Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia
Abstract
:1. Introduction
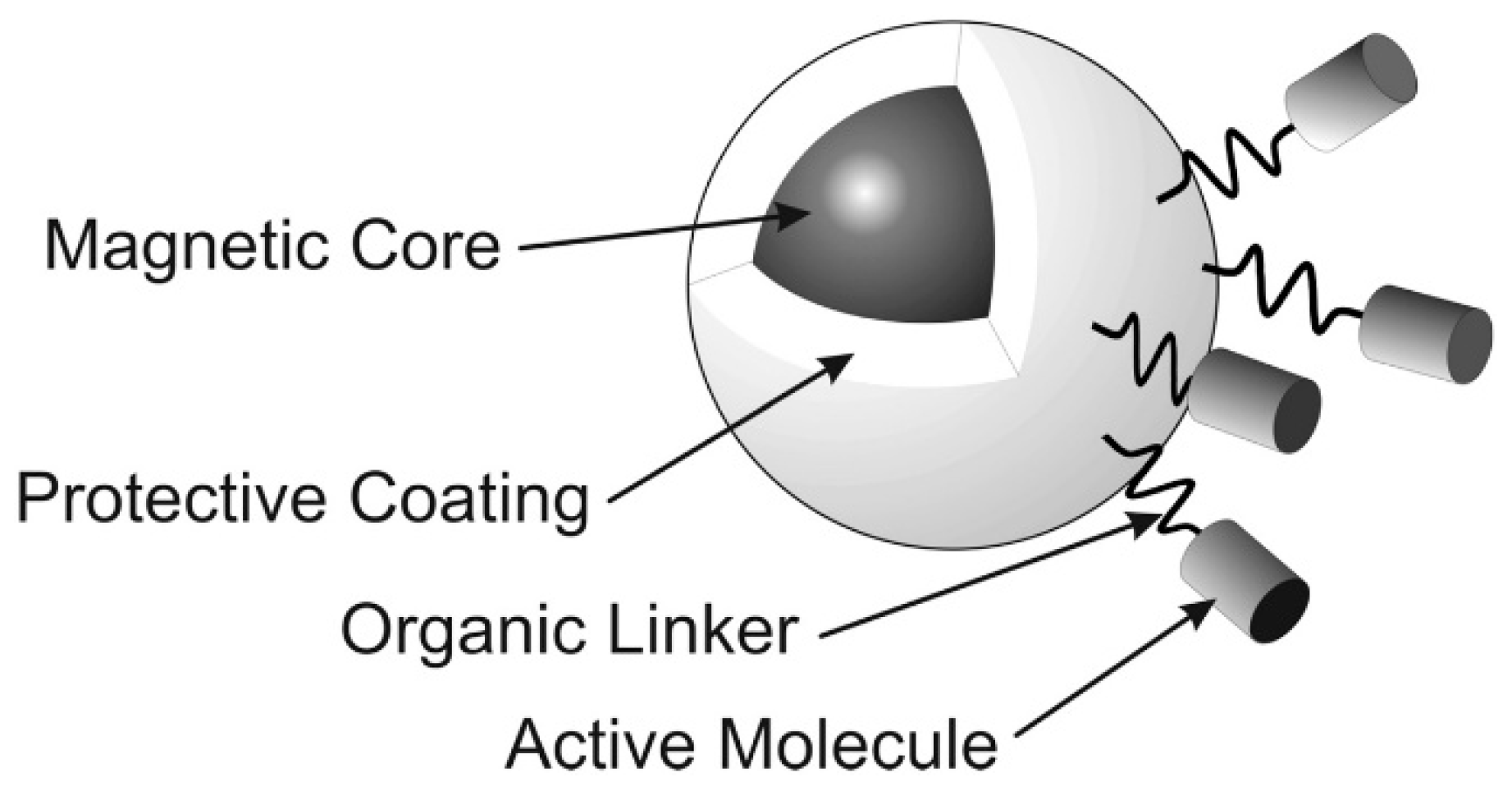
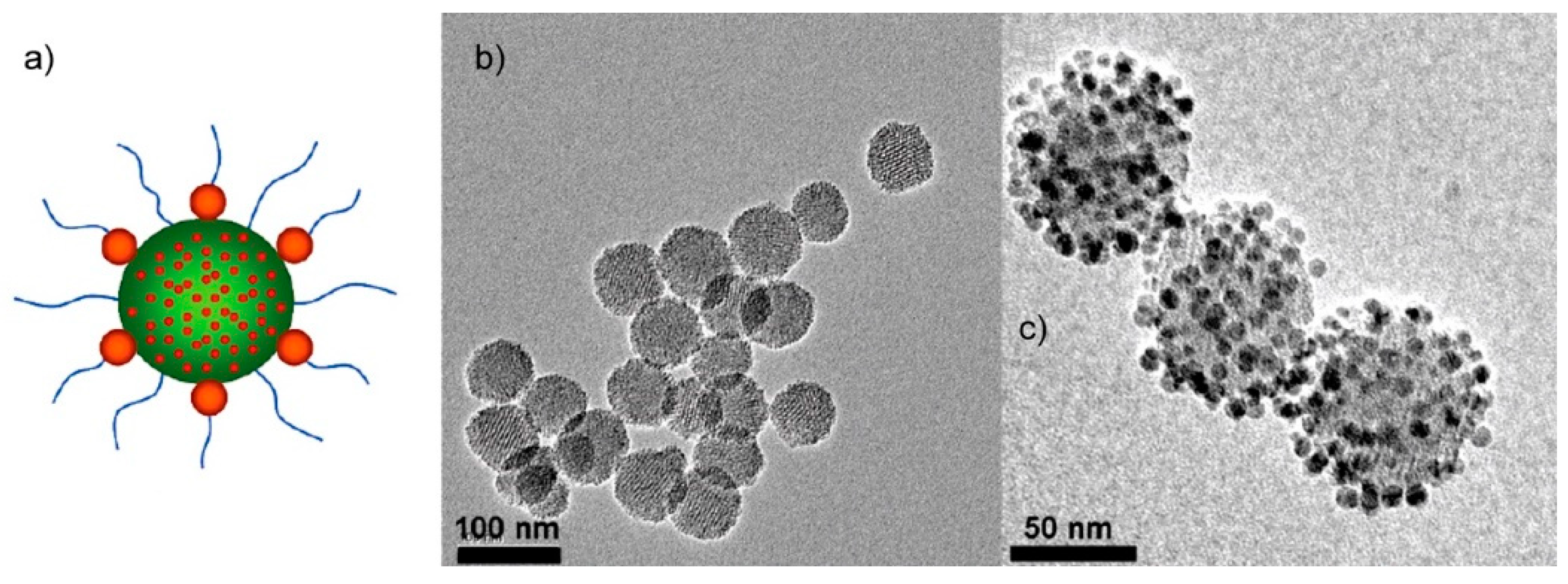
2. Magnetic Nanoparticles
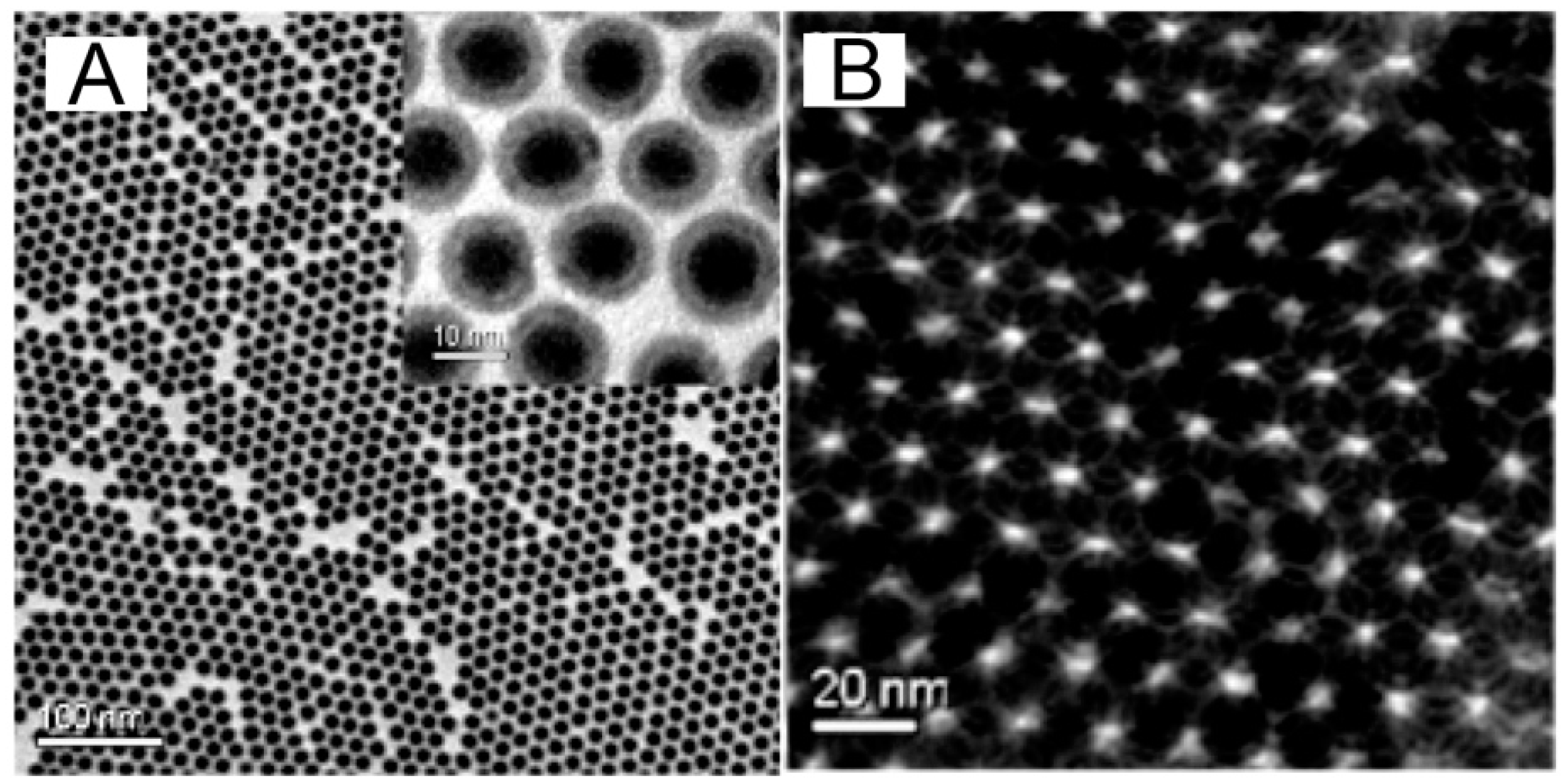
3. Synthesis of Magnetic Nanoparticles
4. Wet Precipitation and Co-Precipitation
5. Utilizing the Reverse Micelle Mechanism for Magnetic Nanoparticle Synthesis
6. Thermal Decomposition and Reduction
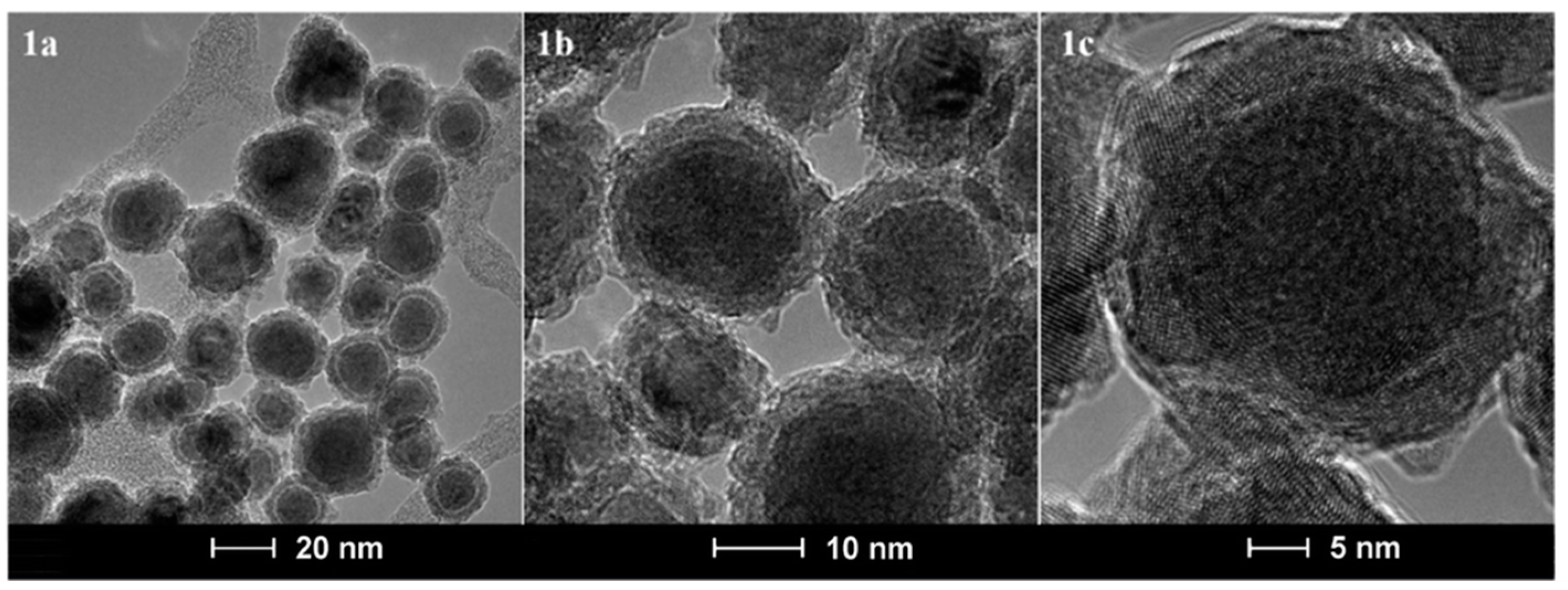
7. Iron/Iron Oxide Core/Shell Nanoparticles
8. Chemical Vapor Condensation Method
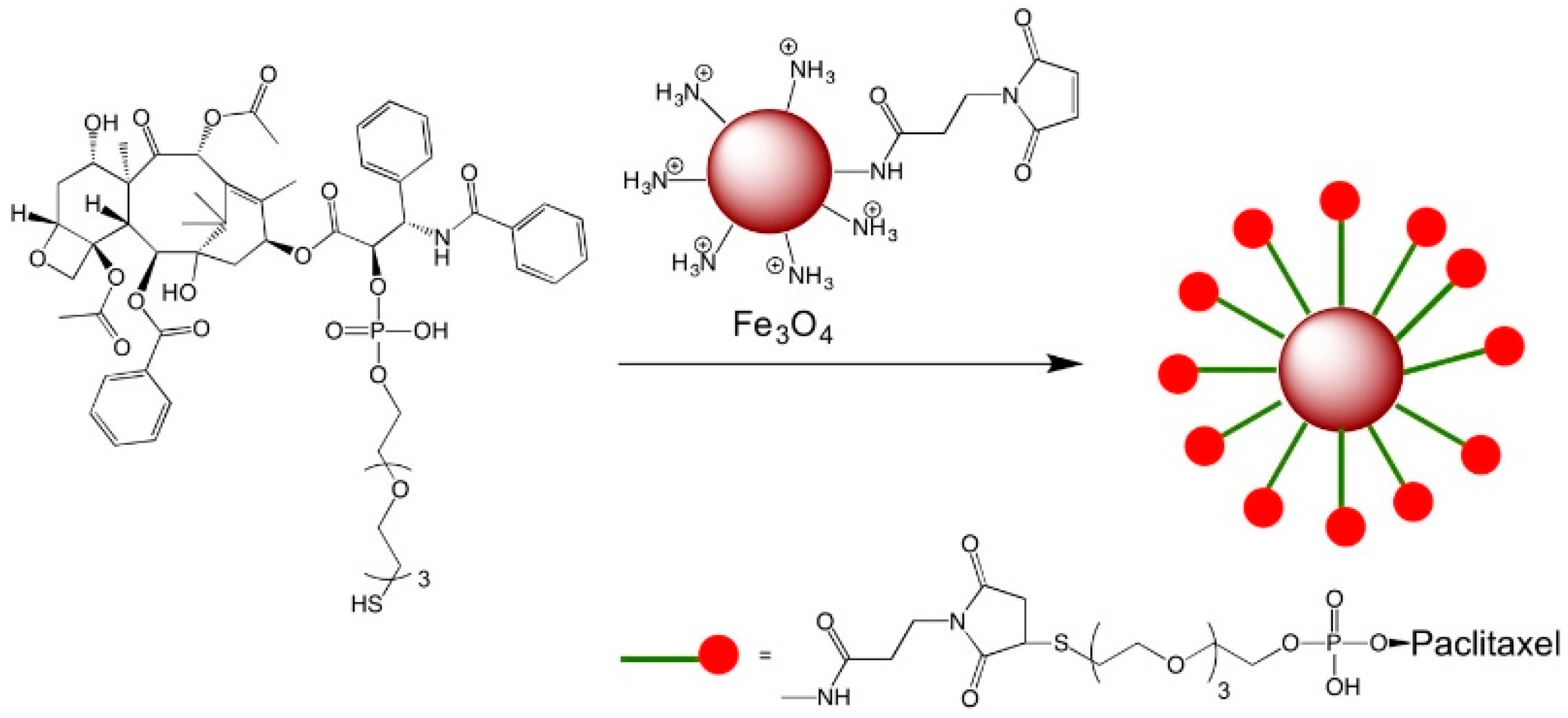
9. Drug Delivery
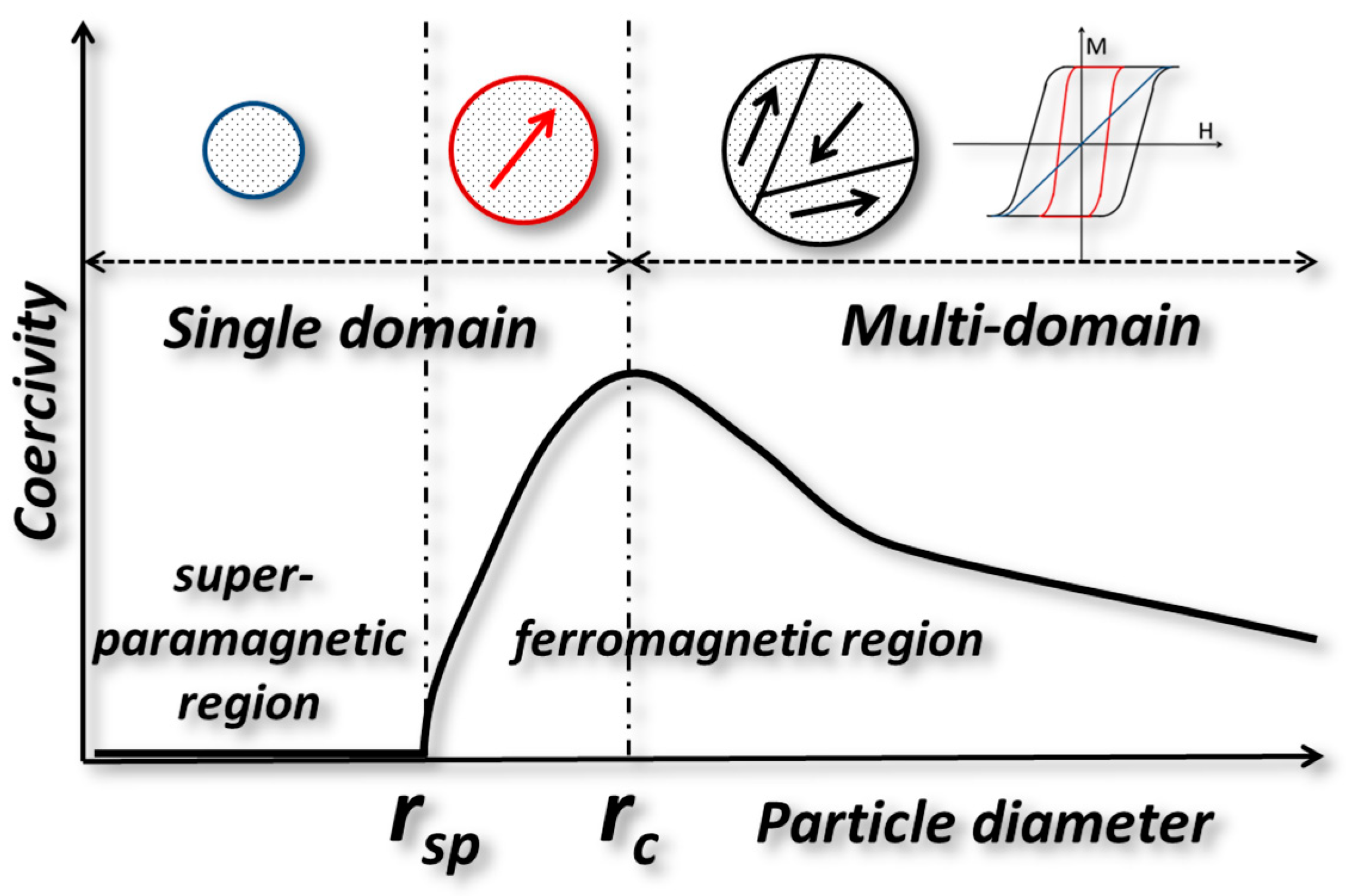
10. Superparamagnetism vs. Ferromagnetism in Magnetic Nanoparticles
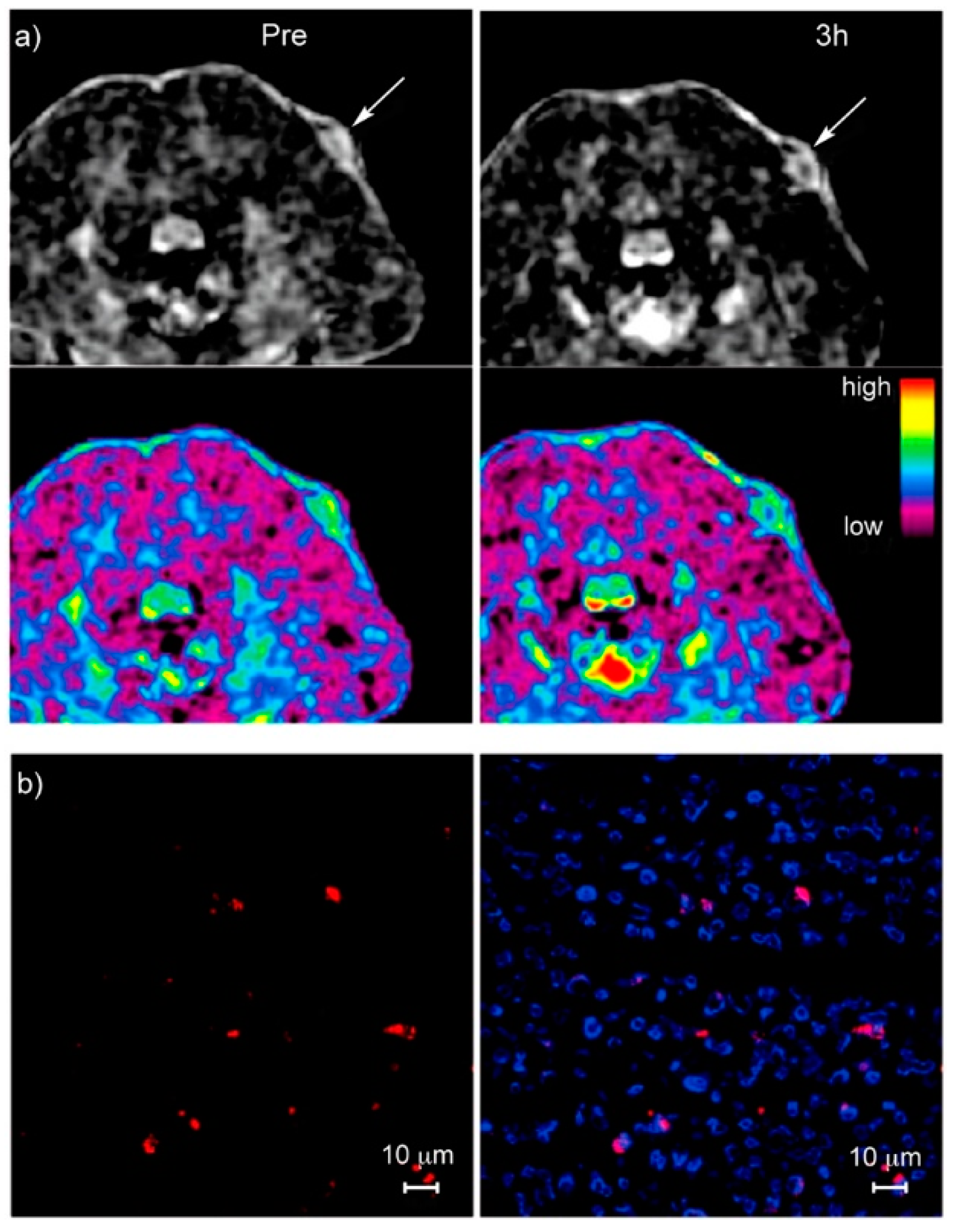
11. Magnetic Nanoparticles in Preclinical Studies
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goodwin, S.; Peterson, C.; Hoh, C.; Bittner, C. Targeting and retention of magnetic targeted carriers (MTCs) enhancing intra-arterial chemotherapy. J. Magn. Magn. Mater. 1999, 194, 132–139. [Google Scholar] [CrossRef]
- Mathiyazhakan, M.; Xu, C. Magnetic Nanoparticles for Drug Delivery; World Scientific Publishing Co. Pte. Ltd.: Hackensack, NJ, USA, 2016; pp. 65–84. [Google Scholar]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lübbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dörken, B.; Herrmann, F.; Gürtler, R. Clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar] [PubMed]
- Yarar, E.; Karakas, G.; Rende, D.; Ozisik, R.; Malta, S. Influence of Surface Coating of Magnetic Nanoparticles on Mechanical Properties of Polymer Nanocomposites; APS March Meeting Abstracts: Boston, MA, USA, 2016. [Google Scholar]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, G.; Chaker, M.; Ozaki, T.; Tijssen, P.; Ma, D. Fluorescent-magnetic multifunctional nanoparticles for imaging and drug delivery. Rev. Nanosci. Nanotechnol. 2013, 2, 346–364. [Google Scholar] [CrossRef]
- Maldiney, T.; Mignet, N. Nanoparticles for Imaging and Imaging Nanoparticles: State of the Art and Current Prospects; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 533–560. [Google Scholar]
- Zeng, L.; Wu, D.; Zou, R.; Chen, T.; Zhang, J.; Wu, A. Paramagnetic and Superparamagnetic Inorganic Nanoparticles for T1-Weighted Magnetic Resonance Imaging. Curr. Med. Chem. 2018, 25, 2970–2986. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kakimi, K.; Nakayama, E.; Jimbow, K. Antitumor immunity by magnetic nanoparticle-mediated hyperthermia. Nanomedicine 2014, 9, 1715–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutz, S.; Mueller, R.; Eberbeck, D.; Hilger, I.; Zeisberger, M. Magnetic nanoparticles adapted for specific biomedical applications. Biomed. Tech. 2015, 60, 405–416. [Google Scholar] [CrossRef]
- Kafrouni, L.; Savadogo, O. Recent progress on magnetic nanoparticles for magnetic hyperthermia. Prog. Biomater. 2016, 5, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B.; et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 119. [Google Scholar] [CrossRef]
- Wang, H.; Shrestha, T.B.; Basel, M.T.; Dani, R.K.; Seo, G.-M.; Balivada, S.; Pyle, M.M.; Prock, H.; Koper, O.B.; Thapa, P.S.; et al. Magnetic-Fe/Fe3O4-nanoparticle-bound SN38 as carboxylesterase-cleavable prodrug for the delivery to tumors within monocytes/macrophages. Beilstein J. Nanotechnol. 2012, 3, 444–455. [Google Scholar] [CrossRef]
- McWilliams, B.T.; Wang, H.; Binns, V.J.; Curto, S.; Bossmann, S.H.; Prakash, P. Experimental investigation of magnetic nanoparticle-enhanced microwave hyperthermia. J. Funct. Biomater. 2017, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wang, H.; Su, Y.; Chen, C.; Xie, L.; Chen, L.; Yu, J.; Toledo, Y.; Abayaweera, G.S.; Zhu, G.; et al. Incorporating 131I into a PAMAM (G5.0) dendrimer-conjugate: Design of a theranostic nanosensor for medullary thyroid carcinoma. RSC Adv. 2017, 7, 16181–16188. [Google Scholar] [CrossRef]
- Mehta, J.P.; Knappett, B.R.; Divitini, G.; Ringe, E.; Midgley, P.A.; Fairen-Jimenez, D.; Wheatley, A.E.H. Advances in the Synthesis and Long-Term Protection of Zero-Valent Iron Nanoparticles. Part. Part. Syst. Charact. 2018, 35, 1–8. [Google Scholar] [CrossRef]
- Kalubowilage, M.; Covarrubias-Zambrano, O.; Malalasekera, A.P.; Wendel, S.O.; Wang, H.; Yapa, A.S.; Chlebanowski, L.; Toledo, Y.; Ortega, R.; Janik, K.E.; et al. Early detection of pancreatic cancers in liquid biopsies by ultrasensitive fluorescence nanobiosensors. Nanomedicine 2018, 14, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bao, S.; Zhang, B.; Chen, Y.; Chen, W.; Wang, C. Coupling Fe@Fe3O4 nanoparticles with multiple-walled carbon nanotubes with width band electromagnetic absorption performance. Appl. Surf. Sci. 2019, 467–468, 836–843. [Google Scholar] [CrossRef]
- Lopez-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogues, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Ong, Q.K.; Lin, X.-M.; Wei, A. Role of Frozen Spins in the Exchange Anisotropy of Core-Shell Fe@Fe3O4 Nanoparticles. J. Phys. Chem. C 2011, 115, 2665–2672. [Google Scholar] [CrossRef]
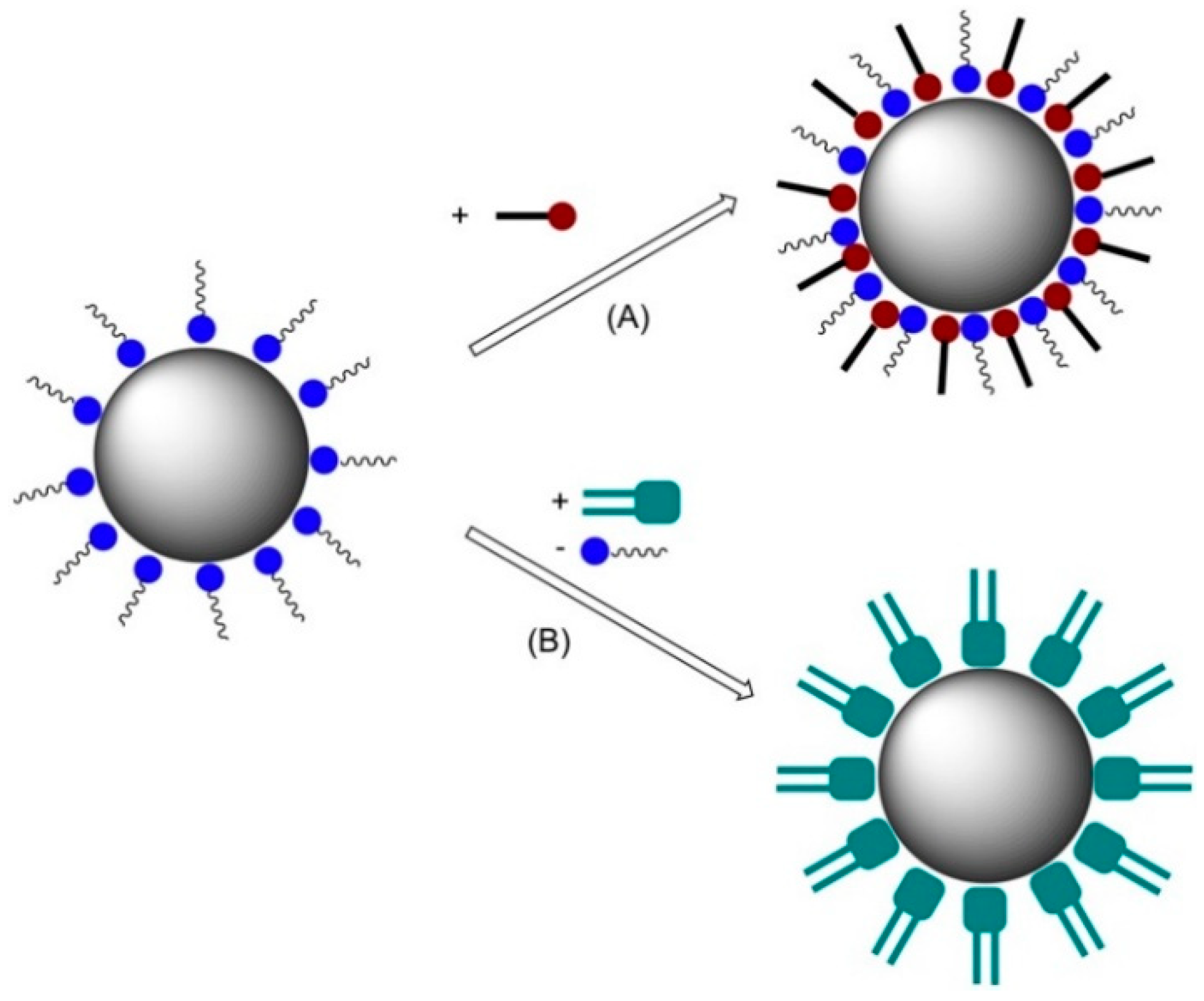
- Malalasekera, A.P.; Bossmann, S.H.; Zhu, G. Magnetic Nanoformulations for Enhanced Drug Delivery and Retention. In Magnetic Nanomaterials: Applications in Catalysis and Life Sciences; Bossmann, S.H., Wang, H., Eds.; RSC: London, UK, 2017. [Google Scholar]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Casals, E.; Puntes, V.F. Inorganic nanoparticle biomolecular corona: Formation, evolution and biological impact. Nanomedicine 2012, 7, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The nanoparticle biomolecule corona: Lessons learned - challenge accepted? Chem. Soc. Rev. 2015, 44, 6094–6121. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-H.; Zhang, X.-Q.; Sun, Q.; Zhang, Y.; Song, Q.; Schüth, F.; Chen, C.; Cheng, F. Precise synthesis of discrete and dispersible carbon-protected magnetic nanoparticles for efficient magnetic resonance imaging and photothermal therapy. Nano Res. 2016, 9, 1460–1469. [Google Scholar] [CrossRef]
- Shen, Z.; Song, J.; Zhou, Z.; Yung, B.C.; Aronova, M.A.; Li, Y.; Dai, Y.; Fan, W.; Liu, Y.; Li, Z.; et al. Dotted Core-Shell Nanoparticles for T1-Weighted MRI of Tumors. Adv. Mater. (Weinheim, Ger.) 2018, 30, 1803163. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Cui, H. Functional nanoparticles for magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 814–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Wang, C.; Bao, C.; Liang, S.; Zhang, L.; Fu, H.; Wang, Y.; Wang, K.; Li, C.; Deng, M.; Liao, Q. HAI-178 antibody-conjugated fluorescent magnetic nanoparticles for targeted imaging and simultaneous therapy of gastric cancer. Nanoscale Res. Lett. 2014, 9, 274. [Google Scholar] [CrossRef]
- Chen, J.; Shi, M.; Liu, P.; Ko, A.; Zhong, W.; Liao, W.; Xing, M.M. Reducible polyamidoamine-magnetic iron oxide self-assembled nanoparticles for doxorubicin delivery. Biomaterials 2014, 35, 1240–1248. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: The effect of nonionic surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Pardoe, H.; Chua-Anusorn, W.; Pierre, T.G.S.; Dobson, J. Structural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcohol. J. Magn. Magn. Mater. 2001, 225, 41–46. [Google Scholar] [CrossRef]
- Jeun, M.; Bae, S.; Tomitaka, A.; Takemura, Y.; Park, K.H.; Paek, S.H.; Chung, K.-W. Effects of particle dipole interaction on the ac magnetically induced heating characteristics of ferrite nanoparticles for hyperthermia. Appl. Phys. Lett. 2009, 95, 082501. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Chaffin, E.; Wang, Y.; Huang, X. Synthesis and properties of magnetic-optical core-shell nanoparticles. RSC Adv. 2017, 7, 17137–17153. [Google Scholar] [CrossRef] [PubMed]
- Sabale, S.; Kandesar, P.; Jadhav, V.; Komorek, R.; Motkuri, R.K.; Yu, X.-Y. Recent developments in the synthesis, properties, and biomedical applications of core/shell superparamagnetic iron oxide nanoparticles with gold. Biomater. Sci. 2017, 5, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Shahin, A.M.; Long, G.J.; Davies, J.E.; Liu, K.; Grandjean, F.; Kauzlarich, S.M. Magnetic and Moessbauer Spectral Study of Core/Shell Structured Fe/Au Nanoparticles. Chem. Mater. 2006, 18, 960–967. [Google Scholar] [CrossRef]
- Baker, I. Magnetic nanoparticle synthesis. In Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–229. [Google Scholar]
- Hajalilou, A.; Kianvash, A.; Lavvafi, H.; Shameli, K. Nanostructured soft magnetic materials synthesized via mechanical alloying: A review. J. Mater. Sci. Mater. Electron. 2018, 29, 1690–1717. [Google Scholar] [CrossRef]
- Latha, G.; Kumar, P.D.; Gopi, K.; Srikanth, P.; Kusumalatha, Y.; Babu, G.V. A review on magnetic micro/nanoparticles. World J. Pharm. Res. 2017, 6, 341–366. [Google Scholar] [CrossRef]
- Jamil, S.; Janjua, M.R.S.A. Synthetic Study and Merits of Fe3O4 Nanoparticles as Emerging Material. J. Cluster Sci. 2017, 28, 2369–2400. [Google Scholar] [CrossRef]
- Geinguenaud, F.; De Montferrand, C.; Jouni, H.; Guenin, E.; Milosevic, I.; Benyettou, F.; Lalatonne, Y.; Motte, L. Magnetic nanoparticle surface functionalization for biomedical applications. In Nanoantenna; Pan Stanford Series on the High-tech of Biotechnology; Jenny Stanford Publishing: Singapore, 2013; Volume 1, pp. 103–149. [Google Scholar]
- Magagnin, L.; Cojocaru, P. Electrochemical synthesis of dispersed metallic nanoparticles. Mod. Aspects Electrochem. 2012, 54, 345–368. [Google Scholar]
- Byrappa, K.; Ohara, S.; Adschiri, T. Nanoparticles synthesis using supercritical fluid technology-towards biomedical applications. Adv. Drug Deliv. Rev. 2008, 60, 299–327. [Google Scholar] [CrossRef] [PubMed]
- Lam, U.T.; Mammucari, R.; Suzuki, K.; Foster, N.R. Processing of Iron Oxide Nanoparticles by Supercritical Fluids. Ind. Eng. Chem. Res. 2008, 47, 599–614. [Google Scholar] [CrossRef]
- Rachakatla, R.S.; Balivada, S.; Seo, G.-M.; Myers, C.B.; Wang, H.; Samarakoon, T.N.; Dani, R.; Pyle, M.; Kroh, F.O.; Walker, B.; et al. Attenuation of Mouse Melanoma by A/C Magnetic Field after Delivery of Bi-Magnetic Nanoparticles by Neural Progenitor Cells. ACS Nano 2010, 4, 7093–7104. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Covarrubias, J.; Prock, H.; Wu, X.; Wang, D.; Bossmann, S.H. Acid-Functionalized Magnetic Nanoparticle as Heterogeneous Catalysts for Biodiesel Synthesis. J. Phys. Chem. C 2015, 119, 26020–26028. [Google Scholar] [CrossRef]
- Wang, H.; Shrestha, T.B.; Basel, M.T.; Pyle, M.; Toledo, Y.; Konecny, A.; Thapa, P.; Ikenberry, M.; Hohn, K.L.; Chikan, V.; et al. Hexagonal magnetite nanoprisms: Preparation, characterization and cellular uptake. J. Mater. Chem. B 2015, 3, 4647–4653. [Google Scholar] [CrossRef]
- Konecny, A.P.; Covarrubias, J.; Wang, H. Magnetic Nanoparticle Design and Application in Magnetic Hyperthermia. In Magnetic Nanomaterials: Applications in Catalysis and Life Sciences; Bossmann, S.H., Wang, H., Eds.; RSC: London, UK, 2017. [Google Scholar]
- Abayaweera, G.S.; Wang, H.; Shrestha, T.B.; Yu, J.; Angle, K.; Thapa, P.; Malalasekera, A.P.; Maurmann, L.; Troyer, D.L.; Bossmann, S.H. Synergy of iron chelators and therapeutic peptide sequences delivered via a magnetic nanocarrier. J. Funct. Biomater. 2017, 8, 23. [Google Scholar] [CrossRef]
- Hasany, S.F.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci. Nanotechnol. 2012, 2, 148–158. [Google Scholar] [CrossRef]
- Charinpanitkul, T.; Sano, N.; Muthakarn, P.; Tanthapanichakoon, W. Enhancing effect of monoolein surfactant on carbon nanoparticle synthesis by arc discharge in liquid. Mater. Res. Bull. 2009, 44, 324–327. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhou, Y. Pitfalls and Challenges in Nanotoxicology: A Case of Cobalt Ferrite (CoFe2O4) Nanocomposites. Chem. Res. Toxicol. 2017, 30, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Odio, O.F.; Reguera, E. Nanostructured spinel ferrites: Synthesis, functionalization, nanomagnetism and environmental applications. In Magnetic Spinels—Synthesis, Properties and Applications; InTechOpen: London, UK, 2017; pp. 185–216. [Google Scholar]
- Piao, Y.; Kim, J.; Na, H.B.; Kim, D.; Baek, J.S.; Ko, M.K.; Lee, J.H.; Shokouhimehr, M.; Hyeon, T. Wrap–bake–peel process for nanostructural transformation from β-FeOOH nanorods to biocompatible iron oxide nanocapsules. Nat. Mater. 2008, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.B.K.; Owen, J.S.; Peters, B. Nucleation and Growth Kinetics from LaMer Burst Data. J. Phys. Chem. A 2017, 121, 7511–7517. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wang, C.; Xie, J.; Sun, S. Synthesis and stabilization of monodisperse Fe nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef]
- Peng, S.; Sun, S. Synthesis and characterization of monodisperse hollow Fe3O4 nanoparticles. Angew. Chem. 2007, 46, 4155–4158. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971. [Google Scholar] [CrossRef]
- Willard, M.; Kurihara, L.; Carpenter, E.; Calvin, S.; Harris, V. Chemically prepared magnetic nanoparticles. Int. Mater. Rev. 2004, 49, 125–170. [Google Scholar] [CrossRef]
- Plank, C.; Schillinger, U.; Scherer, F.; Bergemann, C.; Rémy, J.-S.; Krötz, F.; Anton, M.; Lausier, J.; Rosenecker, J. The magnetofection method: Using magnetic force to enhance gene delivery. Biol. Chem. 2003, 384, 737–747. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169. [Google Scholar]
- Kalantari, K.; Ahmad, M.B.; Shameli, K.; Hussein, M.Z.B.; Khandanlou, R.; Khanehzaei, H. Size-controlled synthesis of Fe3O4 magnetic nanoparticles in the layers of montmorillonite. J. Nanomater. 2014, 2014, 181. [Google Scholar] [CrossRef]
- Sarkar, S.; Choudhury, P.; Dinda, S.; Das, P.K. Tailor-Made Self-Assemblies from Functionalized Amphiphiles: Diversity and Applications. Langmuir 2018, 34, 10449–10468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.K.; Mitra, S.; Mukhopadhyay, R. Dynamic Landscape in Self-Assembled Surfactant Aggregates. Langmuir 2019. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.; Oh, M.C.; Ahn, B. Alkaline metal reagent-assisted synthesis of monodisperse iron oxide nanostructures. Metals 2018, 8, 107. [Google Scholar] [CrossRef]
- Ortiz-Quinonez, J.-L.; Pal, U.; Villanueva, M.S. Structural, Magnetic, and Catalytic Evaluation of Spinel Co, Ni, and Co-Ni Ferrite Nanoparticles Fabricated by Low-Temperature Solution Combustion Process. ACS Omega 2018, 3, 14986–15001. [Google Scholar] [CrossRef]
- Piche, D.; Tavernaro, I.; Fleddermann, J.; Lozano, J.G.; Varambhia, A.; Maguire, M.L.; Koch, M.; Ukai, T.; Hernandez Rodriguez, A.J.; Jones, L.; et al. Targeted T1 Magnetic Resonance Imaging Contrast Enhancement with Extraordinarily Small CoFe2O4 Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 6724–6740. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Zong, Y.; Deng, X.; Zhao, H.; Feng, J.; He, M.; Li, X.; Peng, Y.; Zheng, X. Crystalline-Amorphous Permalloy@Iron Oxide Core-Shell Nanoparticles Decorated on Graphene as High-Efficiency, Lightweight, and Hydrophobic Microwave Absorbents. ACS Appl. Mater. Interfaces 2019, 11, 6374–6383. [Google Scholar] [CrossRef]
- Udukala, D.N. Protease Assays for Cancer Diagnostics. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2014. [Google Scholar]
- Udukala, D.N.; Wang, H.; Wendel, S.O.; Malalasekera, A.P.; Samarakoon, T.N.; Yapa, A.S.; Abayaweera, G.; Basel, M.T.; Maynez, P.; Ortega, R.; et al. Early breast cancer screening using iron/iron oxide-based nanoplatforms with sub-femtomolar limits of detection. Beilstein J. Nanotechnol. 2016, 7, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Voelz, B.E.; Kalubowilage, M.; Bossmann, S.H.; Troyer, D.L.; Chebel, R.C.; Mendonca, L.G.D. Associations between activity of arginase or matrix metalloproteinase-8 (MMP-8) and metritis in periparturient dairy cattle. Theriogenology 2017, 97, 83–88. [Google Scholar] [CrossRef]
- Wang, H.; Udukala, D.N.; Samarakoon, T.N.; Basel, M.T.; Kalita, M.; Abayaweera, G.; Manawadu, H.; Malalasekera, A.; Robinson, C.; Villanueva, D. Nanoplatforms for highly sensitive fluorescence detection of cancer-related proteases. Photochem. Photobiol. Sci. 2014, 13, 231–240. [Google Scholar] [CrossRef]
- Choi, C.-J.; Dong, X.-L.; Kim, B.-K. Microstructure and magnetic properties of Fe nanoparticles synthesized by chemical vapor condensation. Mater. Trans. 2001, 42, 2046–2049. [Google Scholar] [CrossRef]
- Goodwin, S.C.; Bittner, C.A.; Peterson, C.L.; Wong, G. Single-dose toxicity study of hepatic intra-arterial infusion of doxorubicin coupled to a novel magnetically targeted drug carrier. Toxicol. Sci. 2001, 60, 177–183. [Google Scholar] [CrossRef]
- Steinfeld, U.; Pauli, C.; Kaltz, N.; Bergemann, C.; Lee, H.-H. T lymphocytes as potential therapeutic drug carrier for cancer treatment. Int. J. Pharm. 2006, 311, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, C.J.; Steiert, M.; Talmadge, J.E.; Derfus, A.M.; Barry, S.E. Targeted nanoparticles for detecting and treating cancer. Drug Dev. Res. 2006, 67, 70–93. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, H.; Hyung, W.; Park, S.-B.; Haam, S. Magnetic PECA nanoparticles as drug carriers for targeted delivery: Synthesis and release characteristics. J. Microencapsul. 2006, 23, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuan, J.; Yuan, W.; Sui, X.; Wu, S.; Li, Z.; Shen, D. Synthesis, characterization, and controllable drug release of pH-sensitive hybrid magnetic nanoparticles. J. Magn. Magn. Mater. 2009, 321, 2799–2804. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, J.; Yuan, W.; Zhou, M.; Wu, S.; Li, Z.; Xing, X.; Shen, D. Synthesis and characterization of multi-functional hybrid magnetite nanoparticles with biodegradability, superparamagnetism, and fluorescence. Mater. Lett. 2009, 63, 1567–1570. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, P.K.; Behera, S.; Lockey, R.F.; Mohapatra, S.; Mohapatra, S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, Z.-L.; Yang, X.-X.; Huang, P.; Zhou, X.-P.; Du, X.-X. Magnetic chitosan nanoparticles as a drug delivery system for targeting photodynamic therapy. Nanotechnology 2009, 20, 135102. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, N.; Sun, C.; Fichtenholtz, A.; Gunn, J.; Fang, C.; Zhang, M. Methotrexate-immobilized poly (ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small 2006, 2, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Podaru, G.; Chikan, V. Magnetism in Nanomaterials: Heat and Force from Colloidal Magnetic Particles. In Magnetic Nanomaterials: Applications in Catalysis and Life Sciences; Bossmann, S.H., Wang, H., Eds.; Royal Society of Chemistry: London, UK, 2017; Volume 26. [Google Scholar]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sciences 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Karponis, D.; Azzawi, M.; Seifalian, A. An arsenal of magnetic nanoparticles; perspectives in the treatment of cancer. Nanomedicine 2016, 11, 2215–2232. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Y.; Yan, C.; Song, L.; Wen, S.; Zang, F.; Chen, G.; Ding, Q.; Yan, C.; Gu, N. High-performance PEGylated Mn–Zn ferrite nanocrystals as a passive-targeted agent for magnetically induced cancer theranostics. Biomaterials 2014, 35, 9126–9136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K.; et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc. 2009, 132, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Z.; Li, M.; Ren, J.; Qu, X. Fluorescent protein capped mesoporous nanoparticles for intracellular drug delivery and imaging. Chem. A Eur. J. 2013, 19, 15378–15383. [Google Scholar] [CrossRef]
- Hwu, J.R.; Lin, Y.S.; Josephrajan, T.; Hsu, M.-H.; Cheng, F.-Y.; Yeh, C.-S.; Su, W.-C.; Shieh, D.-B. Targeted paclitaxel by conjugation to iron oxide and gold nanoparticles. J. Am. Chem. Soc. 2008, 131, 66–68. [Google Scholar] [CrossRef]
- Bai, Y.-Y.; Zheng, S.; Zhang, L.; Xia, K.; Gao, X.; Li, Z.-H.; Li, C.; He, N.; Ju, S. Non-invasively evaluating therapeutic response of nanorod-mediated photothermal therapy on tumor angiogenesis. J. Biomed. Nanotechnol. 2014, 10, 3351–3360. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Pradhan, A.; Banerjee, R.; Bahadur, D. Dual pH and temperature stimuli-responsive magnetic nanohydrogels for thermo-chemotherapy. J. Nanosci. Nanotechnol. 2014, 14, 4082–4089. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalubowilage, M.; Janik, K.; Bossmann, S.H. Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia. Appl. Sci. 2019, 9, 2927. https://doi.org/10.3390/app9142927
Kalubowilage M, Janik K, Bossmann SH. Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia. Applied Sciences. 2019; 9(14):2927. https://doi.org/10.3390/app9142927
Chicago/Turabian StyleKalubowilage, Madumali, Katharine Janik, and Stefan H. Bossmann. 2019. "Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia" Applied Sciences 9, no. 14: 2927. https://doi.org/10.3390/app9142927





