Bone Tissue Engineering Using Human Cells: A Comprehensive Review on Recent Trends, Current Prospects, and Recommendations
Abstract
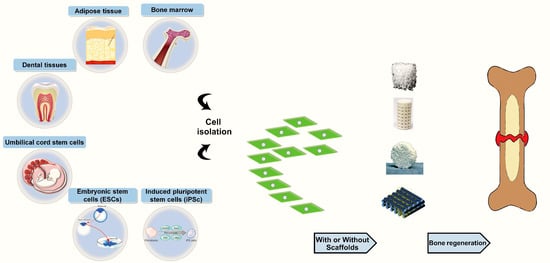
:1. Introduction
2. Differentiated Cells
2.1. Osteoblasts
2.1.1. General Information
2.1.2. Application in BTE
2.2. Osteoclasts
2.2.1. General Information
2.2.2. Application in BTE
3. Undifferentiated Cells (Stem Cells)
3.1. Embryonic Stem Cells (ESCs)
3.1.1. General Information
3.1.2. Application in BTE
3.2. Induced Pluripotent Stem Cells (iPSCs)
3.2.1. General Information
3.2.2. Applications in BTE
3.3. Fetal Stem Cells
3.3.1. Amniotic Fluid-Derived Stem Cells (AFSCs)
3.3.1.1. General Information
3.3.1.2. Applications in BTE
3.3.2. Amniotic Membrane Stem Cells (AMSCs)
3.3.2.1. General Information
3.3.2.2. Applications in BTE
3.3.3. Chorion Mesenchymal Stem Cells (Ch-MSCs)
3.3.3.1. General Information
3.3.3.2. Applications in BTE
3.4. Adult Stem Cells
3.4.1. Bone Marrow Stem Cells (BM-MSCs)
3.4.1.1. General Information
3.4.1.2. Applications in BTE
3.4.2. Adipose Tissue-Derived Mesenchymal Stem Cells (ASCs)
3.4.2.1. General Information
3.4.2.2. Applications in BTE
3.4.3. Umbilical Cord Mesenchymal Stem Cells (UC-MSCs)
3.4.3.1. Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJMSCs)
3.4.3.2. Umbilical Cord Blood-Derived Mesenchymal Stem Cells (UCB-MSCs)
3.4.3.3. Endothelial Progenitor Cells (EPCs)
3.4.3.4. Umbilical Cord Perivascular Cells (HUCPVCs)
3.4.4. Dental Stem/Progenitor Cells
3.4.4.1. General Information
3.4.4.2. Applications in BTE
3.4.5. Periosteum-Derived Progenitor Cells (PDPCs)
3.4.5.1. General Information
3.4.5.2. Applications in BTE
3.4.6. Synovium-Derived Mesenchymal Stem Cells
3.4.6.1. General Information
3.4.6.2. Applications in BTE
3.4.7. Muscle-Derived Stem Cells (MD-SCs)
3.4.7.1. General Information
3.4.7.2. Applications in BTE
4. The Future—Where Are We Going?
Author Contributions
Funding
Conflicts of Interest
References
- Gordeladze, J.O.; Haugen, H.J.; Lyngstadaas, S.P.; Reseland, J.E. Bone Tissue Engineering: State of the Art, Challenges, and Prospects. In Tissue Engineering for Artificial Organs, 1st ed.; Wiley India Private Ltd.: Chennai, India, 2017; pp. 525–551. [Google Scholar]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Xin, X.; Urso, M.; Jiang, X.; Wang, L.; Barnes, B.; Lichtler, A.C.; Rowe, D.W.; Nukavarapu, S.P. A potential translational approach for bone tissue engineering through endochondral ossification. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3925–3928. [Google Scholar]
- Sundelacruz, S.; Kaplan, D.L. Stem cell-and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kerpedjieva, S.S. Acceleration of wound healing by multiple growth factors and cytokines secreted from multipotential stromal cells/mesenchymal stem cells. Adv. Wound Care 2012, 1, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Stappenbeck, T.S.; Miyoshi, H. The role of stromal stem cells in tissue regeneration and wound repair. Science 2009, 324, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, E.M.; Glowacki, J. Cell-free and cell-based approaches for bone regeneration. Nat. Rev. Rheumatol. 2009, 5, 685–697. [Google Scholar] [CrossRef]
- Bruder, S.P.; Fox, B.S. Tissue Engineering of Bone: Cell Based Strategies. Clin. Orthop. Relat. Res. 1999, 367, S68–S83. [Google Scholar] [CrossRef]
- Harada, N.; Watanabe, Y.; Sato, K.; Abe, S.; Yamanaka, K.; Sakai, Y.; Kaneko, T.; Matsushita, T. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 2014, 35, 7800–7810. [Google Scholar] [CrossRef]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef] [Green Version]
- Gawlitta, D.; Farrell, E.; Malda, J.; Creemers, L.B.; Alblas, J.; Dhert, W.J. Modulating endochondral ossification of multipotent stromal cells for bone regeneration. Tissue Eng. Part B 2010, 16, 385–395. [Google Scholar] [CrossRef]
- Tortelli, F.; Tasso, R.; Loiacono, F.; Cancedda, R. The development of tissue-engineered bone of different origin through endochondral and intramembranous ossification following the implantation of mesenchymal stem cells and osteoblasts in a murine model. Biomaterials 2010, 31, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Lotfibakhshaiesh, N.; Hill, R.G.; Milan, P.B.; Hamzehlou, S.; Joghataei, M.T.; Mozafari, M. When size matters: Biological response to strontium- and cobalt-substituted bioactive glass particles. Mater. Today Proc. 2018, 5, 15768–15775. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses entering the mainstream. Drug Discov. Today 2018, 23, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering. Materials 2017, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef]
- Miola, M.; Pakzad, Y.; Banijamali, S.; Kargozar, S.; Vitale-Brovarone, C.; Yazdanpanah, A.; Bretcanu, O.; Ramedani, A.; Vernè, E.; Mozafari, M. Glass-ceramics for cancer treatment: So close, or yet so far? Acta Biomater. 2018, 83, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Marolt, D.; Knezevic, M.; Vunjak-Novakovic, G. Bone tissue engineering with human stem cells. Stem Cell Res. Ther. 2010, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muschler, G.F.; Nakamoto, C.; Griffith, L.G. Engineering principles of clinical cell-based tissue engineering. JBJS 2004, 86, 1541–1558. [Google Scholar] [CrossRef]
- Toscani, D.; Bolzoni, M.; Accardi, F.; Aversa, F.; Giuliani, N. The osteoblastic niche in the context of multiple myeloma. Ann. New York Acad. Sci. 2014, 1335, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E. Osteoblasts: novel roles in orchestration of skeletal architecture. Int. J. Biochem. Cell Biol. 2003, 35, 1301–1305. [Google Scholar] [CrossRef]
- Panaroni, C.; Tzeng, Y.-s.; Saeed, H.; Wu, J.Y. Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches. Curr. Osteoporos. Rep. 2014, 12, 22–32. [Google Scholar] [CrossRef]
- Deckers, M.M.; Van Bezooijen, R.L.; Van Der Horst, G.; Hoogendam, J.; van der Bent, C.; Papapoulos, S.E.; Löwik, C.W. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 2002, 143, 1545–1553. [Google Scholar] [CrossRef]
- Vortkamp, A.; Pathi, S.; Peretti, G.M.; Caruso, E.M.; Zaleske, D.J.; Tabin, C.J. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech. Dev. 1998, 71, 65–76. [Google Scholar] [CrossRef]
- Schroeder, T.M.; Jensen, E.D.; Westendorf, J.J. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res. Part C 2005, 75, 213–225. [Google Scholar] [CrossRef]
- Aubin, J.E. Regulation of osteoblast formation and function. Rev. Endocr. Metab. Disord. 2001, 2, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of gene expression in osteoblasts. Biofactors 2010, 36, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Reviews. Mol. Cell Biol. 2012, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, N.; Hul, M.; Maes, C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res. Part C 2013, 99, 170–191. [Google Scholar] [CrossRef]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; He, B.; Jiang, Y.; Kobayashi, T.; Sorocéanu, M.A.; Zhao, J.; Su, H.; Tong, X.; Amizuka, N.; Gupta, A. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1–34. J. Clin. Investig. 2005, 115, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F.; Colvard, D.S.; Berg, N.J.; Graham, M.L.; Mann, K.G.; Spelsberg, T.C.; Riggs, B.L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science 1988, 241, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Johari, B.; Ahmadzadehzarajabad, M.; Azami, M.; Kazemi, M.; Soleimani, M.; Kargozar, S.; Hajighasemlou, S.; Farajollahi, M.M.; Samadikuchaksaraei, A. Repair of rat critical size calvarial defect using osteoblast-like and umbilical vein endothelial cells seeded in gelatin/hydroxyapatite scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hashemian, S.J.; Soleimani, M.; Milan, P.B.; Askari, M.; Khalaj, V.; Samadikuchaksaraie, A.; Hamzehlou, S.; Katebi, A.R.; Latifi, N.; et al. Acceleration of bone regeneration in bioactive glass/gelatin composite scaffolds seeded with bone marrow-derived mesenchymal stem cells over-expressing bone morphogenetic protein-7. Mater. Sci. Eng. C 2017, 75, 688–698. [Google Scholar] [CrossRef]
- Johari, B.; Kadivar, M.; Lak, S.; Gholipourmalekabadi, M.; Urbanska, A.M.; Mozafari, M.; Ahmadzadehzarajabad, M.; Azarnezhad, A.; Afshari, S.; Zargan, J.; et al. Osteoblast-seeded bioglass/gelatin nanocomposite: a promising bone substitute in critical-size calvarial defect repair in rat. Int. J. Artif. Organs 2016, 39, 524–533. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Samadikuchaksaraie, A.; Hill, R.G.; Shah, P.A.; Milan, P.B.; Mozafari, M.; Fathi, M.; Joghataei, M.T. Synthesis, physico-chemical and biological characterization of strontium and cobalt substituted bioactive glasses for bone tissue engineering. J. Non-Cryst. Solids 2016, 449, 133–140. [Google Scholar] [CrossRef]
- Siggelkow, H.; Rebenstorff, K.; Kurre, W.; Niedhart, C.; Engel, I.; Schulz, H.; Atkinson, M.J.; Hüfner, M. Development of the osteoblast phenotype in primary human osteoblasts in culture: comparison with rat calvarial cells in osteoblast differentiation. J. Cell. Biochem. 1999, 75, 22–35. [Google Scholar] [CrossRef]
- Meyer, U.; Joos, U.; Wiesmann, H. Biological and biophysical principles in extracorporal bone tissue engineering. Int. J. Oral Maxillofac. Surg. 2004, 33, 325–332. [Google Scholar] [CrossRef]
- Jayakumar, P.; Di Silvio, L. Osteoblasts in bone tissue engineering. Proc. Inst. Mech. Eng. Part H 2010, 224, 1415–1440. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, H.P.; Nazer, N.; Klatt, C.; Szuwart, T.; Meyer, U. Bone tissue engineering by primary osteoblast-like cells in a monolayer system and 3-dimensional collagen gel. J. Oral Maxillofac. Surg. 2003, 61, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Swain, R.J.; Evans, N.D.; Boonrungsiman, S.; Jell, G.; Ball, M.D.; Shean, T.A.V.; Oyen, M.L.; Porter, A.; Stevens, M.M. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat. Mater. 2009, 8, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, A.; Alvarez, J.; Greenfield, E.; Teti, A.; Grano, M.; Colucci, S.; Zambonin-Zallone, A.; Ross, F.; Teitelbaum, S.; Cheresh, D. Binding of osteopontin to the osteoclast integrin α v β 3. Osteoporos. Int. 1993, 3, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Kostakis, P.; Pan, B.; Farrugia, A.; Gronthos, S.; Evdokiou, A.; Harrison, K.; Findlay, D.M.; Zannettino, A.C. RANKL expression is related to the differentiation state of human osteoblasts. J. Bone Miner. Res. 2003, 18, 1088–1098. [Google Scholar] [CrossRef]
- Feng, X. RANKing intracellular signaling in osteoclasts. IUBMB Life 2005, 57, 389–395. [Google Scholar] [CrossRef]
- Vaananen, H.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar]
- Troen, B.R. The role of cathepsin K in normal bone resorption. Drug News Perspect 2004, 17, 19–28. [Google Scholar] [CrossRef]
- Detsch, R.; Boccaccini, A.R. The role of osteoclasts in bone tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, F.; Pujic, N.; Liu, Y.; Laroche, N.; Vico, L.; Cancedda, R. Osteoblast and osteoclast differentiation in an in vitro three-dimensional model of bone. Tissue Eng. Part A 2009, 15, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Karpov, M.; Laczka, M.; Leboy, P.S.; Osyczka, A.M. Sol–gel bioactive glasses support both osteoblast and osteoclast formation from human bone marrow cells. J. Biomed. Mater. Res. Part A 2008, 84, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; van den Bergh, W.; Kim, T.B.; Lee, P.D.; Jones, J.R.; Mitchell, C.A. Bioactive glass foam scaffolds are remodelled by osteoclasts and support the formation of mineralized matrix and vascular networks in vitro. Adv. Healthc. Mater. 2013, 2, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, Q. An essential requirement for osteoclasts in refined bone-like tissue reconstruction in vitro. Med. Hypotheses 2006, 67, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Udagawa, N.; Suda, T. A New Member of Tumor Necrosis Factor Ligand Family, ODF/OPGL/TRANCE/RANKL, Regulates Osteoclast Differentiation and Function. Biochem. Biophys. Res. Commun. 1999, 256, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G. Stem cells in tissue engineering. Nature 2001, 414, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Kalishman, J.; Golos, T.G.; Durning, M.; Harris, C.P.; Becker, R.A.; Hearn, J.P. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. USA 1995, 92, 7844–7848. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachibana, M.; Amato, P.; Sparman, M.; Gutierrez, N.M.; Tippner-Hedges, R.; Ma, H.; Kang, E.; Fulati, A.; Lee, H.-S.; Sritanaudomchai, H. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013, 153, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.H.; Brick, D.J.; Nethercott, H.E.; Stover, A.E. Traditional human embryonic stem cell culture. In Human Pluripotent Stem Cells; Humana Press: New York, NY, USA, 2011; pp. 107–123. [Google Scholar]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.; Robey, P.G. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Preparation, culture, and immortalization of mouse embryonic fibroblasts. Curr. Protoc. Mol. Biol. 2005, 70, 28.1.1–28.1.8. [Google Scholar]
- Richards, M.; Fong, C.-Y.; Chan, W.-K.; Wong, P.-C.; Bongso, A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002, 20, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Mallon, B.S.; Park, K.-Y.; Chen, K.G.; Hamilton, R.S.; McKay, R.D. Toward xeno-free culture of human embryonic stem cells. Int. J. Biochem. Cell Biol. 2006, 38, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Meregalli, M.; Farini, A.; Torrente, Y. Stem cell therapy for neuromuscular diseases. In Stem Cells in Clinic and Research; InTech: London, UK, 2011. [Google Scholar]
- Kawaguchi, J.; Mee, P.J.; Smith, A.G. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 2005, 36, 758–769. [Google Scholar] [CrossRef]
- Sottile, V.; Thomson, A.; McWhir, J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells 2003, 5, 149–155. [Google Scholar] [CrossRef]
- Karp, J.M.; Ferreira, L.S.; Khademhosseini, A.; Kwon, A.H.; Yeh, J.; Langer, R.S. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells 2006, 24, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Jukes, J.M.; Both, S.K.; Leusink, A.; Lotus, M.T.; Van Blitterswijk, C.A.; De Boer, J. Endochondral bone tissue engineering using embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6840–6845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Peppo, G.M.; Svensson, S.; Lennerås, M.; Synnergren, J.; Stenberg, J.; Strehl, R.; Hyllner, J.; Thomsen, P.; Karlsson, C. Human embryonic mesodermal progenitors highly resemble human mesenchymal stem cells and display high potential for tissue engineering applications. Tissue Eng. Part A 2010, 16, 2161–2182. [Google Scholar] [CrossRef] [PubMed]
- Taiani, J.; Buie, H.; Campbell, G.; Manske, S.; Krawetz, R.; Rancourt, D.; Boyd, S.; Matyas, J. Embryonic stem cell therapy improves bone quality in a model of impaired fracture healing in the mouse; tracked temporally using in vivo micro-CT. Bone 2014, 64, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Kiskinis, E.; Verstappen, G.; Gu, H.; Boulting, G.; Smith, Z.D.; Ziller, M.; Croft, G.F.; Amoroso, M.W.; Oakley, D.H. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 2011, 144, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Chou, B.-K.; Mali, P.; Huang, X.; Ye, Z.; Dowey, S.N.; Resar, L.M.; Zou, C.; Zhang, Y.A.; Tong, J.; Cheng, L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011, 21, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Narayanan, K.; Leong, M.F.; Wan, A.C. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials 2014, 35, 6006–6014. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.-Y.; Weick, J.P.; Yu, J.; Ma, L.-X.; Zhang, X.-Q.; Thomson, J.A.; Zhang, S.-C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Yang, B.; Hu, K.; Cao, C.; Man, Y.; Wang, P. Deriving osteogenic cells from induced pluripotent stem cells for bone tissue engineering. Tissue Eng. Part B 2017, 23, 1–8. [Google Scholar] [CrossRef]
- Phillips, M.D.; Kuznetsov, S.A.; Cherman, N.; Park, K.; Chen, K.G.; McClendon, B.N.; Hamilton, R.S.; McKay, R.D.G.; Chenoweth, J.G.; Mallon, B.S.; et al. Directed Differentiation of Human Induced Pluripotent Stem Cells Toward Bone and Cartilage: In Vitro Versus In Vivo Assays. Stem Cells Transl. Med. 2014, 3, 867–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, S.; Liu, C.; Krettek, C.; Jagodzinski, M. The Application of Induced Pluripotent Stem Cells for Bone Regeneration: Current Progress and Prospects. Tissue Eng. Part B 2013, 20, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Inamura, M.; Kawabata, K.; Sakurai, F.; Yamanishi, K.; Hayakawa, T.; Mizuguchi, H. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells 2009, 27, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Matsumi, Y.; Hoshikawa, Y.; Takubo, K.; Ryoke, K.; Shiota, G. Involvement of microRNAs in regulation of osteoblastic differentiation in mouse induced pluripotent stem cells. PLoS ONE 2012, 7, e43800. [Google Scholar] [CrossRef]
- Kao, C.-L.; Tai, L.-K.; Chiou, S.-H.; Chen, Y.-J.; Lee, K.-H.; Chou, S.-J.; Chang, Y.-L.; Chang, C.-M.; Chen, S.-J.; Ku, H.-H.; et al. Resveratrol Promotes Osteogenic Differentiation and Protects Against Dexamethasone Damage in Murine Induced Pluripotent Stem Cells. Stem Cells Dev. 2009, 19, 247–258. [Google Scholar] [CrossRef]
- Bilousova, G.; Jun, D.H.; King, K.B.; De Langhe, S.; Chick, W.S.; Torchia, E.C.; Chow, K.S.; Klemm, D.J.; Roop, D.R.; Majka, S.M. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells 2011, 29, 206–216. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; De Mel, S.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447–1460. [Google Scholar] [CrossRef]
- Ilancheran, S.; Moodley, Y.; Manuelpillai, U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta 2009, 30, 2–10. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.; Claas, F.; Fibbe, W.E.; Kanhai, H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Hammerman, M.R. Classic and current opinion in embryonic organ transplantation. Curr. Opin. Organ Transplant. 2014, 19, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Van de Ven, C.; Collins, D.; Bradley, M.B.; Morris, E.; Cairo, M.S. The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp. Hematol. 2007, 35, 1753–1765. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Trohatou, O.; Anagnou, N.P. Amniotic Fluid and Amniotic Membrane Stem Cells: Marker Discovery. Stem Cells Int. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Noort, W.; Scherjon, S.; Kleijburg-Van Der Keur, C.; Kruisselbrink, A.; Van Bezooijen, R.; Beekhuizen, W.; Willemze, R.; Kanhai, H.; Fibbe, W. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. haematologica 2003, 88, 845–852. [Google Scholar]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Abdulrazzak, H.; Moschidou, D.; Jones, G.; Guillot, P.V. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J. R. Soc. Interface 2010, 7, S689–S706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gucciardo, L.; Lories, R.; Ochsenbein-Kölble, N.; Done, E.; Zwijsen, A.; Deprest, J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, A.; Perry, T.E.; Barnes, C.M.; Oh, J.-T.; Ziegler, M.M.; Fishman, S.J.; Fauza, D.O. The placenta as a cell source in fetal tissue engineering. J. Pediatric Surg. 2002, 37, 995–999. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Noort, W.A.; Kruisselbrink, A.B.; Scherjon, S.A.; Beekhuizen, W.; Willemze, R.; Kanhai, H.H.; Fibbe, W.E. Nonexpanded primary lung and bone marrow–derived mesenchymal cells promote the engraftment of umbilical cord blood–derived CD34+ cells in NOD/SCID mice. Exp. Hematol. 2003, 31, 881–889. [Google Scholar] [CrossRef]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karahuseyinoglu, S.; Cinar, O.; Kilic, E.; Kara, F.; Akay, G.G.; Demiralp, D.Ö.; Tukun, A.; Uckan, D.; Can, A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells 2007, 25, 319–331. [Google Scholar] [CrossRef]
- Ilancheran, S.; Michalska, A.; Peh, G.; Wallace, E.M.; Pera, M.; Manuelpillai, U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol. Reprod. 2007, 77, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, F.; Brizzi, L.; Bernabei, P.; Gheri, G.; Di, S.L.; Nutini, L.; Lisi, E.; Di, M.T.; Cariati, E. Identification of hematopoietic progenitor cells in human amniotic fluid before the 12th week of gestation. Ital. J. Anat. Embryol. 1993, 98, 119–126. [Google Scholar]
- Streubel, B.; Martucci-Ivessa, G.; Fleck, T.; Bittner, R. In vitro transformation of amniotic cells to muscle cells--background and outlook. Wien. Med. Wochenschr. 1996, 146, 216–217. [Google Scholar] [PubMed]
- Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [Green Version]
- Rehni, A.K.; Singh, N.; Jaggi, A.S.; Singh, M. Amniotic fluid derived stem cells ameliorate focal cerebral ischaemia-reperfusion injury induced behavioural deficits in mice. Behav. Brain Res. 2007, 183, 95–100. [Google Scholar] [CrossRef]
- De Coppi, P.; Callegari, A.; Chiavegato, A.; Gasparotto, L.; Piccoli, M.; Taiani, J.; Pozzobon, M.; Boldrin, L.; Okabe, M.; Cozzi, E. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J. Urol. 2007, 177, 369–376. [Google Scholar] [CrossRef]
- Klein, J.D.; Turner, C.G.; Ahmed, A.; Steigman, S.A.; Zurakowski, D.; Fauza, D.O. Chest wall repair with engineered fetal bone grafts: an efficacy analysis in an autologous leporine model. J. Pediatr. Surg. 2010, 45, 1354–1360. [Google Scholar] [CrossRef]
- Shaw, S.; Bollini, S.; Nader, K.A.; Gastadello, A.; Mehta, V.; Filppi, E.; Cananzi, M.; Gaspar, H.B.; Qasim, W.; De Coppi, P. Autologous transplantation of amniotic fluid-derived mesenchymal stem cells into sheep fetuses. Cell Transplant. 2011, 20, 1015–1031. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Z.; Cheng, D.; Peng, S.; Wang, H. Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells. PLoS ONE 2011, 6, e19964. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Lee, S.J.; Gomes, M.E.; Reis, R.L.; Atala, A.; Yoo, J.J. Amniotic fluid-derived stem cells as a cell source for bone tissue engineering. Tissue Eng. Part A 2012, 18, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Tejwani, S.; Kolari, R.S.; Sangwan, V.S.; Rao, G.N. Role of amniotic membrane graft for ocular chemical and thermal injuries. Cornea 2007, 26, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Johari, H.G.; Eskandari, S. Effect of amniotic membrane on graft take in extremity burns. Burns 2013, 39, 1137–1141. [Google Scholar] [CrossRef]
- Rinastiti, M.; Santoso, A.; Sosroseno, W. Histological evaluation of rabbit gingival wound healing transplanted with human amniotic membrane. Int. J. Oral Maxillofac. Surg. 2006, 35, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Cargnoni, A.; Di Marcello, M.; Campagnol, M.; Nassuato, C.; Albertini, A.; Parolini, O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant. 2009, 18, 1147–1159. [Google Scholar] [CrossRef]
- Ravishanker, R.; Bath, A.; Roy, R. “Amnion Bank”—The use of long term glycerol preserved amniotic membranes in the management of superficial and superficial partial thickness burns. Burns 2003, 29, 369–374. [Google Scholar] [CrossRef]
- Serena, T.E.; Carter, M.J.; Le, L.T.; Sabo, M.J.; DiMarco, D.T. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014, 22, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Sharma, Y.; Maria, A.; Kaur, P. Effectiveness of human amnion as a graft material in lower anterior ridge vestibuloplasty: a clinical study. J. Maxillofac. Oral Surg. 2011, 10, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Soncini, M.; Kaneko, Y.; Hess, D.C.; Parolini, O.; Borlongan, C.V. Amnion: A potent graft source for cell therapy in stroke. Cell Transplant. 2009, 18, 111–118. [Google Scholar] [CrossRef]
- Insausti, C.L.; Blanquer, M.; García-Hernández, A.M.; Castellanos, G.; Moraleda, J.M. Amniotic membrane-derived stem cells: Immunomodulatory properties and potential clinical application. Stem Cells Cloning Adv. Appl. 2014, 7, 53–63. [Google Scholar] [CrossRef]
- Toda, A.; Okabe, M.; Yoshida, T.; Nikaido, T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J. Pharmacol. Sci. 2007, 105, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, K.-B.; Kim, M.K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014, 47, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banas, R.A.; Trumpower, C.; Bentlejewski, C.; Marshall, V.; Sing, G.; Zeevi, A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum. Immunol. 2008, 69, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Soncini, M.; Evangelista, M.; Schmidt, D. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen. Med. 2009, 4, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. Rep. 2006, 2, 133–141. [Google Scholar] [CrossRef]
- Portmann-Lanz, C.B.; Schoeberlein, A.; Huber, A.; Sager, R.; Malek, A.; Holzgreve, W.; Surbek, D.V. Placental mesenchymal stem cells as potential autologous graft for pre-and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 2006, 194, 664–673. [Google Scholar] [CrossRef]
- Muiños-López, E.; Hermida-Gómez, T.; Fuentes-Boquete, I.; de Toro-Santos, J.; Blanco, F.J.; Díaz-Prado, S.M. Human Amniotic Mesenchymal Stromal Cells as Favorable Source for Cartilage Repair. Tissue Eng. Part A 2017, 23, 901–912. [Google Scholar] [CrossRef]
- Horwitz, E.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.; Krause, D.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Alviano, F.; Fossati, V.; Marchionni, C.; Arpinati, M.; Bonsi, L.; Franchina, M.; Lanzoni, G.; Cantoni, S.; Cavallini, C.; Bianchi, F. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 2007, 7, 11. [Google Scholar] [CrossRef]
- Vidade, A.S.; Souza, A.F.; Sangali, J.R.; Sampaio, R.; Bressan, F.F.; Casals, J.B.; Pierre, N.C.; Mançanares, C.A.; Meirelles, F.; Miglino, M.A. The feline amniotic membrane: a valuable alternative source of mesenchymal stem cells. Placenta 2013, 34, A60. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Corradetti, B.; Bizzaro, D.; Magatti, M.; Ressel, L.; Tassan, S.; Parolini, O.; Cremonesi, F. Characterization and potential applications of progenitor-like cells isolated from horse amniotic membrane. J. Tissue Eng. Regen. Med. 2012, 6, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, B.; Meucci, A.; Bizzaro, D.; Cremonesi, F.; Consiglio, A.L. Mesenchymal stem cells from amnion and amniotic fluid in the bovine. Reproduction 2013, 145, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Rendal-Vázquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Multilineage differentiation potential of cells isolated from the human amniotic membrane. J. Cell. Biochem. 2010, 111, 846–857. [Google Scholar] [CrossRef] [Green Version]
- Jiawen, S.; Jianjun, Z.; Jiewen, D.; Dedong, Y.; Hongbo, Y.; Jun, S.; Xudong, W.; Shen, S.G.; Lihe, G. Osteogenic differentiation of human amniotic epithelial cells and its application in alveolar defect restoration. Stem Cells Transl. Med. 2014, 3, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, N.; Rodríguez-Hernández, A.G.; Enríquez-Jiménez, J.; Alcántara-Quintana, L.E.; Fuentes-Mera, L.; Piña-Barba, M.C.; Zepeda-Rodríguez, A.; Ambrosio, J.R. Nukbone® promotes proliferation and osteoblastic differentiation of mesenchymal stem cells from human amniotic membrane. Biochem. Biophys. Res. Commun. 2013, 434, 676–680. [Google Scholar] [CrossRef]
- Si, J.; Dai, J.; Zhang, J.; Liu, S.; Gu, J.; Shi, J.; Shen, S.G.; Guo, L. Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Bourne, G. The foetal membranes: a review of the anatomy of normal amnion and chorion and some aspects of their function. Postgrad. Med J. 1962, 38, 193–201. [Google Scholar] [CrossRef]
- Cross, J.C. Formation of the placenta and extraembryonic membranes. Ann. N. Y. Acad. Sci. 1998, 857, 23–32. [Google Scholar] [CrossRef]
- Malek, A.; Bersinger, N.A. Human placental stem cells: biomedical potential and clinical relevance. J. Stem Cells 2011, 6, 75–92. [Google Scholar]
- González Paz, L.; Carvajal, C.; Cuenca, J.; Alcayaga-Miranda, F.; Figueroa Fernando, E.; Bartolucci, J.; Salazar-Aravena, L.; Khoury, M. Chorion Mesenchymal Stem Cells Show Superior Differentiation, Immunosuppressive, and Angiogenic Potentials in Comparison With Haploidentical Maternal Placental Cells. Stem Cells Transl. Med. 2015, 4, 1109–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusuma, G.D.; Menicanin, D.; Gronthos, S.; Manuelpillai, U.; Abumaree, M.H.; Pertile, M.D.; Brennecke, S.P.; Kalionis, B. Ectopic Bone Formation by Mesenchymal Stem Cells Derived from Human Term Placenta and the Decidua. PLoS ONE 2015, 10, e0141246. [Google Scholar] [CrossRef] [PubMed]
- Wagers, A.J.; Weissman, I.L. Plasticity of Adult Stem Cells. Cell 2004, 116, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.; Piatetzky-Shapiro, I.; Petrakova, K. Osteogenesis in transplants of bone marrow cells. Development 1966, 16, 381–390. [Google Scholar]
- Friedenstein, A.; Chailakhjan, R.; Lalykina, K. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Owen, M. Marrow stromal stem cells. J. Cell Sci. 1988, 1988, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Eleotério, R.B.; Sepúlveda, R.V.; Reis, E.C.; Valente, F.L.; Borges, A.P. Isolation, expansion and differentiation of mesenchymal stromal cells from rabbits’ bone marrow. Pesqui. Veterinária Bras. 2016, 36, 423–430. [Google Scholar] [CrossRef]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011, 52, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Saito, T.; Caplan, A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995, 18, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, E.; Niebur, G.; McHugh, P. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cells Mater. 2012, 23, 901–912. [Google Scholar] [CrossRef]
- Stock, P.; Brückner, S.; Winkler, S.; Dollinger, M.M.; Christ, B. Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury. Int. J. Mol. Sci. 2014, 15, 7004–7028. [Google Scholar] [CrossRef] [PubMed]
- Phadnis, S.M.; Joglekar, M.V.; Dalvi, M.P.; Muthyala, S.; Nair, P.D.; Ghaskadbi, S.M.; Bhonde, R.R.; Hardikar, A.A. Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo. Cytotherapy 2011, 13, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Muñiz, C.; Teodosio, C.; Mayado, A.; Amaral, A.T.; Matarraz, S.; Bárcena, P.; Sanchez, M.L.; Alvarez-Twose, I.; Diez-Campelo, M.; García-Montero, A.C. Ex vivo identification and characterization of a population of CD13 high CD105+ CD45− mesenchymal stem cells in human bone marrow. Stem Cell Res. Ther. 2015, 6, 169. [Google Scholar] [CrossRef]
- Clause, K.C.; Liu, L.J.; Tobita, K. Directed stem cell differentiation: The role of physical forces. Cell Commun. Adhes. 2010, 17, 48–54. [Google Scholar] [CrossRef]
- Shin, H.; Temenoff, J.S.; Bowden, G.C.; Zygourakis, K.; Farach-Carson, M.C.; Yaszemski, M.J.; Mikos, A.G. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg–Gly–Asp modified hydrogels without dexamethasone and β-glycerol phosphate. Biomaterials 2005, 26, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.K.; Capilla, E.; Rosen, C.J.; Gilsanz, V.; Pessin, J.E.; Judex, S.; Rubin, C.T. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. 2009, 24, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-y.; Wu, X.-y.; Tong, J.-b.; Yang, X.-x.; Zhao, J.-l.; Zheng, Q.-f.; Zhao, G.-b.; Ma, Z.-j. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Shen, R.; Song, L.; Lu, M.; Wang, J.; Zhao, S.; Tang, Y.; Meng, X.; Li, Z.; He, Z.-X. Bone Marrow Mesenchymal Stem Cells (BM-MSCs) improve heart function in swine myocardial infarction model through paracrine effects. Sci. Rep. 2016, 6, 28250. [Google Scholar]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Brouki Milan, P.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Part B 2018, 106, 61–72. [Google Scholar] [CrossRef]
- Gronthos, S.; Graves, S.; Ohta, S.; Simmons, P. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood 1994, 84, 4164–4173. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Kirkham, G.; Cartmell, S. Genes and proteins involved in the regulation of osteogenesis. In Topics in Tissue Engineering; Oulu University: Oulu, Finland, 2007; Volume 3. [Google Scholar]
- Phinney, D.G. Biochemical heterogeneity of mesenchymal stem cell populations: Clues to their therapeutic efficacy. Cell Cycle 2007, 6, 2884–2889. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Zwingenberger, S.; Gibon, E.; Goodman, S.B.; Li, G. Mesenchymal stem cells homing to improve bone healing. J. Orthop. Transl. 2017, 9, 19–27. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Krebsbach, P.H.; Satomura, K.; Kerr, J.; Riminucci, M.; Benayahu, D.; Robey, P.G. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J. Bone Miner. Res. 1997, 12, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Zannettino, A.C.; Hay, S.J.; Shi, S.; Graves, S.E.; Kortesidis, A.; Simmons, P.J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 2003, 116, 1827–1835. [Google Scholar] [CrossRef] [Green Version]
- Stockmann, P.; Park, J.; Von Wilmowsky, C.; Nkenke, E.; Felszeghy, E.; Dehner, J.-F.; Schmitt, C.; Tudor, C.; Schlegel, K.A. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells–a comparison of different tissue sources. J. Cranio-Maxillo-Facial Surg. 2012, 40, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Muraglia, A.; Corsi, A.; Bianco, P.; Marcacci, M.; Martin, I.; Boyde, A.; Ruspantini, I.; Chistolini, P.; Rocca, M. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J. Biomed. Mater. Res. 2000, 49, 328–337. [Google Scholar] [CrossRef]
- Niemeyer, P.; Fechner, K.; Milz, S.; Richter, W.; Suedkamp, N.P.; Mehlhorn, A.T.; Pearce, S.; Kasten, P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 2010, 31, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, E.M.; Prockop, D.J.; Fitzpatrick, L.A.; Koo, W.W.; Gordon, P.L.; Neel, M.; Sussman, M.; Orchard, P.; Marx, J.C.; Pyeritz, R.E. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999, 5, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedzki, T.; Dabrowski, Z.; Miszta, H.; Pawlikowski, M. Bone healing after bone marrow stromal cell transplantation to the bone defect. Biomaterials 1993, 14, 115–121. [Google Scholar] [CrossRef]
- Rodriguez, A.-M.; Elabd, C.; Amri, E.-Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef]
- Rodbell, M. The Metabolism of Isolated Fat Cells. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Grottkau, B.E.; Lin, Y. Osteogenesis of adipose-derived stem cells. Bone Res. 2013, 1, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-C.; Estes, B.T.; Awad, H.A.; Guilak, F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng. Part A 2008, 15, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.J.; McIlhenny, S.; Tulenko, T.; Golesorkhi, N.; Zhang, P.; Larson, R.; Lombardi, J.; Shapiro, I.; DiMuzio, P.J. Endothelial differentiation of adipose-derived stem cells: Effects of endothelial cell growth supplement and shear force. J. Surg. Res. 2009, 152, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Dusting, G.J.; Stubbs, S.; Arunothayaraj, S.; Han, X.L.; Collas, P.; Morrison, W.A.; Dilley, R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010, 14, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Cho, H.-H.; Cho, Y.-B.; Park, J.-S.; Jeong, H.-S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Reis, R.L.; Sousa, N.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef]
- Zhao, L.; Johnson, T.; Liu, D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res. Ther. 2017, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Glotzbach, J.P.; Sorkin, M.; Longaker, M.T.; Gurtner, G.C. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann. Plast. Surg. 2014, 72, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Palmieri, A.; Vinci, R.; Azzi, L.; Taglabue, A.; Carinci, F. Adipose derived stem cells: Basic science fundaments and clinical application. An update. Minerva Stomatol 2014, 63, 273–281. [Google Scholar]
- Bowles, A.C.; Scruggs, B.A.; Bunnell, B.A. Mesenchymal Stem Cell-Based Therapy in a Mouse Model of Experimental Autoimmune Encephalomyelitis (EAE). In Animal Models for Stem Cell Therapy; Christ, B., Oerlecke, J., Stock, P., Eds.; Springer: New York, NY, USA, 2014; pp. 303–319. [Google Scholar]
- Osinga, R.; Di Maggio, N.; Todorov, A.; Allafi, N.; Barbero, A.; Laurent, F.; Schaefer, D.J.; Martin, I.; Scherberich, A. Generation of a Bone Organ by Human Adipose-Derived Stromal Cells Through Endochondral Ossification. Stem Cells Transl. Med. 2016, 5, 1090–1097. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, H. Adipose-derived stem cells for tissue repair and regeneration: Ten years of research and a literature review. J. Nippon Med Sch. 2009, 76, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Aust, L.; Devlin, B.; Foster, S.; Halvorsen, Y.; Hicok, K.; Du Laney, T.; Sen, A.; Willingmyre, G.; Gimble, J. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gomillion, C.T.; Burg, K.J. Stem cells and adipose tissue engineering. Biomaterials 2006, 27, 6052–6063. [Google Scholar] [CrossRef]
- Im, G., II; Shin, Y.-W.; Lee, K.-B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr. Cartil. 2005, 13, 845–853. [Google Scholar] [CrossRef] [Green Version]
- De Girolamo, L.; Sartori, M.F.; Albisetti, W.; Brini, A.T. Osteogenic differentiation of human adipose-derived stem cells: Comparison of two different inductive media. J. Tissue Eng. Regen. Med. 2007, 1, 154–157. [Google Scholar] [CrossRef]
- Chou, Y.-F.; Zuk, P.A.; Chang, T.-L.; Benhaim, P.; Wu, B.M. Adipose-derived stem cells and BMP2: Part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect. Tissue Res. 2011, 52, 109–118. [Google Scholar] [CrossRef]
- Morcos, M.W.; Al-Jallad, H.; Hamdy, R. Comprehensive Review of Adipose Stem Cells and Their Implication in Distraction Osteogenesis and Bone Regeneration. BioMed Res. Int. 2015, 2015, 842975. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.M.; Shi, Y.-Y.; Aalami, O.O.; Chou, Y.-F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.-P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Cranio-Maxillo-Facial Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Eterno, V.; Zambelli, A.; Pavesi, L.; Villani, L.; Zanini, V.; Petrolo, G.; Manera, S.; Tuscano, A.; Amato, A. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget 2014, 5, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.-D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef] [PubMed]
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef]
- Jyothi Prasanna, S.; Sowmya Jahnavi, V. Wharton’s jelly mesenchymal stem cells as off-the-shelf cellular therapeutics: A closer look into their regenerative and immunomodulatory properties. Open Tissue Eng. Regen. Med. J. 2011, 4. [Google Scholar] [CrossRef]
- Liu, S.; Jia, Y.; Yuan, M.; Guo, W.; Huang, J.; Zhao, B.; Peng, J.; Xu, W.; Lu, S.; Guo, Q. Repair of Osteochondral Defects Using Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells in a Rabbit Model. BioMed Res. Int. 2017, 2017, 8760383. [Google Scholar] [CrossRef]
- Cardoso, T.C.; Ferrari, H.F.; Garcia, A.F.; Novais, J.B.; Silva-Frade, C.; Ferrarezi, M.C.; Andrade, A.L.; Gameiro, R. Isolation and characterization of Wharton’s jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012, 12, 18. [Google Scholar] [CrossRef]
- Iacono, E.; Brunori, L.; Pirrone, A.; Pagliaro, P.P.; Ricci, F.; Tazzari, P.L.; Merlo, B. Isolation, characterization and differentiation of mesenchymal stem cells from amniotic fluid, umbilical cord blood and Wharton’s jelly in the horse. Reproduction 2012, 143, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Cunto, M.; Zambelli, D.; Ricci, F.; Tazzari, P.L.; Merlo, B. Could fetal fluid and membranes be an alternative source for mesenchymal stem cells (MSCs) in the feline species? A preliminary study. Vet. Res. Commun. 2012, 36, 107–118. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Cui, B.; Law, F.; Jiang, X.; Yang, L.; Xie, Q.; Huang, T. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin. Med. J. 2005, 118, 1987–1993. [Google Scholar] [PubMed]
- Dhitiseith, D.; Honsawek, S. Differential Expression of Osteogenic Differentiation in Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells Treated with Demineralized Bone. Adv. Mater. Res. 2008, 55–57, 697–700. [Google Scholar]
- Zhang, Y.-N.; Lie, P.-C.; Wei, X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy 2009, 11, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, P.; Alitheen, N.B.; Ali, A.M.; Omar, A.R.; Mohit, M.; Janzamin, E.; Samani, F.S.; Torshizi, Z.; Nematollahi-Mahani, S.N. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. Vitr. Cell. Dev. Biol. Anim. 2012, 48, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 2008, 26, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, K.D.; Yuan, M.; Peng, J.; Zhang, L.; Sui, X.; Zhao, B.; Xu, W.; Wang, A.; Lu, S. Characteristics of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. J. Biosci. Bioeng. 2014, 117, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Troyer, D.L.; Weiss, M.L. Concise review: Wharton’s Jelly-derived cells are a primitive stromal cell population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef]
- Ciavarella, S.; Dammacco, F.; De Matteo, M.; Loverro, G.; Silvestris, F. Umbilical cord mesenchymal stem cells: Role of regulatory genes in their differentiation to osteoblasts. Stem Cells Dev. 2009, 18, 1211–1220. [Google Scholar] [CrossRef]
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS ONE 2014, 9, e98565. [Google Scholar] [CrossRef]
- Kouroupis, D.; Churchman, S.M.; English, A.; Emery, P.; Giannoudis, P.V.; McGonagle, D.; Jones, E.A. Assessment of umbilical cord tissue as a source of mesenchymal stem cell/endothelial cell mixtures for bone regeneration. Regen. Med. 2013, 8, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Toupadakis, C.A.; Wong, A.; Genetos, D.C.; Cheung, W.K.; Borjesson, D.L.; Ferraro, G.L.; Galuppo, L.D.; Leach, J.K.; Owens, S.D.; Yellowley, C.E. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res. 2010, 71, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, S.; Pan, L.; Jia, S. Enhancement of bone formation by genetically engineered human umbilical cord–derived mesenchymal stem cells expressing osterix. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e221–e229. [Google Scholar] [CrossRef]
- Diao, Y.; Ma, Q.; Cui, F.; Zhong, Y. Human umbilical cord mesenchymal stem cells: Osteogenesis in vivo as seed cells for bone tissue engineering. J. Biomed. Mater. Res. Part A 2009, 91, 123–131. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Manuchehrabadi, N.; Weir, M.D.; Zhu, Z.; Xu, H.H.K. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials 2013, 34, 9917–9925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Sibov, T.T.; Severino, P.; Marti, L.; Pavon, L.; Oliveira, D.; Tobo, P.; Campos, A.; Paes, A.; Amaro, E., Jr.; Gamarra, L. Mesenchymal stem cells from umbilical cord blood: Parameters for isolation, characterization and adipogenic differentiation. Cytotechnology 2012, 64, 511–521. [Google Scholar] [CrossRef]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef]
- Sparrow, R.L.; Cauchi, J.A.; Ramadi, L.T.; Waugh, C.M.; Kirkland, M.A. Influence of mode of birth and collection on WBC yields of umbilical cord blood units. Transfusion 2002, 42, 210–215. [Google Scholar] [CrossRef]
- McGuckin, C.P.; Pearce, D.; Forraz, N.; Tooze, J.A.; Watt, S.M.; Pettengell, R. Multiparametric analysis of immature cell populations in umbilical cord blood and bone marrow. Eur. J. Haematol. 2003, 71, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.M.; Kim, B.-C.; Park, J.-H.; Kwon, I.K.; Mantalaris, A.; Hwang, Y.-S. Stem cells in bone tissue engineering. Biomed. Mater. 2010, 5, 062001. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Barry, F.; O’brien, T. UC blood-derived mesenchymal stromal cells: An overview. Cytotherapy 2007, 9, 717–726. [Google Scholar] [CrossRef]
- Armson, B.A.; Allan, D.S.; Casper, R.F. Umbilical Cord Blood: Counselling, Collection, and Banking. J. Obstet. Gynaecol. Can. 2015, 37, 832–844. [Google Scholar] [CrossRef]
- Lee, M.; Jeong, S.Y.; Ha, J.; Kim, M.; Jin, H.J.; Kwon, S.-J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2014, 446, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.-J.; Byeon, Y.-E.; Lim, J.-H.; Ryu, H.-H.; Kim, W.H.; Koyama, Y.; Kikuchi, M.; Kang, K.-S.; Kweon, O.-K. Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs. J. Vet. Sci. 2008, 9, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Van Phuc, P.; Nhung, T.H.; Loan, D.T.T.; Chung, D.C.; Ngoc, P.K. Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. Vitr. Cell. Dev. Biol. Anim. 2011, 47, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Miyoshi, S.; Hida, N.; Uyama, T.; Okamoto, K.; Ikegami, Y.; Miyado, K.; Segawa, K.; Terai, M.; Sakamoto, M. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells 2007, 25, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Lee, Y.-S.; Kang, K.-S. In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood. J. Vet. Sci. 2006, 7, 343–348. [Google Scholar] [CrossRef]
- Koch, T.G.; Heerkens, T.; Thomsen, P.D.; Betts, D.H. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007, 7, 26. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Sun, J.; Zhou, H.; Zhang, W.; Cui, L.; Cao, Y. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood-derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng. Part A 2010, 16, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ye, X.; Zhu, Y.; Li, Y.; Sun, J.; Cui, L.; Cao, Y. Osteogenic differentiation of GFP-labeled human umbilical cord blood derived mesenchymal stem cells after cryopreservation. Cryobiology 2011, 63, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.-Q.; Zang, W.-J.; Bao, L.-J.; Li, D.-L.; Xu, X.-L.; Yu, X.-J. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol. Int. 2006, 30, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.J.; Hong, S.H.; Jeong, J.A.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2004, 321, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia-and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.; Kishore, R. Endothelial Progenitor Cells: Procedure for Cell Isolation and Applications. In Adult Stem Cells; Humana Press: New York, NY, USA, 2017; pp. 85–89. [Google Scholar]
- Murohara, T. Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors. Trends Cardiovasc. Med. 2001, 11, 303–307. [Google Scholar] [CrossRef]
- Naruse, K.; Hamada, Y.; Nakashima, E.; Kato, K.; Mizubayashi, R.; Kamiya, H.; Yuzawa, Y.; Matsuo, S.; Murohara, T.; Matsubara, T. Therapeutic Neovascularization Using Cord Blood–Derived Endothelial Progenitor Cells for Diabetic Neuropathy. Diabetes 2005, 54, 1823–1828. [Google Scholar] [CrossRef]
- Matsumoto, T.; Mugishima, H. Non-hematopoietic stem cells in umbilical cord blood. Int. J. Stem Cells 2009, 2, 83–89. [Google Scholar] [CrossRef]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef]
- Lin, Y.; Weisdorf, D.J.; Solovey, A.; Hebbel, R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Investig. 2000, 105, 71. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kawamoto, A.; Kuroda, R.; Ishikawa, M.; Mifune, Y.; Iwasaki, H.; Miwa, M.; Horii, M.; Hayashi, S.; Oyamada, A. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am. J. Pathol. 2006, 169, 1440–1457. [Google Scholar] [CrossRef] [PubMed]
- Mifune, Y.; Matsumoto, T.; Kawamoto, A.; Kuroda, R.; Shoji, T.; Iwasaki, H.; Kwon, S.M.; Miwa, M.; Kurosaka, M.; Asahara, T. Local delivery of granulocyte colony stimulating factor-mobilized CD34-positive progenitor cells using bioscaffold for modality of unhealing bone fracture. Stem Cells 2008, 26, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kuroda, R.; Mifune, Y.; Kawamoto, A.; Shoji, T.; Miwa, M.; Asahara, T.; Kurosaka, M. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone 2008, 43, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Duttenhoefer, F.; Lara de Freitas, R.; Meury, T.; Loibl, M.; Benneker, L.M.; Hermann, M.; Richards, R.; Alini, M.; Verrier, S. 3D scaffolds co-seeded with human endothelial progenitor and mesenchymal stem cells: Evidence of prevascularisation within 7 days. Eur. Cells Mater. 2013, 26, 64–65. [Google Scholar] [CrossRef]
- Zebardast, N.; Lickorish, D.; Davies, J.E. Human umbilical cord perivascular cells (HUCPVC) A mesenchymal cell source for dermal wound healing. Organogenesis 2010, 6, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Sarugaser, R.; Lickorish, D.; Baksh, D.; Hosseini, M.M.; Davies, J.E. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 2005, 23, 220–229. [Google Scholar] [CrossRef]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef]
- Ennis, J.; Sarugaser, R.; Gomez, A.; Baksh, D.; Davies, J. Isolation, characterization, and differentiation of human umbilical cord perivascular cells (HUCPVCs). Methods Cell Biol. 2008, 86, 121–136. [Google Scholar]
- Milan, P.B.; Lotfibakhshaiesh, N.; Joghataie, M.; Ai, J.; Pazouki, A.; Kaplan, D.; Kargozar, S.; Amini, N.; Hamblin, M.; Mozafari, M. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016, 45, 234–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yannarelli, G.; Dayan, V.; Pacienza, N.; Lee, C.-J.; Medin, J.; Keating, A. Human umbilical cord perivascular cells exhibit enhanced cardiomyocyte reprogramming and cardiac function after experimental acute myocardial infarction. Cell Transplant. 2013, 22, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Mozafari, M.; Brouki Milan, P.; Hamzehlou, S.; Barati, M.; Baino, F.; Hill, R.G.; Joghataei, M.T. Strontium- and cobalt-substituted bioactive glasses seeded with human umbilical cord perivascular cells to promote bone regeneration via enhanced osteogenic and angiogenic activities. Acta Biomater. 2017, 58, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Ujiie, Y.; Nishikawa, S.; Inoue, K.; Shirakawa, S.; Hanada, N.; Liddell, R.; Davies, J.E.; Gomi, K. Bone formation by human umbilical cord perivascular cells. J. Biomed. Mater. Res. Part A 2015, 103, 2807–2814. [Google Scholar] [CrossRef]
- Tsang, W.P.; Shu, Y.; Kwok, P.L.; Zhang, F.; Lee, K.K.H.; Tang, M.K.; Li, G.; Chan, K.M.; Chan, W.-Y.; Wan, C. CD146+ human umbilical cord perivascular cells maintain stemness under hypoxia and as a cell source for skeletal regeneration. PLoS ONE 2013, 8, e76153. [Google Scholar] [CrossRef] [PubMed]
- Gökçinar-Yagci, B.; Özyüncü, Ö.; Çelebi-Saltik, B. Isolation, characterisation and comparative analysis of human umbilical cord vein perivascular cells and cord blood mesenchymal stem cells. Cell Tissue Bank. 2016, 17, 345–352. [Google Scholar] [CrossRef]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Suardita, K.; Ishii, M.; Sugiyama, M.; Igarashi, A.; Oda, R.; Nishimura, M.; Saito, M.; Nakagawa, K.; Yamanaka, K. Alveolar bone marrow as a cell source for regenerative medicine: Differences between alveolar and iliac bone marrow stromal cells. J. Bone Miner. Res. 2005, 20, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Yagi, K.; Kojima, M.; Yagyuu, T.; Ohshima, A.; Sobajima, S.; Tadokoro, M.; Katsube, Y.; Isoda, K.; Kondoh, M. Multipotent cells from the human third molar: Feasibility of cell-based therapy for liver disease. Differentiation 2008, 76, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Gnanasegaran, N.; Govindasamy, V.; Nathan, P.; Musa, S.; Kasim, N.H.A. Dental Stem Cell Differentiation Toward Endodermal Cell Lineages: Approaches to Control Hepatocytes and Beta Cell Transformation. In Dental Stem Cells; Springer: Cham, Switzerland, 2016; pp. 243–268. [Google Scholar]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-C.; Chang, K.-C.; Tsai, S.-J.; Chang, H.-H.; Lin, C.-P. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J. Formos. Med Assoc. 2014, 113, 956–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry–part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Osorio, E.; Ledesma-Martínez, E.; Mendoza-Núñez, V.M. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar]
- Wang, X.; Sha, X.-J.; Li, G.-H.; Yang, F.-S.; Ji, K.; Wen, L.-Y.; Liu, S.-Y.; Chen, L.; Ding, Y.; Xuan, K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch. Oral Biol. 2012, 57, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Kim, S.-O.; Kim, S.-H.; Choi, H.-J.; Son, H.-K.; Jung, H.-S.; Kim, C.-S.; Lee, J.-H. In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Eng. Part A 2012, 18, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Silvério, K.G.; Rodrigues, T.L.; Coletta, R.D.; Benevides, L.; Da Silva, J.S.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Mesenchymal stem cell properties of periodontal ligament cells from deciduous and permanent teeth. J. Periodontol. 2010, 81, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liu, Y.; Lu, W.; Yang, F.; Yu, J.; Wang, X.; Ma, Q.; Yang, Z.; Wen, L.; Xuan, K. Periodontal tissue engineering with stem cells from the periodontal ligament of human retained deciduous teeth. J. Periodontal Res. 2013, 48, 105–116. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.M.; Jung, I.H.; Kim, J.C.; Choi, S.H.; Cho, K.S.; Kim, C.S. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J. Clin. Periodontol. 2011, 38, 721–731. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal Ligament Stem Cells: Current Status, Concerns, and Future Prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Estrela, C.; Alencar, A.H.G.d.; Kitten, G.T.; Vencio, E.F.; Gava, E. Mesenchymal stem cells in the dental tissues: Perspectives for tissue regeneration. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef]
- Mitrano, T.I.; Grob, M.S.; Carrión, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J. Periodontol. 2010, 81, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [PubMed]
- Haekkinen, L.; Uitto, V.J.; Larjava, H. Cell biology of gingival wound healing. Periodontology 2000 2000, 24, 127–152. [Google Scholar] [CrossRef]
- Wang, F.; Yu, M.; Yan, X.; Wen, Y.; Zeng, Q.; Yue, W.; Yang, P.; Pei, X. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev. 2011, 20, 2093–2102. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Xu, X.; Chen, C.; Ansari, S.; Zadeh, H.H.; Snead, M.L.; Shi, S. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials 2014, 35, 2642–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, M.J.; Imaizumi, M.; Tsuchiya, S.; Morsczeck, C. Dental follicle stem cells and tissue engineering. J. Oral Sci. 2010, 52, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Mori, G.; Ballini, A.; Carbone, C.; Oranger, A.; Brunetti, G.; Di Benedetto, A.; Rapone, B.; Cantore, S.; Di Comite, M.; Colucci, S. Osteogenic differentiation of dental follicle stem cells. Int. J. Med. Sci. 2012, 9, 480–487. [Google Scholar] [CrossRef]
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation 2009, 77, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise Reviews: Characteristics and Potential Applications of Human Dental Tissue-Derived Mesenchymal Stem Cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Riccio, M.; Resca, E.; Maraldi, T.; Pisciotta, A.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human dental pulp stem cells produce mineralized matrix in 2D and 3D cultures. Eur. J. Histochem. 2010, 54, e46. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Tomasello, L.; Pizzo, G.; Pizzolanti, G.; Giordano, C.; Campisi, G. Dental pulp stem cells for bone tissue engineering: A review of the current literature and a look to the future. Regen. Med. 2018, 13, 207–218. [Google Scholar]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cells Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Eslaminejad, M.B.; Bordbar, S.; Nazarian, H. Odontogenic differentiation of dental pulp-derived stem cells on tricalcium phosphate scaffolds. J. Dent. Sci. 2013, 8, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Bai, Y.; Zhang, D. Osteogenic stimulation of human dental pulp stem cells with a novel gelatin-hydroxyapatite-tricalcium phosphate scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-T.; Chou, W.-L.; Chou, C.-M. Osteoblastic differentiation of stem cells from human exfoliated deciduous teeth induced by thermosensitive hydrogels with strontium phosphate. Mater. Sci. Eng. C 2015, 52, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-T.; Wu, P.-S.; Ko, C.-S.; Huang, T.-Y. Osteogenic differentiation and mineralization of human exfoliated deciduous teeth stem cells on modified chitosan scaffold. Mater. Sci. Eng. C 2014, 41, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-T.; Wu, P.-S.; Huang, T.-Y. Osteogenic differentiation of stem cells from human exfoliated deciduous teeth on poly (ε-caprolactone) nanofibers containing strontium phosphate. Mater. Sci. Eng. C 2015, 46, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Chadipiralla, K.; Yochim, J.M.; Bahuleyan, B.; Huang, C.-Y.C.; Garcia-Godoy, F.; Murray, P.E.; Stelnicki, E.J. Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res. 2010, 340, 323–333. [Google Scholar] [CrossRef]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Tang, R.; Wei, F.; Wei, L.; Wang, S.; Ding, G. Osteogenic differentiated periodontal ligament stem cells maintain their immunomodulatory capacity. J. Tissue Eng. Regen. Med. 2014, 8, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.-H.; Lee, S.-H.; Jun, C.-M.; Oh, N.; Yun, J.-H. Characterization of the enhanced bone regenerative capacity of human periodontal ligament stem cells engineered to express the gene encoding bone morphogenetic protein 2. Tissue Eng. Part A 2014, 20, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, K.H.; Gwak, E.H.; Rhee, S.H.; Lee, J.C.; Shin, S.Y.; Koo, K.T.; Lee, Y.M.; Seol, Y.J. Ex vivo bone morphogenetic protein 2 gene delivery using periodontal ligament stem cells for enhanced re-osseointegration in the regenerative treatment of peri-implantitis. J. Biomed. Mater. Res. Part A 2015, 103, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.-H.; Zhou, Q.; Wang, Z.-L. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: A comparative study in the rat. J. Biomater. Appl. 2014, 29, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-T.; Kim, J.; Seonwoo, H.; Chang, J.U.; Choi, H.; Hexiu, J.; Cho, W.J.; Choung, P.-H.; Chung, J.H. Enhanced osteogenesis of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering using fluid shear stress in a rocking culture method. Tissue Eng. Part C 2012, 19, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, J.S.; Chung, Y.S.; Sin, Y.W.; Ryu, K.H.; Lee, J.; You, H.K. Growth and osteogenic differentiation of alveolar human bone marrow-derived mesenchymal stem cells on chitosan/hydroxyapatite composite fabric. J. Biomed. Mater. Res. Part A 2013, 101, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Oh, S.Y.; Lee, J.S.; Park, S.Y.; Choi, E.Y.; Cho, K.S.; Kim, C.S. In vivo bone formation by human alveolar-bone-derived mesenchymal stem cells obtained during implant osteotomy using biphasic calcium phosphate ceramics or Bio-Oss as carriers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 515–524. [Google Scholar] [CrossRef]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef]
- Yang, H.; Gao, L.-N.; An, Y.; Hu, C.-H.; Jin, F.; Zhou, J.; Jin, Y.; Chen, F.-M. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 2013, 34, 7033–7047. [Google Scholar] [CrossRef]
- Handa, K.; Saito, M.; Tsunoda, A.; Yamauchi, M.; Hattori, S.; Sato, S.; Toyoda, M.; Teranaka, T.; Narayanan, A.S. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect. Tissue Res. 2002, 43, 406–408. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Ohshima, S.; Yamakoshi, Y.; Simmer, J.P.; Honda, M.J. Osteogenic differentiation capacity of porcine dental follicle progenitor cells. Connect. Tissue Res. 2010, 51, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Choi, Y.S.; Shin, H.C.; Lee, C.W.; Kim, D.-I. Isolation of human periosteum-derived progenitor cells using immunophenotypes for chondrogenesis. Biotechnol. Lett. 2005, 27, 607–611. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Vanlauwe, J.; Eyckmans, J.; Khan, I.M.; Archer, C.W.; Jones, E.A.; McGonagle, D.; Mitsiadis, T.A.; Pitzalis, C. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheumatol. 2006, 54, 1209–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tate, M.L.K.; Ritzman, T.F.; Schneider, E.; Knothe, U.R. Testing of a new one-stage bone-transport surgical procedure exploiting the periosteum for the repair of long-bone defects. JBJS 2007, 89, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, H.; Reed, A.; Joyner, C.; Simpson, A. Anatomical effects of periosteal elevation. J. Orthop. Res. 2000, 18, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-W.; Hah, Y.-S.; Kim, D.R.; Kim, J.-R.; Byun, J.-H. Osteogenic phenotypes and mineralization of cultured human periosteal-derived cells. Arch. Oral Biol. 2007, 52, 983–989. [Google Scholar] [CrossRef]
- Augello, A.; Kurth, T.B.; De Bari, C. Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur. Cell Mater. 2010, 20, 121–133. [Google Scholar] [CrossRef]
- Bilkay, U.; Tokat, C.; Helvaci, E.; Ozek, C.; Zekioglu, O.; Onat, T.; Songur, E. Osteogenic capacities of tibial and cranial periosteum: A biochemical and histologic study. J. Craniofac. Surg. 2008, 19, 453–458. [Google Scholar] [CrossRef]
- Srisa-Art, M.; Bonzani, I.C.; Williams, A.; Stevens, M.M.; Edel, J.B. Identification of rare progenitor cells from human periosteal tissue using droplet microfluidics. Analyst 2009, 134, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Noh, S.-E.; Lim, S.-M.; Lee, C.-W.; Kim, C.-S.; Im, M.-W.; Lee, M.-H.; Kim, D.-I. Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol. Lett. 2008, 30, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-x.; Ringe, J.; Liang, Z.; Loch, A.; Chen, L.; Sittinger, M. Osteogenic potential of human periosteum-derived progenitor cells in PLGA scaffold using allogeneic serum. J. Zhejiang Univ. Sci. B 2006, 7, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirasawa, S.; Sekiya, I.; Sakaguchi, Y.; Yagishita, K.; Ichinose, S.; Muneta, T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem. 2006, 97, 84–97. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheumatol. 2001, 44, 1928–1942. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Varshney, R.R.; Ren, L.; Cai, D.; Wang, D.-A. Synovium-derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Eng. Part B 2009, 15, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheumatol. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djouad, F.; Bony, C.; Häupl, T.; Uzé, G.; Lahlou, N.; Louis-Plence, P.; Apparailly, F.; Canovas, F.; Rème, T.; Sany, J. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res. Ther. 2005, 7, R1304. [Google Scholar] [CrossRef]
- Jo, C.; Ahn, H.; Kim, H.; Seong, S.; Lee, M. Surface characterization and chondrogenic differentiation of mesenchymal stromal cells derived from synovium. Cytotherapy 2007, 9, 316–327. [Google Scholar] [CrossRef]
- McGonagle, D.; Baboolal, T.G.; Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 719–730. [Google Scholar] [CrossRef]
- Archer, C.W.; Dowthwaite, G.P.; Francis-West, P. Development of synovial joints. Birth Defects Res. Part C Embryo Today Rev. 2003, 69, 144–155. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Zhu, F.; Yang, X. Osteogenic differentiation of synovial mesenchymal stem cells in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi 2005, 23, 145–147, 151. [Google Scholar]
- De Bari, C.; Dell’Accio, F.; Karystinou, A.; Guillot, P.V.; Fisk, N.M.; Jones, E.A.; McGonagle, D.; Khan, I.M.; Archer, C.W.; Mitsiadis, T.A. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis Rheumatol. 2008, 58, 240–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsusaki, M.; Kadowaki, K.; Tateishi, K.; Higuchi, C.; Ando, W.; Hart, D.A.; Tanaka, Y.; Take, Y.; Akashi, M.; Yoshikawa, H. Scaffold-Free Tissue-Engineered Construct–Hydroxyapatite Composites Generated by an Alternate Soaking Process: Potential for Repair of Bone Defects. Tissue Eng. Part A 2008, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Usas, A.; Huard, J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials 2007, 28, 5401–5406. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Usas, A.; Tang, Y.; Lu, A.; Tan, J.; Schneppendahl, J.; Kozemchak, A.M.; Wang, B.; Cummins, J.H.; Tuan, R.S. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials 2014, 35, 6859–6870. [Google Scholar] [CrossRef] [Green Version]
- Mastrogiacomo, M.; Derubeis, A.; Cancedda, R. Bone and cartilage formation by skeletal muscle derived cells. J. Cell. Physiol. 2005, 204, 594–603. [Google Scholar] [CrossRef]
- Musgrave, D.; Pruchnic, R.; Bosch, P.; Ziran, B.; Whalen, J.; Huard, J. Human skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J. Bone Joint Surg. 2002, 84, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells. Expert Opin. Biol. Ther. 2010, 10, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Lavasani, M.; Lu, A.; Thompson, S.D.; Robbins, P.D.; Huard, J.; Niedernhofer, L.J. Isolation of muscle-derived stem/progenitor cells based on adhesion characteristics to collagen-coated surfaces. In Stem Cells and Aging; Humana Press: Totowa, NJ, USA, 2013; pp. 53–65. [Google Scholar]
- Bueno, D.F.; Kerkis, I.; Costa, A.M.; Martins, M.T.; Kobayashi, G.S.; Zucconi, E.; Fanganiello, R.D.; Salles, F.T.; Almeida, A.B.; do Amaral, C.E.R. New source of muscle-derived stem cells with potential for alveolar bone reconstruction in cleft lip and/or palate patients. Tissue Eng. Part A 2008, 15, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Ke, Z.-Y.; Yin, L.-J.; Deng, Z.-L. BMP9-Induced Osteogenetic Differentiation and Bone Formation of Muscle-Derived Stem Cells. J. Biomed. Biotechnol. 2012, 2012, 610952. [Google Scholar]
- Corsi, K.A.; Pollett, J.B.; Phillippi, J.A.; Usas, A.; Li, G.; Huard, J. Osteogenic potential of postnatal skeletal muscle–derived stem cells is influenced by donor sex. J. Bone Miner. Res. 2007, 22, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, J.H.; Ahn, H.H.; Lee, J.Y.; Khang, G.; Lee, B.; Lee, H.B.; Kim, M.S. The osteogenic differentiation of rat muscle-derived stem cells in vivo within in situ-forming chitosan scaffolds. Biomaterials 2008, 29, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6-to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef]
- Vacanti, C.A.; Bonassar, L.J.; Vacanti, M.P.; Shufflebarger, J. Replacement of an avulsed phalanx with tissue-engineered bone. N. Engl. J. Med. 2001, 344, 1511–1514. [Google Scholar] [CrossRef]
- Hentz, V.R.; Chang, J. Tissue Engineering for Reconstruction of the Thumb. N. Engl. J. Med. 2001, 344, 1547–1548. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S. Lost in translation: The gap in scientific advancements and clinical application. Front. Bioeng. Biotechnol. 2016, 4, 43. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Dunnill, P. Assessing the value of autologous and allogeneic cells for regenerative medicine. Regen. Med. 2009, 4, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Csobonyeiova, M.; Polak, S.; Zamborsky, R.; Danisovic, L. iPS cell technologies and their prospect for bone regeneration and disease modeling: A mini review. J. Adv. Res. 2017, 8, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; Zhao, L.; Weir, M.D.; Sun, J.; Chen, W.; Man, Y.; Xu, H.H. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015, 18, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Cell Type | Advantages | Disadvantages |
|---|---|---|
| Autologous osteoblasts |
|
|
| Embryonic stem cells |
|
|
| Fetal stem cells (AFSCs, AMSCs, Ch-MSCs) |
|
|
| Adult stem cells (BM-MSCs, ASCs, WJMSCs, UCB-MSCs, EPCs, HUCPVCs, SMSCs, PDPCs, MD-SCs, Dental stem/progenitor cells) |
|
|
| Induced pluripotent stem cells (iPSCs) |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Brouki Milan, P.; Kim, H.-W.; Baino, F. Bone Tissue Engineering Using Human Cells: A Comprehensive Review on Recent Trends, Current Prospects, and Recommendations. Appl. Sci. 2019, 9, 174. https://doi.org/10.3390/app9010174
Kargozar S, Mozafari M, Hamzehlou S, Brouki Milan P, Kim H-W, Baino F. Bone Tissue Engineering Using Human Cells: A Comprehensive Review on Recent Trends, Current Prospects, and Recommendations. Applied Sciences. 2019; 9(1):174. https://doi.org/10.3390/app9010174
Chicago/Turabian StyleKargozar, Saeid, Masoud Mozafari, Sepideh Hamzehlou, Peiman Brouki Milan, Hae-Won Kim, and Francesco Baino. 2019. "Bone Tissue Engineering Using Human Cells: A Comprehensive Review on Recent Trends, Current Prospects, and Recommendations" Applied Sciences 9, no. 1: 174. https://doi.org/10.3390/app9010174