Polyurethane-Supported Graphene Oxide Foam Functionalized with Carbon Dots and TiO2 Particles for Photocatalytic Degradation of Dyes
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Regents and Materials
2.2. Preparation of CQDs/TiO2 Particles
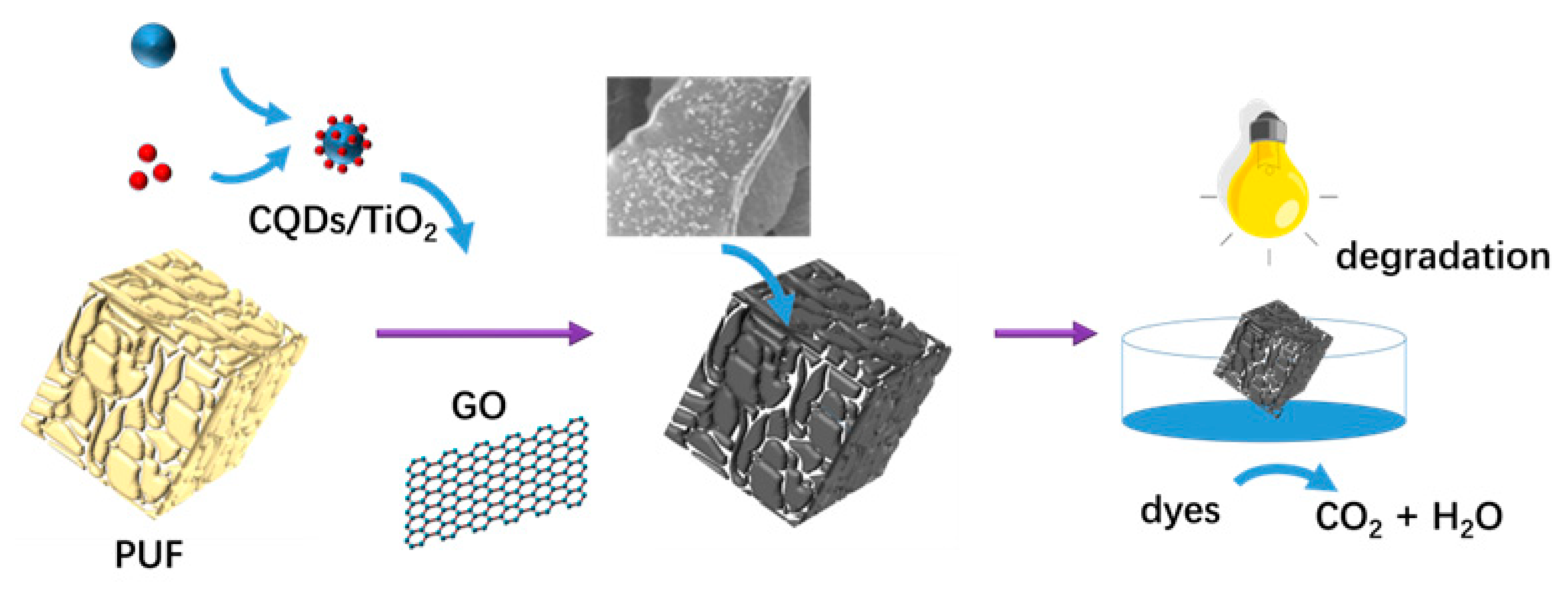
2.3. Fabrication of 3D GO and CQDs/TiO2/GO Foams
2.4. Adsorption and Photocatalytic Degradation and Dyes
2.5. Characterization Techniques
3. Results and Discussion
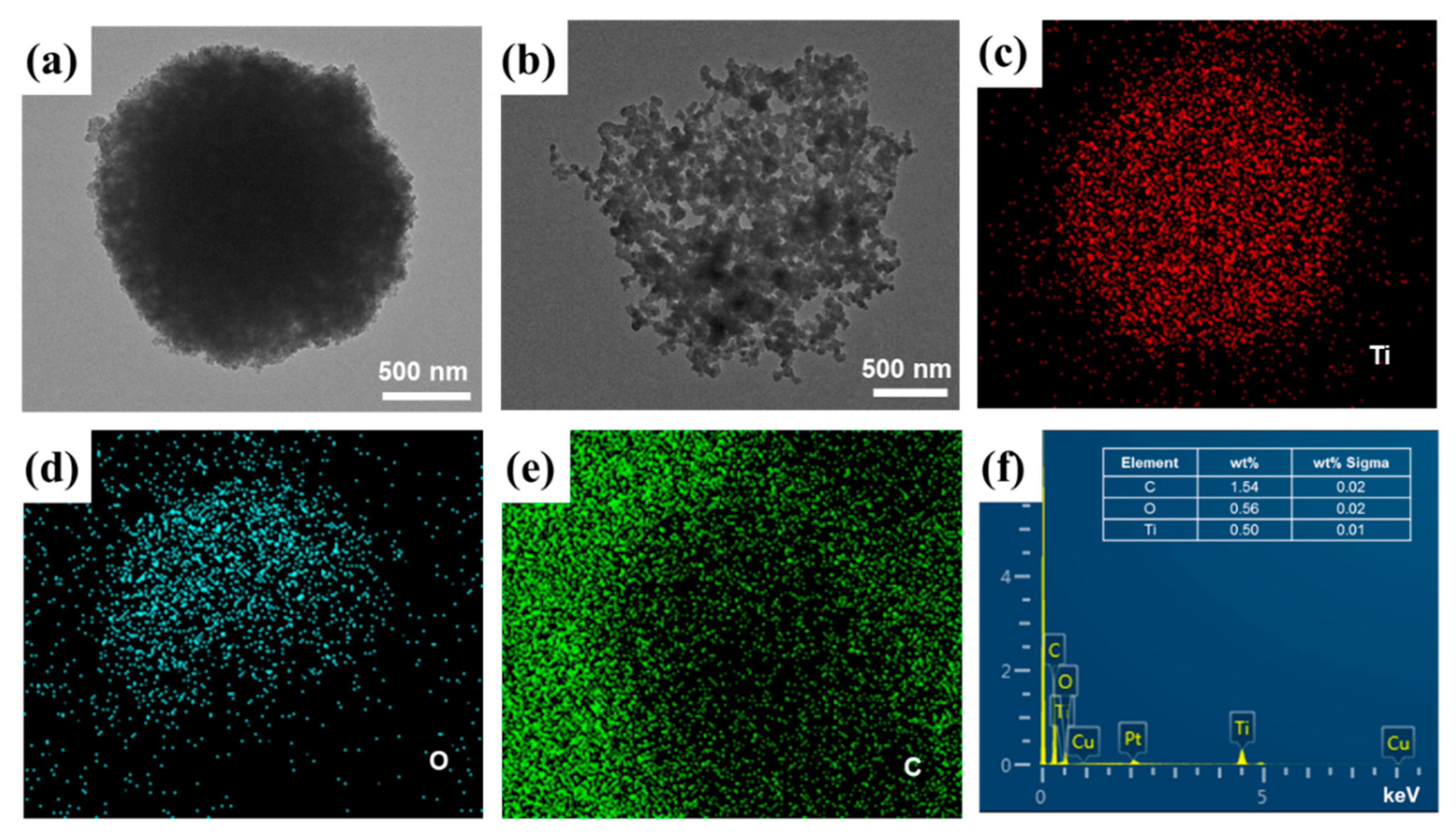
3.1. Morphological Characterizations of CQDs/TiO2 Particles
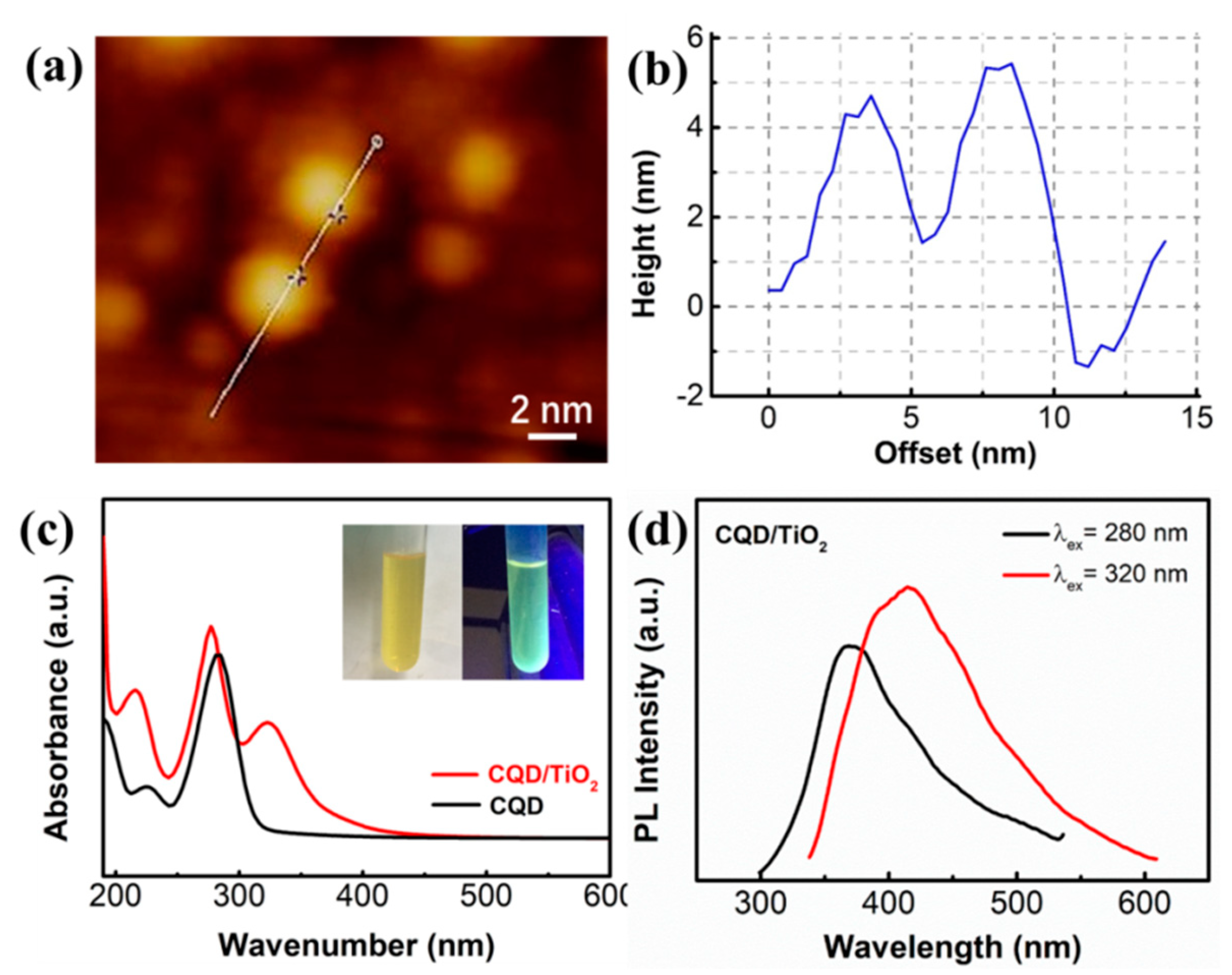
3.2. Spectrum Characterizations of CQDs/TiO2 Particles
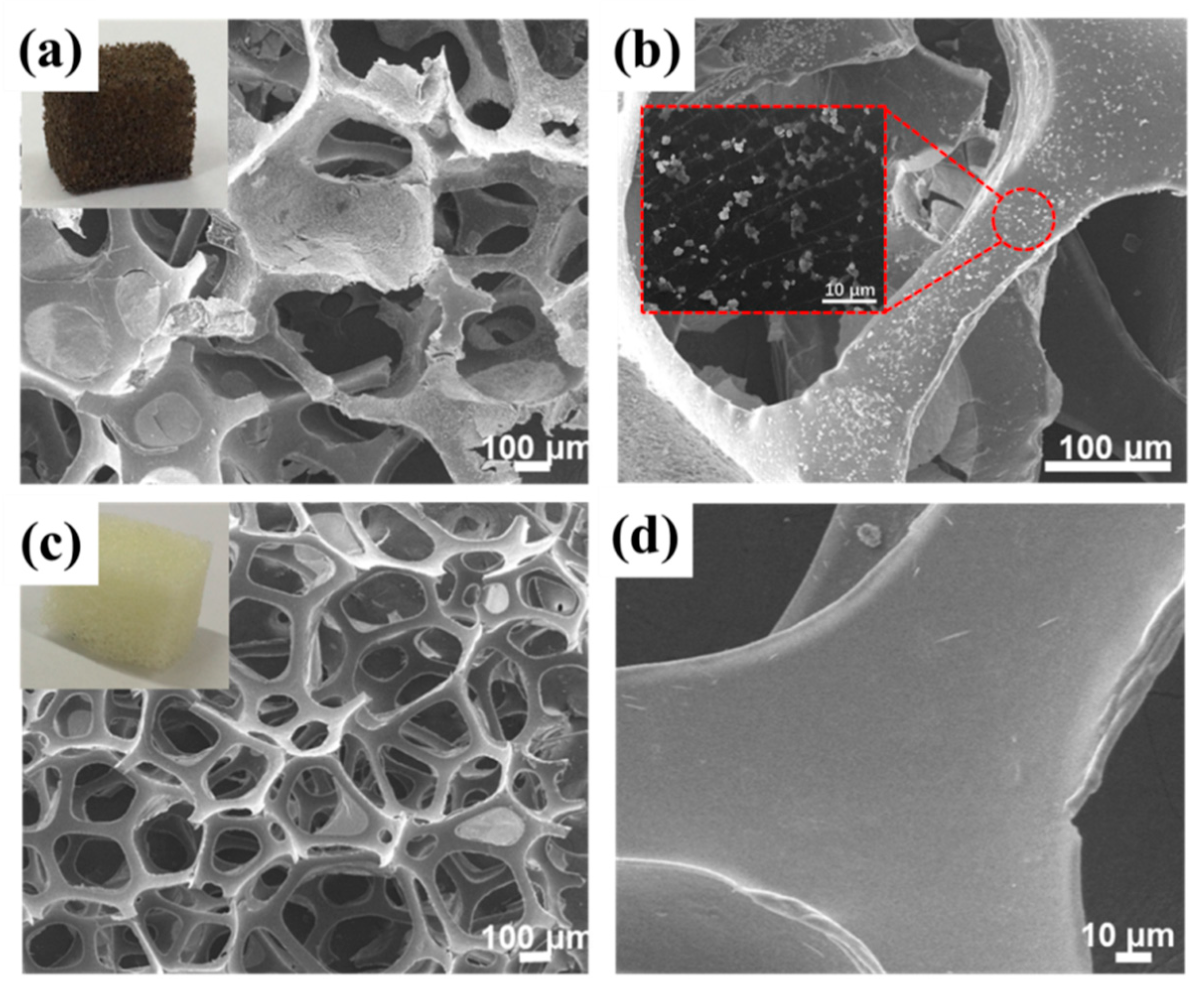
3.3. Characterization of 3D CQDs/TiO2/GO Foams
3.4. Adsorption and Photocatalytic Degradation of Dyes
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- An, S.; Joshi, B.N.; Lee, J.G.; Lee, M.W.; Kim, Y.I.; Kim, M.W.; Jo, H.S.; Yoon, S.S. A comprehensive review on wettability, desalination, and purification using graphene-based materials at water interfaces. Catal. Today 2017, 295, 14–25. [Google Scholar] [CrossRef]
- Dutta, K.; De, S. Smart responsive materials for water purification: An overview. J. Mater. Chem. A 2017, 5, 22095–22112. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wu, A.G.; Ciacchi, L.C.; Wei, G. Recent advances in nanoporous membranes for water purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Montecchio, F.; Persson, H.; Engvall, K.; Delin, J.; Lanza, R. Development of a stagnation point flow system to screen and test TiO2-based photocatalysts in air purification applications. Chem. Eng. J. 2016, 306, 734–744. [Google Scholar] [CrossRef]
- Ren, H.J.; Koshy, P.; Chen, W.F.; Qi, S.H.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.Q.; Liu, J.; Pareek, V.K.; Wang, S.B. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Tan, L.; Yu, C.F.; Wang, M.; Zhang, S.Y.; Sun, J.Y.; Dong, S.Y.; Sun, J.H. Synergistic effect of adsorption and photocatalysis of 3D g-C3C4 -agar hybrid aerogels. Appl. Surf. Sci. 2019, 467, 286–292. [Google Scholar] [CrossRef]
- Kuo, M.Y.; Hsiao, C.F.; Chiu, Y.H.; Lai, T.H.; Fang, M.J.; Wu, J.Y.; Chen, J.W.; Wu, C.L.; Wei, K.H.; Lin, H.C.; et al. Au@Cu2O core@shell nanocrystals as dual-functional catalysts for sustainable environmental applications. Appl. Catal. B 2019, 242, 499–506. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photoca22talysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Godin, R.; Moss, B.; Kafizas, A.; Zafeiratos, S.; Durrant, J.R.; Petit, C. Titanium dioxide/carbon nitride nanosheet nanocomposites for gas phase CO2 photoreduction under uv-visible irradiation. Appl. Catal. B 2019, 242, 369–378. [Google Scholar] [CrossRef]
- Negishi, N.; Miyazaki, Y.; Kato, S.; Yang, Y.N. Effect of HCO3− concentration in groundwater on TiO2 photocatalytic water purification. Appl. Catal. B 2019, 242, 449–459. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, C.; Zhao, H.B.; Gao, G.F.; Jiang, T.S. Fabrication of ternary structure photocatalyst CDs/CQD@mpg-C3N4 with enhanced photocatytic performance based on synergistic effect. ChemistrySelect 2018, 3, 11824–11834. [Google Scholar] [CrossRef]
- Zhang, L.W.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and mechanisms of charge transfer processes in photocatalytic systems: A review. J. Photochem. Photobiol. C 2012, 13, 263–276. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, Y.H.; Li, Z.H. Carbon quantum dots (cqds) and co(dmgh)(2)pycl synergistically promote photocatalytic hydrogen evolution over hexagonal ZnIn2S4. Appl. Surf. Sci. 2018, 462, 255–262. [Google Scholar] [CrossRef]
- Li, K.; Liu, W.; Ni, Y.; Li, D.; Lin, D.; Su, Z.; Wei, G. Technical synthesis and biomedical applications of graphene quantum dots. J. Mater. Chem. B 2017, 5, 4811–4826. [Google Scholar] [CrossRef]
- Hu, X.T.; Shi, J.Y.; Shi, Y.Q.; Zou, X.B.; Arslan, M.; Zhang, W.; Huang, X.W.; Li, Z.H.; Xu, Y.W. Use of a smartphone for visual detection of melamine in milk based on Au@carbon quantum dots nanocomposites. Food Chem. 2019, 272, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Zhao, Y.F.; Zhou, C.; Shang, L.; Peng, Y.; Cao, Y.H.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 3344–3351. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhang, Y.G.; Chen, W.; Huang, S.M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Wei, Q.; Oribayo, O.; Feng, X.S.; Rempel, G.L.; Pan, Q.M. Synthesis of polyurethane foams loaded with TiO2 nanoparticles and their modification for enhanced performance in oil spill cleanup. Ind. Eng. Chem. Res. 2018, 57, 8918–8926. [Google Scholar] [CrossRef]
- Qian, X.F.; Ren, M.; Yue, D.T.; Zhu, Y.; Han, Y.; Bian, Z.F.; Zhao, Y.X. Mesoporous TiO2 films coated on carbon foam based on waste polyurethane for enhanced photocatalytic oxidation of vocs. Appl. Catal. B 2017, 212, 1–6. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.F.; Li, Y.; Su, Z.Q.; Wei, G. Recent advances in the synthesis and applications of graphene-polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Li, D.P.; Zhang, W.S.; Yu, X.Q.; Wang, Z.P.; Su, Z.Q.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.; Zhang, Z.F.; Li, D.P.; Zhang, W.S.; Yu, X.Q.; Liu, W.; Gong, C.C.; Wei, G.; Su, Z.Q. Biomimetic ultralight, highly porous, shape-adjustable, and biocompatible 3D graphene minerals via incorporation of self-assembled peptide nanosheets. Adv. Funct. Mater. 2018, 28, 1801056. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.Y.; Wei, G.; Su, Z.Q. Reduced graphene oxide-based double network polymeric hydrogels for pressure and temperature sensing. Sensors 2018, 18, 3162. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.H.; Zhang, K.; Li, L.; Chen, X.X.; Hu, J.L.; Yan, D.Y.; Xiao, G.Y.; He, X.H. High performance graphene-based foam fabricated by a facile approach for oil absorption. J. Mater. Chem. A 2017, 5, 11263–11270. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.H.; Lee, S.B.; Kuo, W.S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Zhang, Y.; Steckbeck, S.; Su, Z.Q.; Li, Z. Biomimetic graphene-fept nanohybrids with high solubility, ferromagnetism, fluorescence, and enhanced electrocatalytic activity. J. Mater. Chem. 2012, 22, 17190–17195. [Google Scholar] [CrossRef]
- Ivanda, M.; Music, S.; Popovic, S.; Gotic, M. Xrd, raman and ft-ir spectroscopic observations of nanosized tio2 synthesized by the sol-gel method based on an esterification reaction. J. Mol. Struct. 1999, 481, 645–649. [Google Scholar] [CrossRef]
- Roy, N.; Sohn, Y.; Pradhan, D. Synergy of low-energy {101} and high-energy {001} TiO2 crystal facets for enhanced photocatalysis. ACS Nano 2013, 7, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S. Stacked bioglass/TiO2 nanocoatings on titanium substrate for enhanced osseointegration and its electrochemical corrosion studies. Appl. Surf. Sci. 2015, 349, 561–569. [Google Scholar] [CrossRef]
- Li, H.T.; He, X.D.; Kang, Z.H.; Huang, H.; Liu, Y.; Liu, J.L.; Lian, S.Y.; Tsang, C.H.A.; Yang, X.B.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Pan, J.; Sheng, Y.; Zhang, J.; Wei, J.; Huang, P.; Zhang, X.; Feng, B. Preparation of carbon quantum dots/TiO2nanotubes composites and their visible light catalytic applications. J. Mater. Chem. A 2014, 2, 18082–18086. [Google Scholar] [CrossRef]



| Samples | Average Thickness/nm | Average Length /nm | Percentage of High-Energy Lattice Plane |
|---|---|---|---|
| 10 h CQDs /TiO2 | 5.3 | 6.1 | 22% |
| 12 h CQDs /TiO2 | 6.5 | 9.5 | 30% |
| 14 h CQDs /TiO2 | 5.8 | 9.1 | 32% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Sun, S.; Zhou, L.; Li, P.; Su, Z.; Wei, G. Polyurethane-Supported Graphene Oxide Foam Functionalized with Carbon Dots and TiO2 Particles for Photocatalytic Degradation of Dyes. Appl. Sci. 2019, 9, 293. https://doi.org/10.3390/app9020293
Liu T, Sun S, Zhou L, Li P, Su Z, Wei G. Polyurethane-Supported Graphene Oxide Foam Functionalized with Carbon Dots and TiO2 Particles for Photocatalytic Degradation of Dyes. Applied Sciences. 2019; 9(2):293. https://doi.org/10.3390/app9020293
Chicago/Turabian StyleLiu, Tianjiao, Shuwei Sun, Lin Zhou, Peng Li, Zhiqiang Su, and Gang Wei. 2019. "Polyurethane-Supported Graphene Oxide Foam Functionalized with Carbon Dots and TiO2 Particles for Photocatalytic Degradation of Dyes" Applied Sciences 9, no. 2: 293. https://doi.org/10.3390/app9020293






