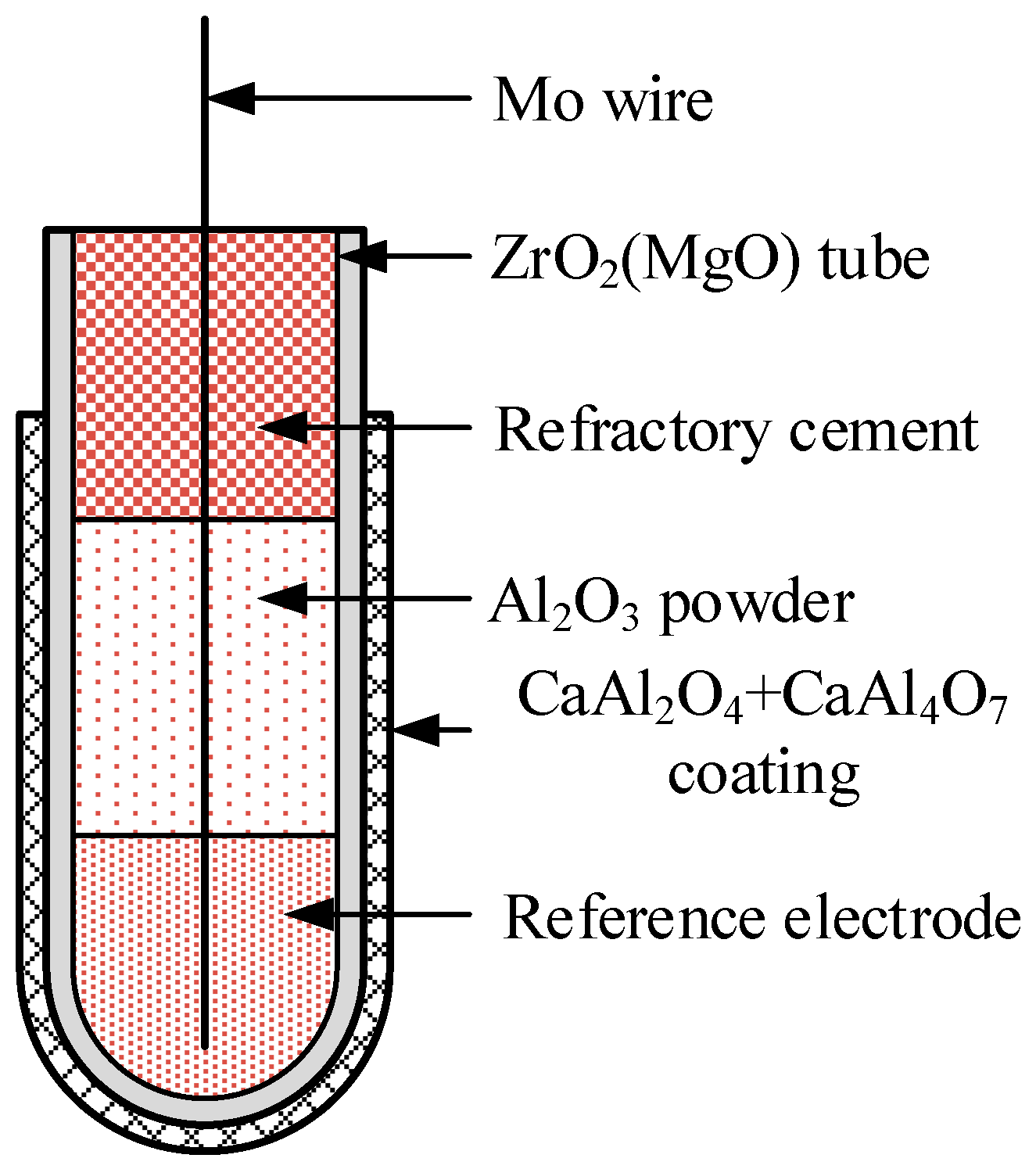
2.1. Fabrication of the ZrO2(MgO)/CaAl2O4+CaAl4O7 Bilayer Structure
2.2. Measurement of Sulfur Content
2.3. Detection and Analysis
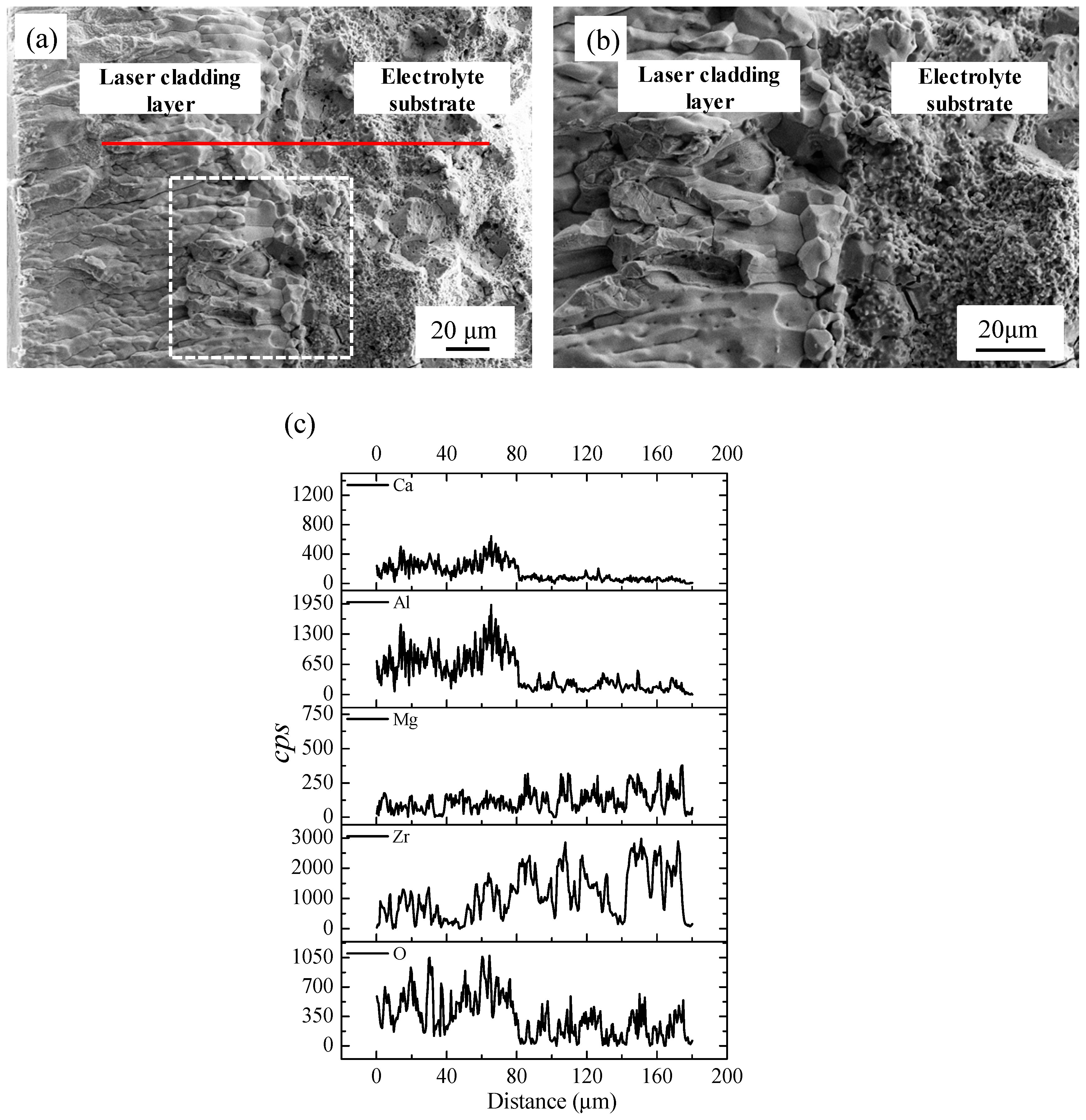
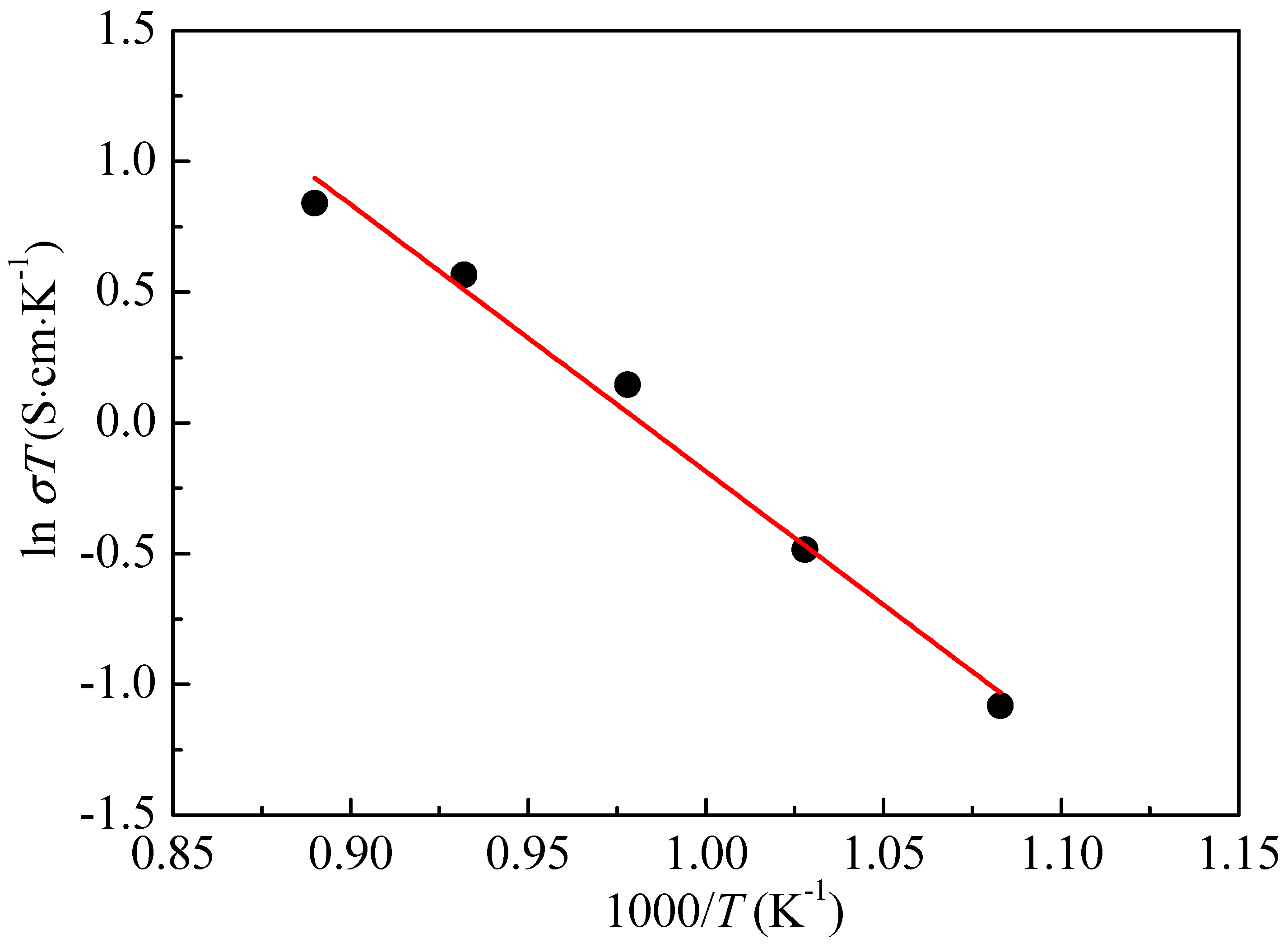
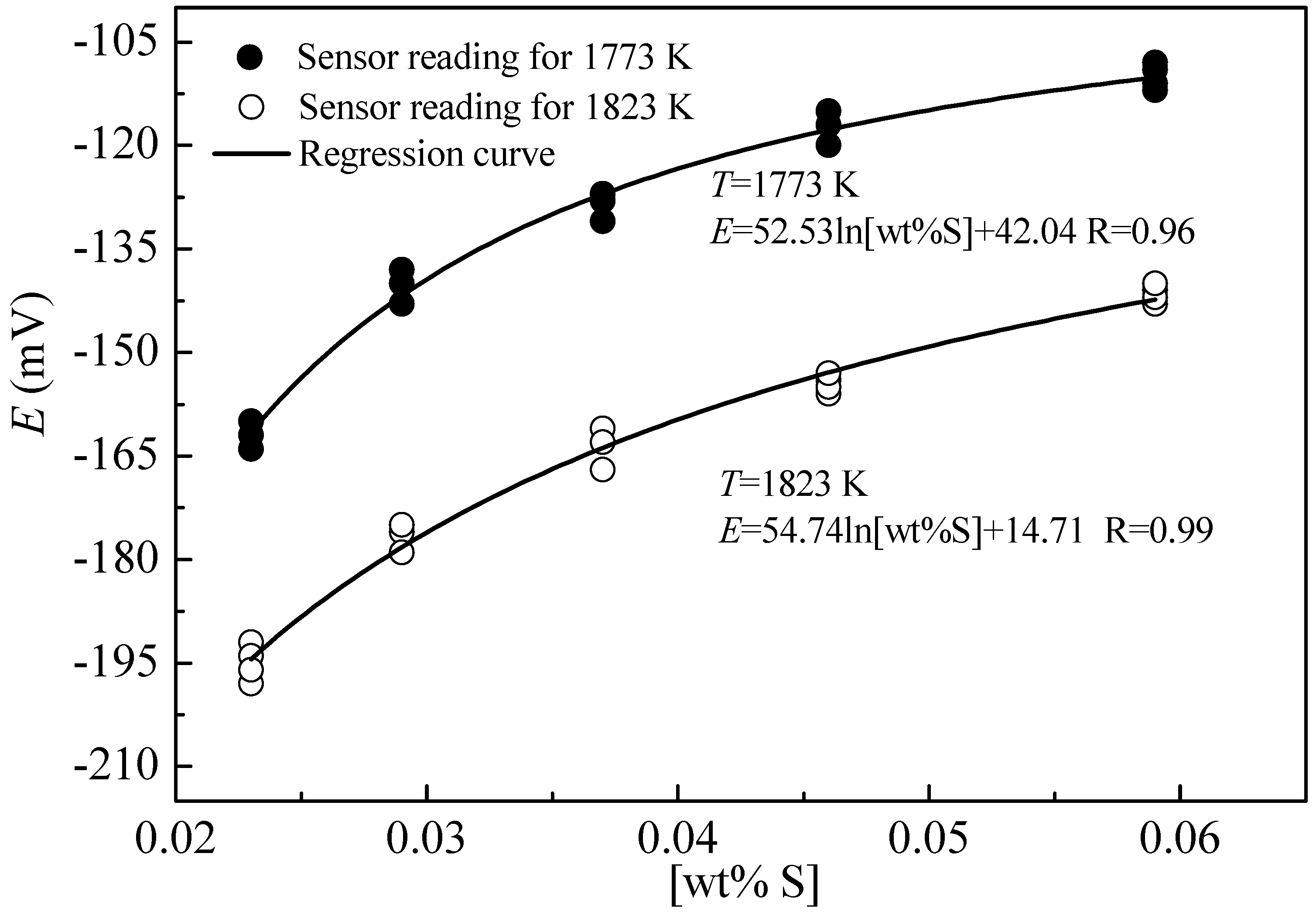
3. Results and Discussion
4. Conclusions
- (1)
- The laser cladding layer was composed of Ca0.15Zr0.85O1.85, CaAl2O4 and CaAl4O7 phases, which achieved good densification and adheres to the substrate firmly.
- (2)
- The fine bilayer structure showed a high ionic conductivity that increased linearly with the increase of temperature.
- (3)
- The sulfur sensor was assembled using the fabricated bilayer structure to measure the sulfur content in the carbon-saturated liquid iron, and the sulfur content could be calculated through the following empirical equation based on the EMF measured with the sensor:
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fischer, W.A.; Janke, D. Metallurgische Elektrochemie; Springer: Berlin, Germany, 1975. [Google Scholar]
- Ono, K.; Oishi, T.; Moriyama, J. Measurements on galvanic cells involving solid-sulphide electrolytes. Solid State Ion. 1981, 3, 555–558. [Google Scholar] [CrossRef]
- Ono, K.; Moriyama, J. Rapid electrochemical determination of dissolved sulphur in liquid copper by solid-sulphide electrolytes. Solid State Ion. 1981, 2, 127–130. [Google Scholar] [CrossRef]
- Egami, A.; Onoye, T.; Narita, K. Solid electrolyte for the determination of sulfur in liquid iron. Solid State Ion. 1981, 3, 617–620. [Google Scholar] [CrossRef]
- Gu, L.J.; Tang, Z.H. Study on the determination of sulfur activities in liquid iron by means of a sulfur concentration cell with a solid electrolyte CaS(TiS2). Kang T’ieh/Iron Steel 1984, 19, 13–19. [Google Scholar]
- Gozzi, D.; Granati, P. Sulfur determination in carbon-saturated iron by solid-state electrochemical sensor. Metall. Mater. Trans. B 1994, 25, 561–568. [Google Scholar] [CrossRef]
- Hong, Y.R.; Jin, C.J.; Li, L.S.; Sun, J.L. An application of the electrochemical sulfur sensor in steelmaking. Sens. Actuator B Chem. 2002, 87, 13–17. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Feng, G.S.; Zhang, J.L.; Cao, C.G. Development of sulfur sensor for quick analysis of hot metal. Iron Steel 2000, 35, 57–61. [Google Scholar]
- Liu, T.; Li, L.; Yu, J.K. An electrochemical sulfur sensor based on ZrO2(MgO) as solid electrolyte and ZrS2+MgS as auxiliary electrode. Sens. Actuator B Chem. 2009, 139, 501–504. [Google Scholar] [CrossRef]
- Liu, T.; Yu, J.K.; Chen, M. Synthesizing sulphides by a temperature gradient method. Ceram. Int. 2007, 33, 321–325. [Google Scholar] [CrossRef]
- Mao, F.X.; Yu, J.K.; Liu, T.; Jiang, Y.F. Synthesis of an auxiliary electrode by laser cladding coating for in-situ electrochemical sulfur sensing. Mater. Lett. 2015, 138, 276–278. [Google Scholar] [CrossRef]
- Michalek, K.; Čamek, L.; Gryc, K.; Tkadleckova, M.; Huczala, T.; Troszok, V. Desulphurization of the high-alloy and middle-alloy steels under the conditions of an EAF by means of synthetic slag based on CaO−Al2O3. Mater. Tehnol. 2012, 46, 297–303. [Google Scholar]
- Yan, P.C.; Huang, S.G.; Dyck, V.J.; Guo, M.X.; Blanpain, B. Desulphurisation and inclusion behaviour of stainless steel refining by using CaO-Al2O3 based slag at low sulphur levels. Trans. Iron Steel Inst. Jpn. 2014, 54, 72–81. [Google Scholar] [CrossRef]
- Yan, P.C.; Guo, X.L.; Huang, S.G.; Dyck, V.J.; Guo, M.X.; Blanpain, B. Desulphurisation of stainless steel by using CaO-Al2O3 based slags during secondary metallurgy. ISIJ Int. 2013, 53, 459–467. [Google Scholar] [CrossRef]
- Wen, T.P.; Yu, J.K.; Jin, E.D.; Liu, T.; Hou, X.H.; Sun, Q.Y. Application of solid electrochemical sulfur sensor in the liquid iron. Sens. Actuator B Chem. 2019, 279, 177–182. [Google Scholar] [CrossRef]
- Wang, C.Z. Solid Electrolyte and Chemical Sensors; Metallurgical Industry Press: Beijing, China, 2000; pp. 73–117. [Google Scholar]

| Contents | Parameter |
|---|---|
| Average power | 500 W |
| Impulse frequency | 14 Hz |
| Pulse width | 3.0 ms |
| Defocusing amount | 50 mm |
| Scanning speed | 2.5 mm·s−1 |
| Overlapping ratio | 45% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, T.; Yu, J.; Jin, E.; Yuan, L.; Zhou, Y.; Tian, C. Fabrication of ZrO2(MgO)/CaAl2O4+CaAl4O7 Bilayer Structure Used for Sulfur Sensor by Laser Cladding. Appl. Sci. 2019, 9, 1036. https://doi.org/10.3390/app9061036
Wen T, Yu J, Jin E, Yuan L, Zhou Y, Tian C. Fabrication of ZrO2(MgO)/CaAl2O4+CaAl4O7 Bilayer Structure Used for Sulfur Sensor by Laser Cladding. Applied Sciences. 2019; 9(6):1036. https://doi.org/10.3390/app9061036
Chicago/Turabian StyleWen, Tianpeng, Jingkun Yu, Endong Jin, Lei Yuan, Yuting Zhou, and Chen Tian. 2019. "Fabrication of ZrO2(MgO)/CaAl2O4+CaAl4O7 Bilayer Structure Used for Sulfur Sensor by Laser Cladding" Applied Sciences 9, no. 6: 1036. https://doi.org/10.3390/app9061036





