Porous Titanium for Biomedical Applications: Evaluation of the Conventional Powder Metallurgy Frontier and Space-Holder Technique
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physical and Microstructural Properties
3.2. Mechanical Properties
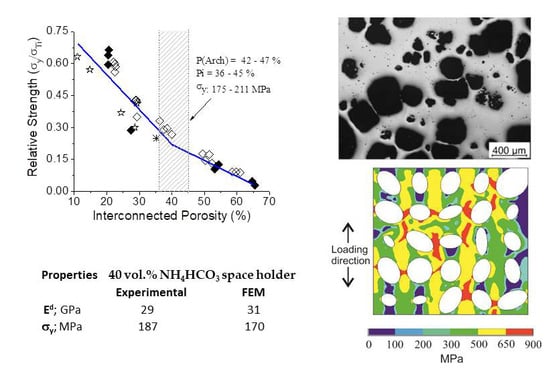
3.3. Finite Element Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torres, Y.; Trueba, P.; Pavón, J.; Montealegre, I.; Rodríguez-Ortiz, J. Designing, processing and characterisation of titanium cylinders with graded porosity: An alternative to stress-shielding solutions. Mater. Des. 2014, 63, 316–324. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Y.; Wang, X.; Hodgson, P.; Wen, C.E. Mechanical properties and bioactive surface modification via alkali-heat treatment of a porous Ti–18Nb–4Sn alloy for biomedical applications. Acta Biomater. 2008, 4, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Schmidutz, F.; Agarwal, Y.; Müller, P.; Gueorguiev, B.; Richards, R.; Sprecher, C. Stress-shielding induced bone remodeling in cementless shoulder resurfacing arthroplasty: A finite element analysis and in vivo results. J. Biomech. 2014, 47, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Yánez, A.; Martel, O.; Afonso, H.; Monopoli, D. Computational study and experimental validation of porous structures fabricated by electron beam melting: A challenge to avoid stress shielding. Mater. Sci. Eng. C 2014, 45, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Castillo, S.; Torres, Y. Different models for simulation of mechanical behaviour of porous materials. J. Mech. Behav. Biomed. Mater. 2018, 80, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, W.; Grynpas, M.D.; Tully, A.E. Hydroxyapatite reinforced polyethylene—A mechanically compatible implant material for bone replacement. Biomaterials 1981, 2, 185–186. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, R.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Aguilar, C.; Guerra, C.; Lascano, S.; Guzman, D.; Rojas, P.A.; Thirumurugan, M.; Bejar, L.; Medina, A. Synthesis and characterization of Ti–Ta–Nb–Mn foams. Mater. Sci. Eng. C 2016, 58, 420–431. [Google Scholar] [CrossRef]
- Trueba, P. Desarrollo de titanio con porosidad gradiente radial y longitudinal para aplicaciones biomédicas. Ph.D. Thesis, University of Seville, Seville, Spain, 2017. [Google Scholar]
- Naebe, M.; Shirvanimoghaddam, K. Functionally graded materials: A review of fabrication and properties. Appl. Mater. Today 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Dewidar, M.M.; Lim, J.K. Properties of solid core and porous surface Ti–6Al–4V implants manufactured by powder metallurgy. J. Alloys Compd. 2008, 454, 442–446. [Google Scholar] [CrossRef]
- Wenjuan, N.; Chenguang, B.; GuiBao, Q.; Qiang, W. Processing and properties of porous titanium using space holder technique. Mater. Sci. Eng. A 2009, 506, 148–151. [Google Scholar]
- Yavari, S.A.; van der Stok, J.; Chai, Y.C.; Wauthle, R.; Birgani, Z.T.; Habibovic, P.; Mulier, M.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Bone regeneration performance of surface-treated porous titanium. Biomaterials 2014, 35, 6172–6181. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.; Pavón, J.J.; Nieto, I.; Rodríguez, J.A. Conventional Powder Metallurgy Process and Characterization of Porous Titanium for Biomedical Applications. Metall. Mater. Trans. B 2011, 42, 891–900. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [Green Version]
- Torres, Y.; Pavón, J.; Rodríguez, J. Processing and characterization of porous titanium for implants by using NaCl as space holder. J. Mater. Process. Technol. 2012, 212, 1061–1069. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.; Rodriguez, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C 2014, 37, 148–155. [Google Scholar] [CrossRef]
- Oh, I.H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, P.; Wang, L.; Xing, W.; Fan, Y.; Xu, N. Preparation conditions and porosity of porous titanium sintered under positive pressure. Mater. Manufact. Proce. 2013, 28, 1166–1170. [Google Scholar] [CrossRef]
- Kato, K.; Ochiai, S.; Yamamoto, A.; Daigo, Y.; Honma, K.; Matano, S.; Omori, K. Novel multilayer Ti foam with cortical bone strength and cytocompatibility. Acta Biomater. 2013, 9, 5802–5809. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.; Rodríguez, J.A.; Arias, S.; Echeverry, M.; Robledo, S.; Amigó, V.; Pavón, J.J. Processing, Characterization and biological testing of porous titanium obtained by space-holder technique. J. Mater. Sci. 2012, 47, 6565–6576. [Google Scholar] [CrossRef]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Laptev, A.; Bram, M.; Buchkremer, D.; Stover, D. Green strength of powder compacts provided for production of highly porous titanium parts. Powder Metall. 2005, 48, 358. [Google Scholar] [CrossRef]
- Jia, J.; Siddiq, A.R.; Kennedy, A.R. Porous titanium manufactured by a novel powder tapping method using spherical salt bead space holders: Characterisation and mechanical properties. J. Mech. Behav. Biomed. Mater. 2015, 48, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Jung, H.-D.; Kang, M.-H.; Kim, H.-E.; Koh, Y.-H.; Estrin, Y. Fabrication of porous titanium scaffold with controlled porous structure and net-shape using magnesium as spacer. Mater. Sci. Eng. C 2013, 33, 2808–2815. [Google Scholar] [CrossRef]
- Mansourighasri, A.; Muhamad, N.; Sulong, A.B. Processing titanium foams using tapioca starch as a space holder. J. Mater. Process. Technol. 2012, 212, 83–89. [Google Scholar] [CrossRef]
- Reddy, T.H.; Pal, S.; Kumar, K.C.; Mohan, M.K.; Kokol, V. Finite Element Analysis for Mechanical Response of Magnesium Foams with Regular Structure Obtained by Powder Metallurgy Method. Procedia Eng. 2016, 149, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, M.; Takahashi, M.; Okuno, O. Elastic Moduli of Cast Ti-Au, Ti- Ag, and Ti- Cu alloys. Dent. Mater. 2006, 22, 641–646. [Google Scholar] [CrossRef]
- Muñoz, S.; Pavón, J.; Rodríguez-Ortiz, J.; Civantos, A.; Allain, J.; Torres, Y. On the influence of space holder in the development of porous titanium implants: Mechanical, computational and biological evaluation. Mater. Charact. 2015, 108, 68–78. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Pabst, W.; Gregorová, E. New relation for the porosity dependence of the effective tensile modulus of brittle materials. J. Mater. Sci. 2004, 39, 3501–3503. [Google Scholar] [CrossRef]
- Knudsen, F. Dependence of mechanical strength of brittle polycrystalline specimens on porosity and grain size. J. Am. Ceram. Soc. 1959, 42, 376–387. [Google Scholar] [CrossRef]
- Spriggs, R. Expression for effect of porosity on elastic modulus of polycrystalline refractory materials, particularly aluminum oxide. J. Am. Ceram. Soc. 1961, 44, 628–629. [Google Scholar] [CrossRef]
- Eudier, M. The mechanical properties of sintered low-alloy steels. Powder Metall. 1962, 5, 278–290. [Google Scholar] [CrossRef]
- Fleck, N.; Smith, R. The mechanical properties of sintered low-alloy steels. Powder Metall. 1981, 3, 121–125. [Google Scholar] [CrossRef]
- Itälä, A.; Ylanen, H.; Ekholm, C.; Karlsson, K.; Aro, H. Pore diameter of more than 100 micron is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Stangl, R.; Rinne, B.; Kastl, S.; Hendrich, C. The influence of pore geometry in cp Ti-implants a cell culture investigation. Eur. Cells Mater. 2001, 2, 1–9. [Google Scholar] [CrossRef]
- Götz, H.E.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Properties of open-cell porous metals and alloys for orthopaedic applications. J. Mater. Sci. Mater. Med. 2013, 24, 2293–2325. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Gil, E.; Torres, Y.; Cordero-Arias, L.; Pavón, J.; Rodríguez-Ortiz, J.A.; Borjas, S. Optimization of electrophoretic deposition and characterization of CHITOSAN/45S5 BIOGLASS© composite coatings on porous titanium for biomedical applications. In Proceedings of the International Conference on Electrophoretic Deposition V: Fundamentals and Applications (EPD 2014), Hernstein, Austria, 5–10 October 2014. [Google Scholar]
- Kubicki, B. Stress concentration at pores in sintered materials. Powder Metall. 1995, 38, 295–298. [Google Scholar] [CrossRef]
- Dieter, G. Mechanical Metallurgy; McGraw-Hill Book Company: New York, NY, USA, 1988. [Google Scholar]
- Straffelini, G. Strain hardening behaviour of powder metallurgy alloys. Powder Metall. 2005, 48, 189–192. [Google Scholar] [CrossRef]





| d[10], μm | d[50], μm | d[90], μm | |
|---|---|---|---|
| Ti powder | 9.7 | 23.3 | 48.4 |
| NaCl | 183.0 | 384.0 | 701.0 |
| NH4HCO3 | 73.0 | 233.0 | 497.0 |
| Processing Conditions | Archimedes | Image Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Pi, % | P(Arch), % | P(IA), % | Deq, µm | Ff | Cpore | λ, μm | ||
| Loose Sintering | 1000 °C | 44.1 | 44.8 | 45.3 | 17 | 0.70 | 0.2 | 25 |
| 1200 °C | 28.5 | 35.2 | 30.0 | 15 | 0.82 | 0.1 | 41 | |
| 13 MPa | 1000 °C | 28.0 | 29.3 | 30.8 | 14 | 0.79 | 0.1 | 36 |
| 1200 °C | 6.2 | 13.6 | 13.1 | 10 | 0.93 | 0.0 | 66 | |
| Space holder | 30 vol.% NaCl | 20.5 | 28.5 | 28.4 | 47.0 | 0.90 | 0.3 | 157 |
| 40 vol.% NaCl | 27.5 | 35.8 | 35.1 | 78.0 | 0.74 | 0.2 | 181 | |
| 30 vol.% NH4HCO3 | 22.4 | 27.8 | 29.1 | 18.1 | 0.90 | 0.3 | 57 | |
| 40 vol.% NH4HCO3 | 29.3 | 37.6 | 36.6 | 32.0 | 0.84 | 0.3 | 80 | |
| Processing Conditions | Experimental | |||
|---|---|---|---|---|
| σy, MPa | Ec, GPa | Ed, GPa | ||
| Loose Sintering | 1000 °C | 67 | 9.6 | 29.1 |
| 1200 °C | 165 | 25.1 | 50.5 | |
| 13 MPa | 1000 °C | 200 | 12.5 | 50.1 |
| 1200 °C | 350 | 26.1 | 59.4 | |
| Space holder | 30 vol.% NaCl | 415 | 4.6 | 45.1 |
| 40 vol.% NaCl | 187 | 5.3 | 29.0 | |
| 30 vol.% NH4HCO3 | 389 | 15.9 | 38.9 | |
| 40 vol.% NH4HCO3 | 272 | 5.8 | 30.0 | |
| Properties | Experimental | FEM |
|---|---|---|
| E, GPa | 31 | 39 |
| σy, MPa | 170 | 153 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lascano, S.; Arévalo, C.; Montealegre-Melendez, I.; Muñoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous Titanium for Biomedical Applications: Evaluation of the Conventional Powder Metallurgy Frontier and Space-Holder Technique. Appl. Sci. 2019, 9, 982. https://doi.org/10.3390/app9050982
Lascano S, Arévalo C, Montealegre-Melendez I, Muñoz S, Rodriguez-Ortiz JA, Trueba P, Torres Y. Porous Titanium for Biomedical Applications: Evaluation of the Conventional Powder Metallurgy Frontier and Space-Holder Technique. Applied Sciences. 2019; 9(5):982. https://doi.org/10.3390/app9050982
Chicago/Turabian StyleLascano, Sheila, Cristina Arévalo, Isabel Montealegre-Melendez, Sergio Muñoz, José A. Rodriguez-Ortiz, Paloma Trueba, and Yadir Torres. 2019. "Porous Titanium for Biomedical Applications: Evaluation of the Conventional Powder Metallurgy Frontier and Space-Holder Technique" Applied Sciences 9, no. 5: 982. https://doi.org/10.3390/app9050982