Photo-Fenton like Catalyst System: Activated Carbon/CoFe2O4 Nanocomposite for Reactive Dye Removal from Textile Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Activated Carbon/CoFe2O4 (AC/CFO) Nanocomposite
2.2.1. Procedure for the Preparation of Nanocomposite
2.2.2. Characterization of the Nanocomposite
2.3. Photo-Fenton-Like System
2.4. Analytical Methods
3. Results and Discussion
3.1. Characterization of Nanocomposite
3.1.1. FESEM and Morphology of AC/CFO
3.1.2. The XRD Analysis of AC/CFO
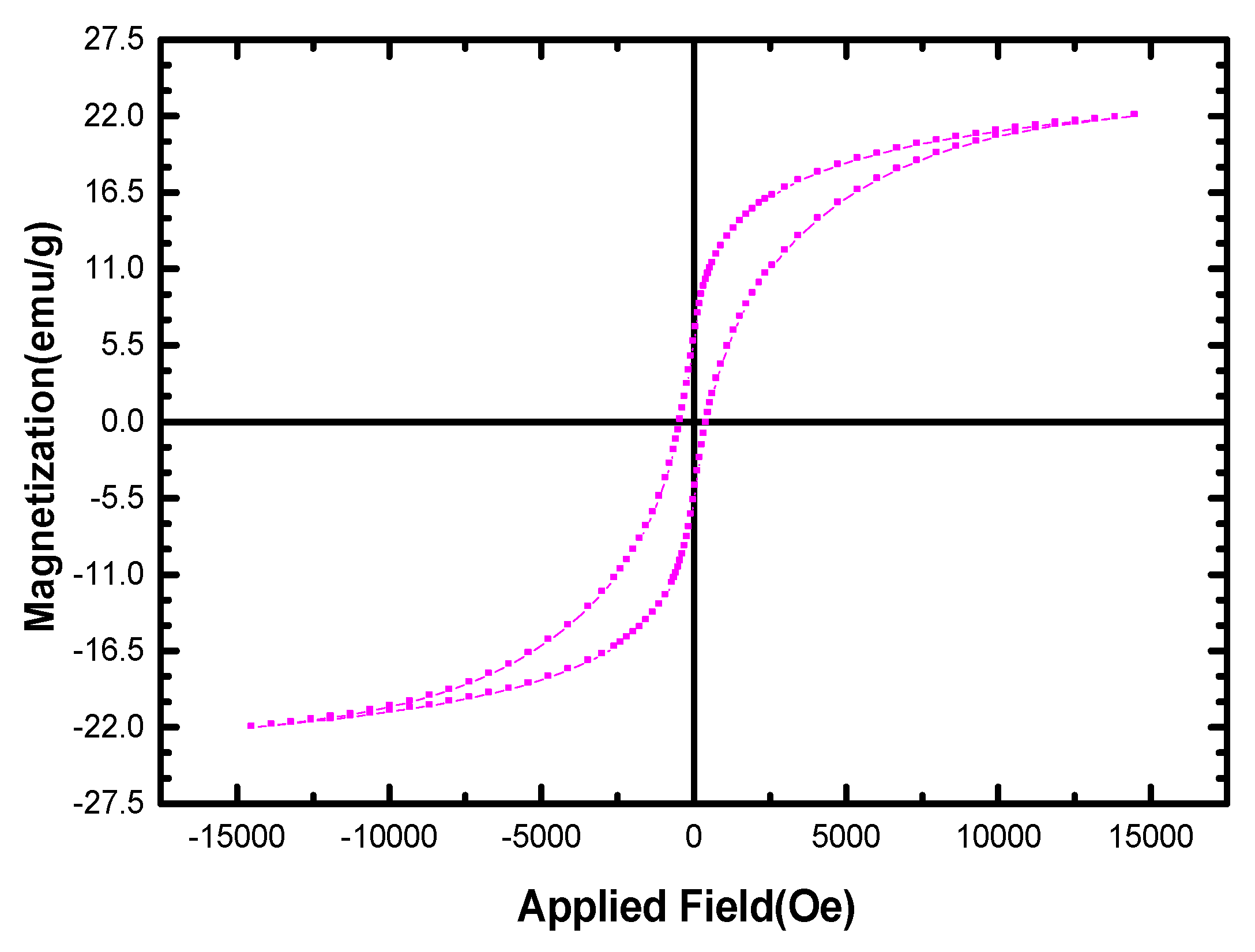
3.1.3. Magnetic Properties of AC/CFO
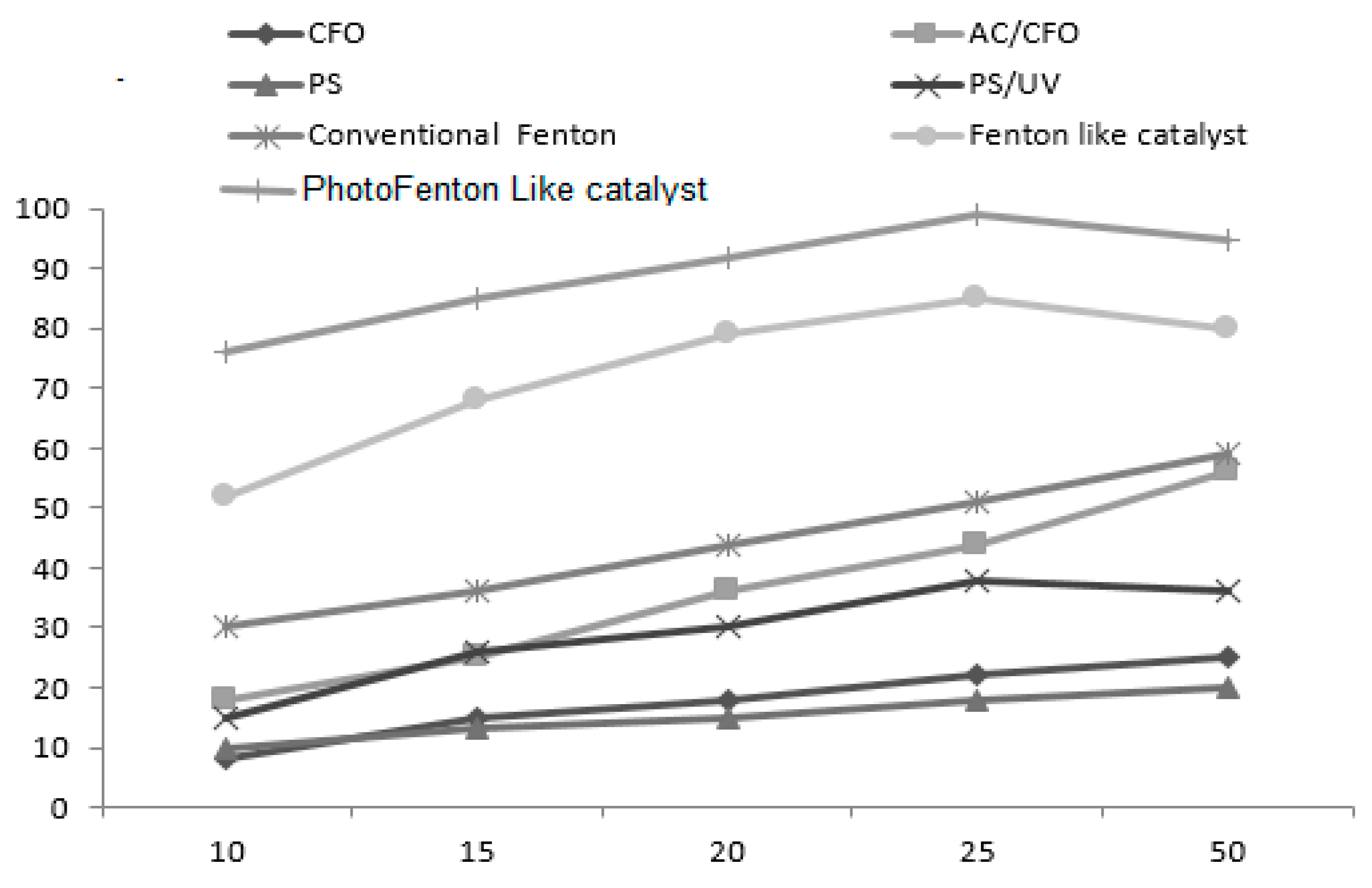
3.2. Catalytic Activity of Dye in the Presence of AC/CFO
3.3. Effects of Operational Parameters Photo-Fenton like Catalyst
3.3.1. Effect of Solution pH
3.3.2. Effect of Catalyst Dosage
3.3.3. Effect of Initial S2O82− Concentration
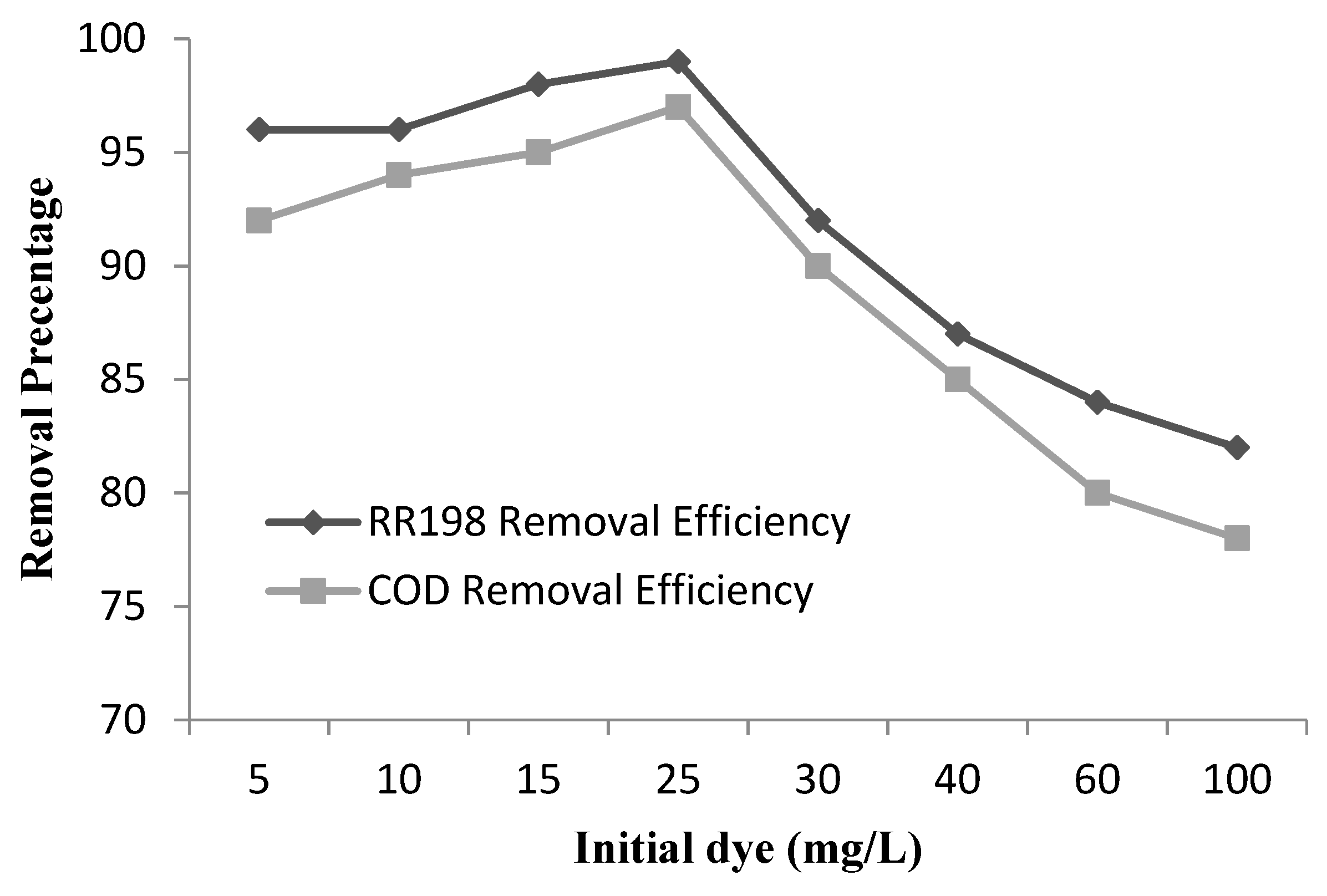
3.3.4. Effect of Initial Dye Concentration and Kinetics of Degradation of RR198
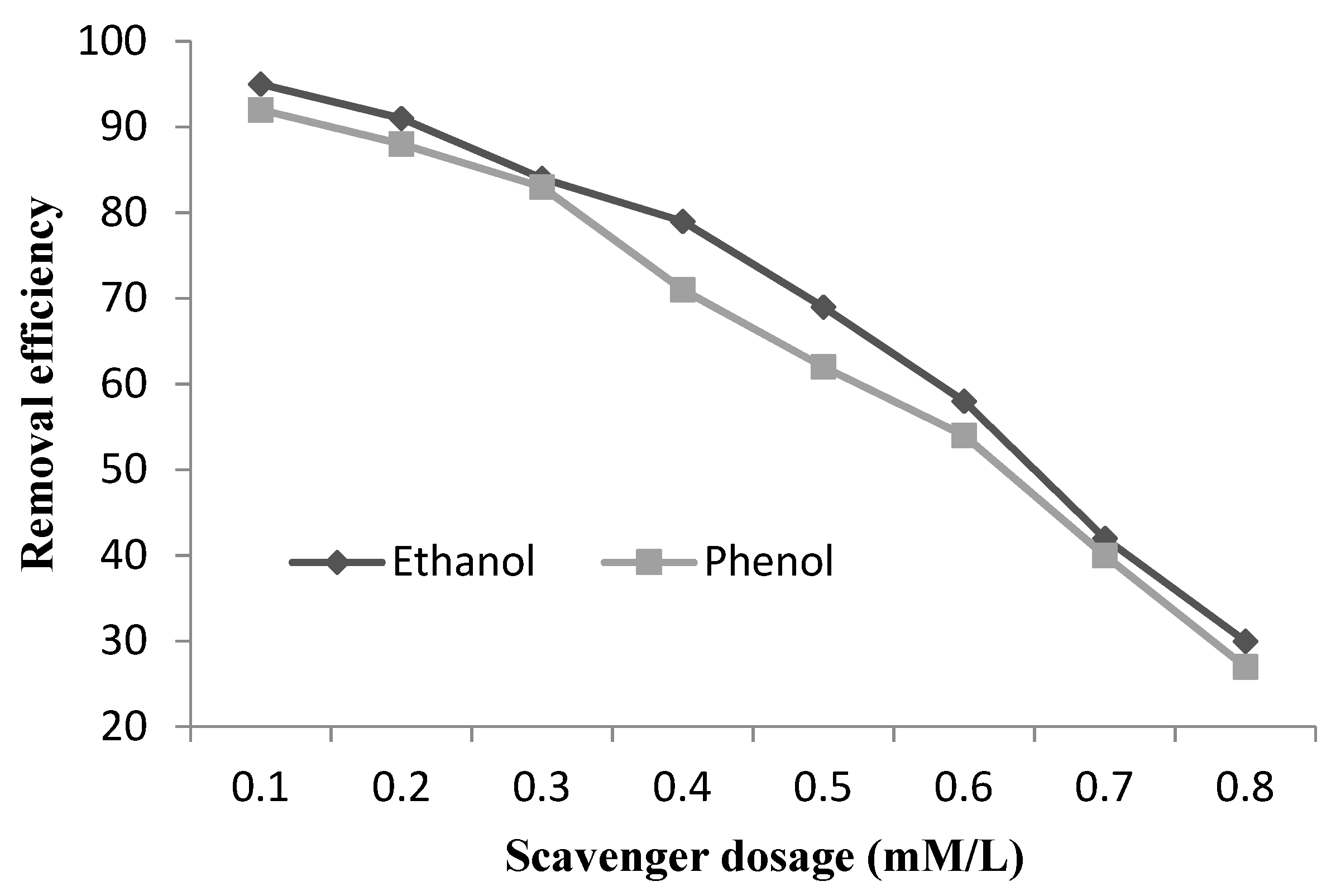
3.4. Determination of Radicals Present in the PF like Catalyst System
3.5. Stability of the Catalyst
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Photocatalytic C-H activation and oxidative esterification using Pd@g-C3N4. Catal. Today 2018, 309, 248–252. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, D.; O’Connor, D.; Li, X.; Pehkonen, S.; Varma, R.S.; Wang, X. Green and size-specific synthesis of stable fe–cu oxides as earth-abundant adsorbents for malachite green removal. Acs Sustain. Chem. Eng. 2018, 6, 9229–9236. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Alijani, H.; Sharifi, I.; Sharifi, F.; Pourseyedi, S.; Kharazi, S.; Lima Nobre, M.A.; Khatami, M. Leishmanicidal activity of biogenic Fe3O4 nanoparticles. Sci. Pharm. 2017, 85, 36. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Alijani, H.Q.; Sharifi, I. Biosynthesis of bimetallic and core–shell nanoparticles: Their biomedical applications—A review. IET Nanobiotech 2018, 12, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Alijani, H.Q.; Fakheri, B.; Mobasseri, M.M.; Heydarpour, M.; Farahani, Z.K.; Khan, A.U. Super-paramagnetic iron oxide nanoparticles (spions): Greener synthesis using stevia plant and evaluation of its antioxidant properties. J. Clean. Prod. 2019, 208, 1171–1177. [Google Scholar] [CrossRef]
- Rahimi, S.; Poormohammadi, A.; Salmani, B.; Ahmadian, M.; Rezaei, M. Comparing the photocatalytic process efficiency using batch and tubular reactors in removal of methylene blue dye and cod from simulated textile wastewater. J. Water Reuse Desalin. 2016, 6, 574–582. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Modarres, M.; Amiri, M.S.; Darroudi, M. Phyto-synthesis of silver nanoparticles using aerial extract of salvia leriifolia benth and evaluation of their antibacterial and photo-catalytic properties. Res. Chem. Intermed. 2019, 45, 1105–1116. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Fabrication of mgo nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol. 2019, 190, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Yosefi, N.; Toolabi, A.; Khanjani, N.; Rahimi, S.; Fatehizadeh, A. Adsorption of direct yellow 9 and acid orange 7 from aqueous solutions by modified pumice. Asian J. Chem. 2012, 24, 3094–3098. [Google Scholar]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, F.; Ahmed, M.J.; Khanday, W.; Asif, M.; Hameed, B. Mesoporous carbonaceous material from fish scales as low-cost adsorbent for reactive orange 16 adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 47–54. [Google Scholar] [CrossRef]
- Samadi, M.T.; Zolghadrnasab, H.; Godini, K.; Poormohammadi, A.; Ahmadian, M.; Shanesaz, S. Kinetic and adsorption studies of reactive black 5 removal using multi-walled carbon nanotubes from aqueous solution. Der Pharma Chem. 2015, 7, 267–274. [Google Scholar]
- Gu, M.; Yin, Q.; Wang, Z.; He, K.; Wu, G. Color and nitrogen removal from synthetic dye wastewater in an integrated mesophilic hydrolysis/acidification and multiple anoxic/aerobic process. Chemosphere 2018, 212, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Jabbari, A.; Zarghan, M.; Moosavi-Movahedi, A.A. Copper nanoparticles–carbon microparticles nanocomposite for electrooxidation and sensitive detection of sotalol. Sens. Actuators B Chem. 2009, 140, 245–251. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly synthesis of silver and gold nanoparticles by euphrasia officinalis leaf extract and its biomedical applications. Artif. Cellsnanomedicineand Biotechnol. 2018, 46, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; Santilli, C.V.; Borges, P.D.; Tronto, J.; Pinto, F.G. Adsorption of acid yellow 42 dye on calcined layered double hydroxide: Effect of time, concentration, ph and temperature. Appl. Clay Sci. 2017, 140, 132–139. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Venkateswarlu, K.; Thavamani, P.; Lee, Y.B.; Naidu, R.; Megharaj, M. Quercus robur acorn peel as a novel coagulating adsorbent for cationic dye removal from aquatic ecosystems. Ecol. Eng. 2017, 101, 3–8. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Sun, Y.; Zhang, S.; Liang, J.; Liu, Y.; An, Y. Evaluation of a novel dextran-based flocculant on treatment of dye wastewater: Effect of kaolin particles. Sci. Total Environ. 2018, 640, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation of dye industry effluent using mesoporous bimetallic ru-cu/sba-15 catalyst. Process. Saf. Environ. Prot. 2018, 118, 125–132. [Google Scholar] [CrossRef]
- Sattarahmady, N.; Heli, H.; Moradi, S.E. Cobalt hexacyanoferrate/graphene nanocomposite—Application for the electrocatalytic oxidation and amperometric determination of captopril. Sens. Actuators B Chem. 2013, 177, 1098–1106. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on fenton and improvements to the fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Malakootian, M.; Yousefi, N.; Fatehizadeh, A.; Van Ginkel, S.W.; Ghorbani, M.; Rahimi, S.; Ahmadian, M. Nickel (ii) removal from industrial plating effluent by fenton process. Environ. Eng. Manag. J. 2015, 14, 837–842. [Google Scholar]
- Moreira, F.C.; Boaventura, R.A.; Brillas, E.; Vilar, V.J. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-organic frameworks for highly efficient heterogeneous fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Munoz, M.; De Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176, 249–265. [Google Scholar] [CrossRef]
- Han, C.; Huang, G.; Zhu, D.; Hu, K. Facile synthesis of MoS2/Fe3O4 nanocomposite with excellent photo-fenton-like catalytic performance. Mater. Chem. Phys. 2017, 200, 16–22. [Google Scholar] [CrossRef]
- Chen, L.; Cai, T.; Cheng, C.; Xiong, Z.; Ding, D. Degradation of acetamiprid in uv/h2o2 and uv/persulfate systems: A comparative study. Chem. Eng. J. 2018, 351, 1137–1146. [Google Scholar] [CrossRef]
- Fakhri, A.; Naji, M.; Tahami, S. Ultraviolet/ultrasound-activated persulfate for degradation of drug by zinc selenide quantum dots: Catalysis and microbiology study. J. Photochem. Photobiol. B Biol. 2017, 170, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.J.; Kakavandi, B.; Jaafarzadeh, N.; Kalantary, R.R.; Ahmadi, M.; Babaei, A.A. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Seid-Mohammadi, A.; Asgari, G.; Poormohammadi, A.; Ahmadian, M.; Rezaeivahidian, H. Removal of phenol at high concentrations using uv/persulfate from saline wastewater. Desalin. Water Treat. 2016, 57, 19988–19995. [Google Scholar] [CrossRef]
- Shiraz, A.D.; Takdastan, A.; Borghei, S.M. Photo-fenton like degradation of catechol using persulfate activated by uv and ferrous ions: Influencing operational parameters and feasibility studies. J. Mol. Liq. 2018, 249, 463–469. [Google Scholar] [CrossRef]
- Rahimi, S.; Ehrampoush, M.H.; Ghaneian, M.T.; Reshadat, S.; Fatehizadeh, A.; Ahmadian, M.; Ghanizadeh, G.; Rahimi, S. Application of TiO2/uv-c photocatalytic process in removal of reactive red 198 dye from synthetic textile wastewater. Asian J. Chem. 2013, 25, 7427–7432. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods in Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 32. [Google Scholar]
- Ai, L.; Huang, H.; Chen, Z.; Wei, X.; Jiang, J. Activated carbon/CoFe2O4 composites: Facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem. Eng. J. 2010, 156, 243–249. [Google Scholar] [CrossRef]
- Moussavi, S.; Ehrampoush, M.; Mahvi, A.; Ahmadian, M.; Rahimi, S. Adsorption of humic acid from aqueous solution on single-walled carbon nanotubes. Asian J. Chem. 2013, 25, 5319–5324. [Google Scholar] [CrossRef]
- Xu, J.; Xin, P.; Gao, Y.; Hong, B.; Jin, H.; Jin, D.; Peng, X.; Li, J.; Gong, J.; Ge, H. Magnetic properties and methylene blue adsorptive performance of CoFe2O4/activated carbon nanocomposites. Mater. Chem. Phys. 2014, 147, 915–919. [Google Scholar] [CrossRef]
- Babu, S.G.; Aparna, P.; Satishkumar, G.; Ashokkumar, M.; Neppolian, B. Ultrasound-assisted mineralization of organic contaminants using a recyclable laFeO3 and Fe3+/persulfate fenton-like system. Ultrason. Sonochem. 2017, 34, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Su, E.-C.; Lee, J.-T.; Gong, Y.-J.; Huang, B.-S.; Wey, M.-Y. Photocatalytic conversion of ethylenediaminetetraacetic acid dissolved in real electroplating wastewater to hydrogen in a solar light-responsive system. Water Sci. Technol. 2018, 77, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Niu, H.; Chen, J.; Song, J.; Mao, C.; Zhang, S.; Gao, Y. Immobilizing laFeO3 nanoparticles on carbon spheres for enhanced heterogeneous photo-fenton like performance. Appl. Surf. Sci. 2017, 404, 138–145. [Google Scholar] [CrossRef]
- Palas, B.; Ersöz, G.; Atalay, S. Photo fenton-like oxidation of tartrazine under visible and uv light irradiation in the presence of laCuO3 perovskite catalyst. Process. Saf. Environ. Prot. 2017, 111, 270–282. [Google Scholar] [CrossRef]
- Wang, X.; Min, J.; Li, S.; Zhu, X.; Cao, X.; Yuan, S.; Zuo, X.; Deng, X. Sono-assisted synthesis of cuo nanorods–graphene oxide as a synergistic activator of persulfate for bisphenol a removal. J. Environ. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Tian, J.; Wu, C.; Yu, H.; Gao, S.; Li, G.; Cui, F.; Qu, F. Applying ultraviolet/persulfate (UV/PS) pre-oxidation for controlling ultrafiltration membrane fouling by natural organic matter (nom) in surface water. Water Res. 2018, 132, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Y.; Wang, Y.; Shi, T.-N.; He, X.-L.; Qi, S.-Y. Process optimization on methyl orange discoloration in Fe3O4/rgo-H2O2 fenton-like system. Water Sci. Technol. 2018, 77, 2929–2939. [Google Scholar] [PubMed]
- Fang, Z.-d.; Zhang, K.; Liu, J.; Fan, J.-y.; Zhao, Z.-w. Fenton-like oxidation of azo dye in aqueous solution using magnetic Fe3O4-MnO2 nanocomposites as catalysts. Water Sci. Eng. 2017, 10, 326–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Tran, H.P.; Du, X.; Hussain, I.; Huang, S.; Zhou, S.; Wen, W. Efficient pyrite activating persulfate process for degradation of p-chloroaniline in aqueous systems: A mechanistic study. Chem. Eng. J. 2017, 308, 1112–1119. [Google Scholar] [CrossRef]
- Liu, F.; Yi, P.; Wang, X.; Gao, H.; Zhang, H. Degradation of acid orange 7 by an ultrasound/zno-gac/persulfate process. Sep. Purif. Technol. 2018, 194, 181–187. [Google Scholar] [CrossRef]






| Parameter mgL−1 | BOD | COD | TSS | Ca2+ | Mg2+ | Cl− | Na+ | K | NO3 |
|---|---|---|---|---|---|---|---|---|---|
| Raw WW | 185 | 880 | 479.2 | 240 | 35 | 298 | 250 | 21 | 51 |
| Dye | Kinetic Model | Dye | Kinetic Model | Dye | Kinetic Model |
|---|---|---|---|---|---|
| Intra-particle diffusion | pseudo-second order | pseudo-first order | |||
| = + | = | ||||
| 0.5 | ki | 24.2 | qe, Cal (mg/g) | 22 | qe, Cal (mg/g) |
| 16.7 | Ci | 0.009 | k1(min−1) | 0.07 | k1(min−1) |
| 0.6 | R2 | 0.99 | R2 | 0.98 | R2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, M.R.; Varma, R.S.; Ahmadian, M.; Pourkhosravani, M.; Asadzadeh, S.N.; Karimi, P.; Khatami, M. Photo-Fenton like Catalyst System: Activated Carbon/CoFe2O4 Nanocomposite for Reactive Dye Removal from Textile Wastewater. Appl. Sci. 2019, 9, 963. https://doi.org/10.3390/app9050963
Heidari MR, Varma RS, Ahmadian M, Pourkhosravani M, Asadzadeh SN, Karimi P, Khatami M. Photo-Fenton like Catalyst System: Activated Carbon/CoFe2O4 Nanocomposite for Reactive Dye Removal from Textile Wastewater. Applied Sciences. 2019; 9(5):963. https://doi.org/10.3390/app9050963
Chicago/Turabian StyleHeidari, Mohammad Reza., Rajender S. Varma, Mohammad Ahmadian, Mohsen Pourkhosravani, Seyedeh N. Asadzadeh, Pouria Karimi, and Mehrdad Khatami. 2019. "Photo-Fenton like Catalyst System: Activated Carbon/CoFe2O4 Nanocomposite for Reactive Dye Removal from Textile Wastewater" Applied Sciences 9, no. 5: 963. https://doi.org/10.3390/app9050963






