Photocatalytic Activity of Monosized AuZnO Composite Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Synthesis of PEO-PPO-PEO-Coated AuZnO Composite Nanoparticles
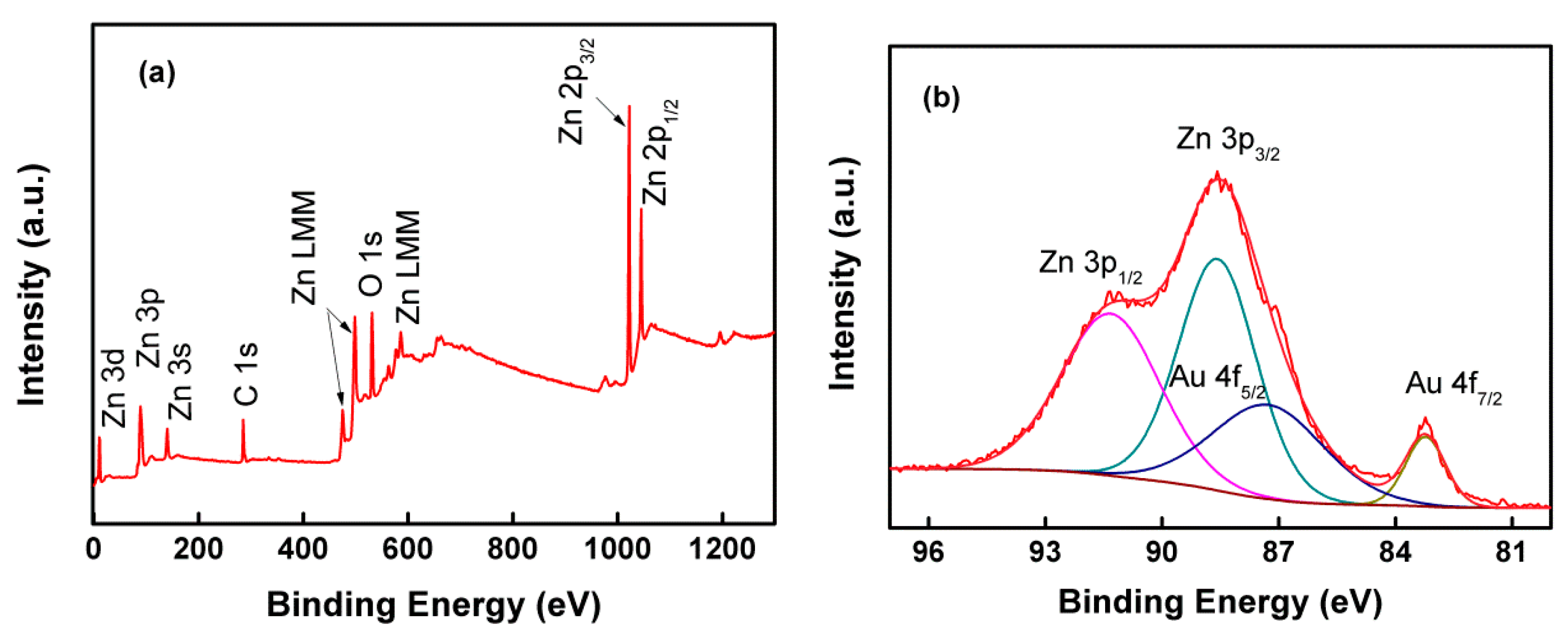
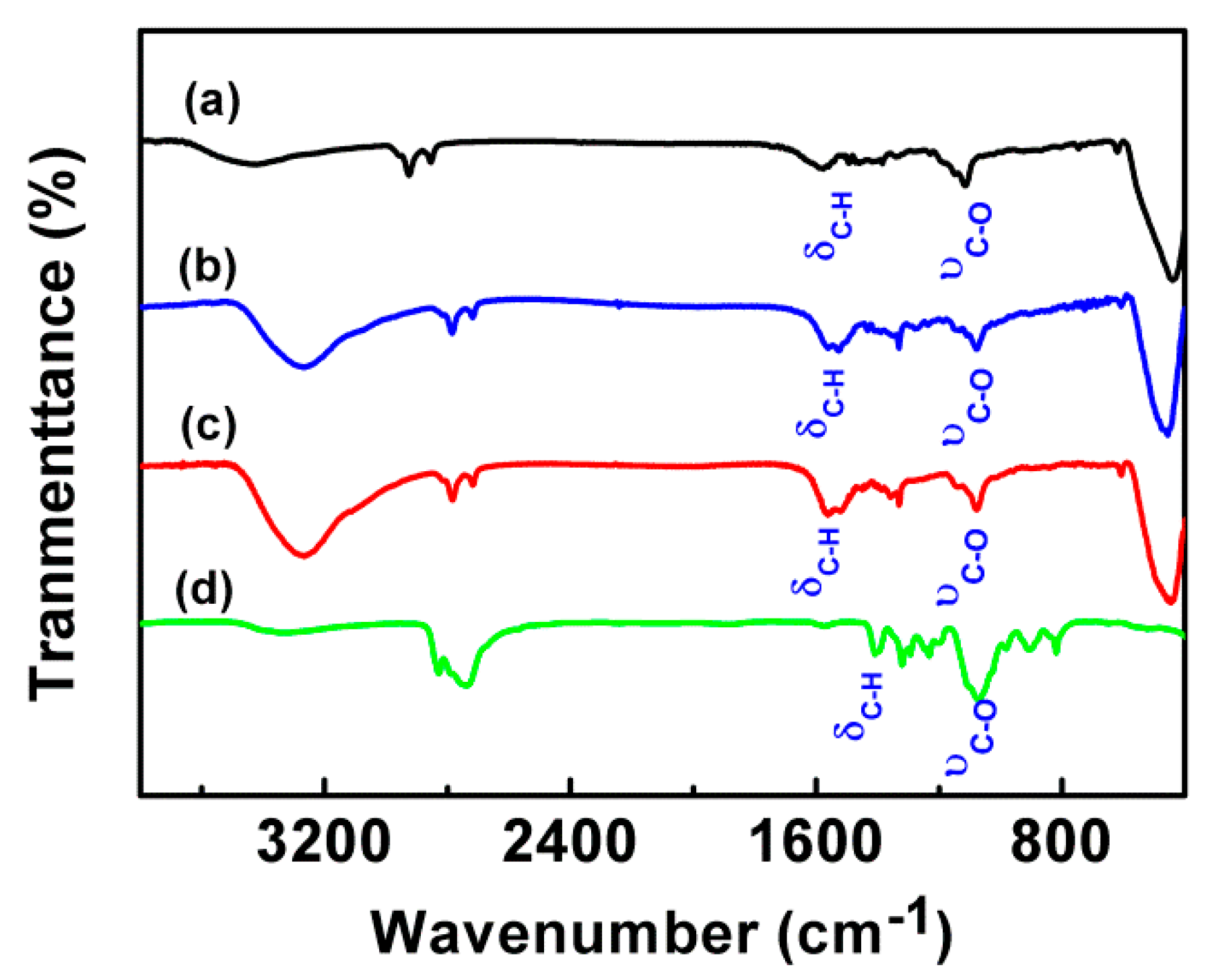
2.3. Characterization
2.4. Measurement of Photocatalytic Activity
3. Results and Discussion
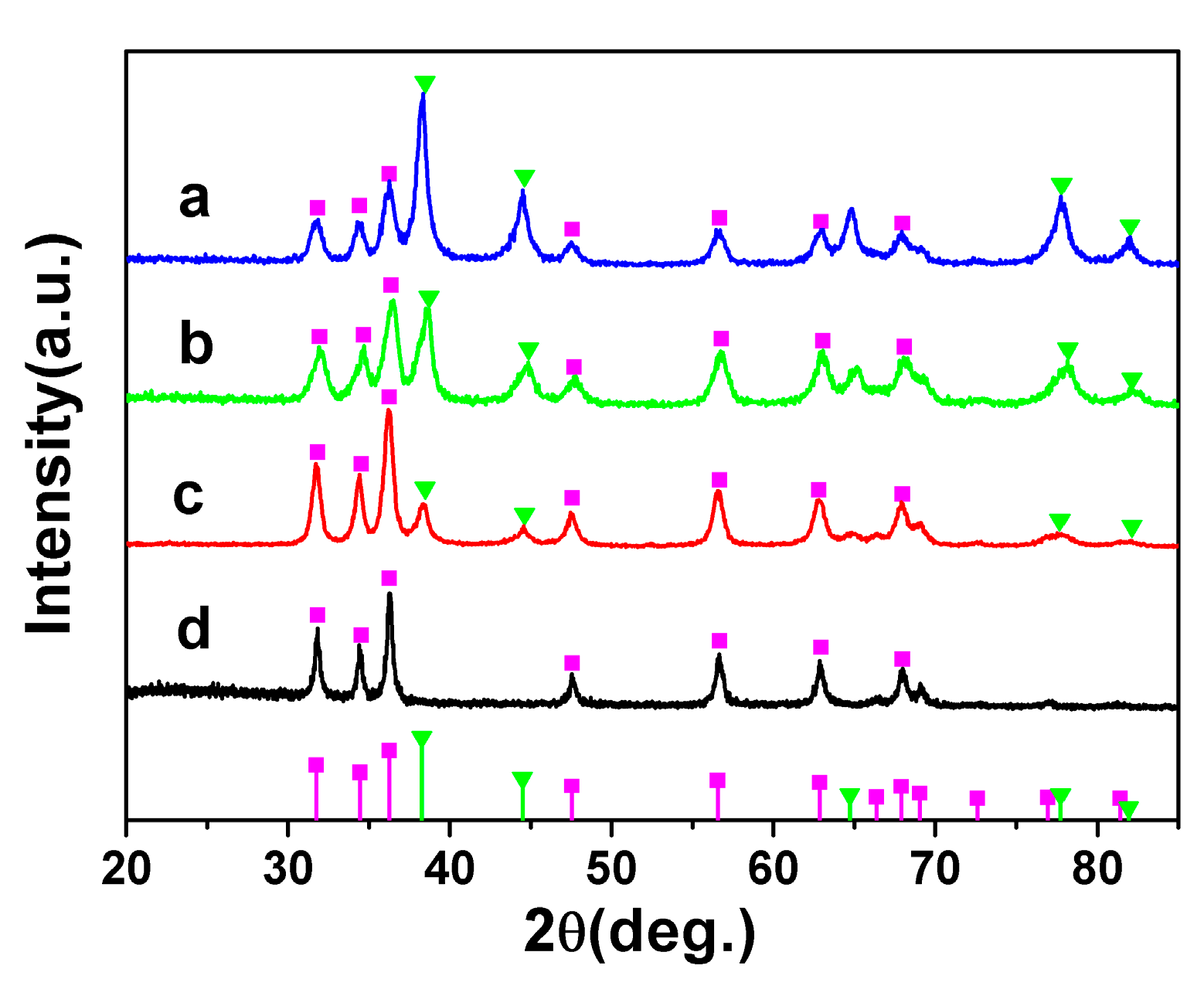
3.1. Transmission Electron Microscope Morphology and Nanostructures of AuZnO Composite Nanoparticles
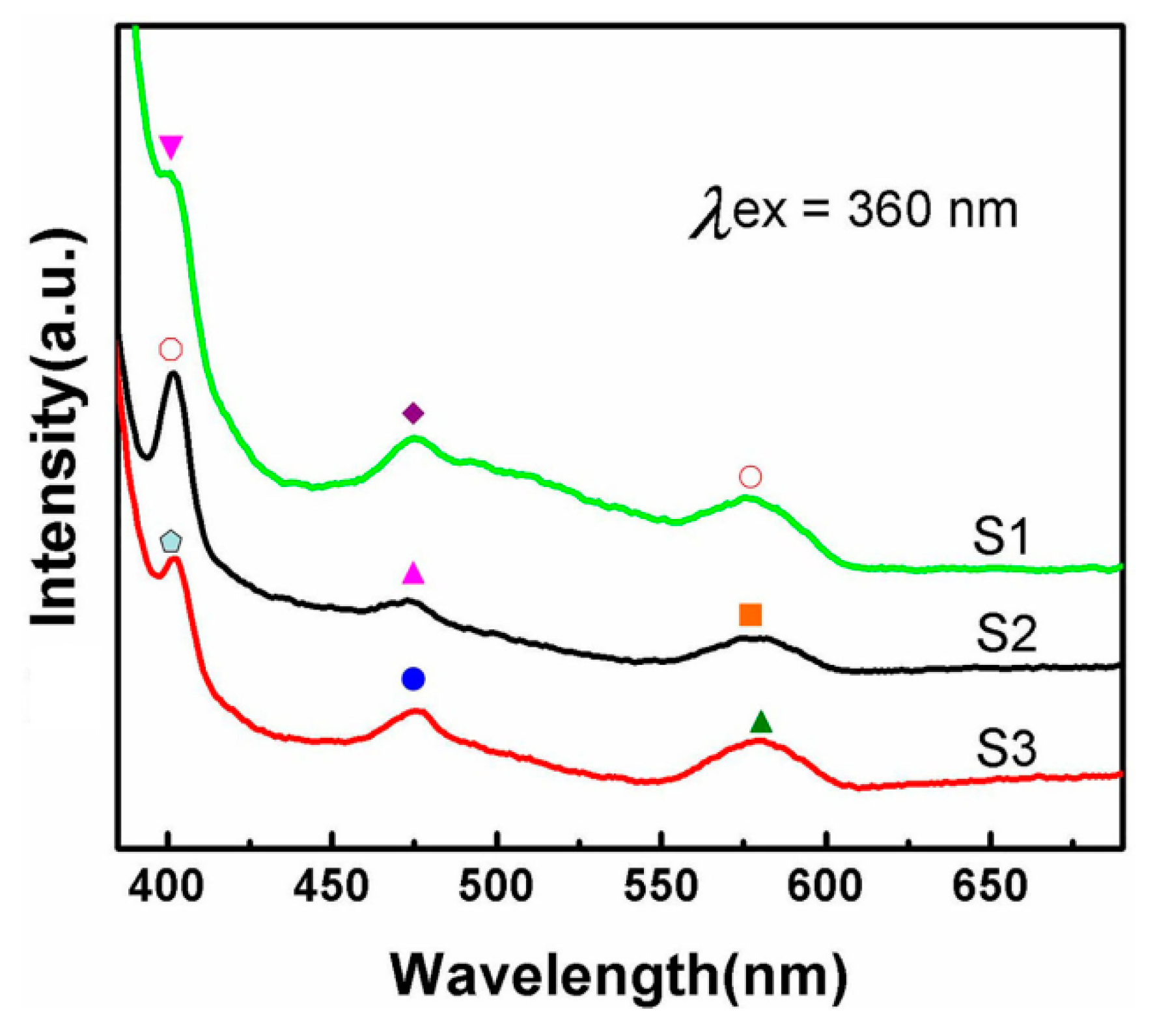
3.2. Optical Properties of AuZnO Composite Nanoparticles
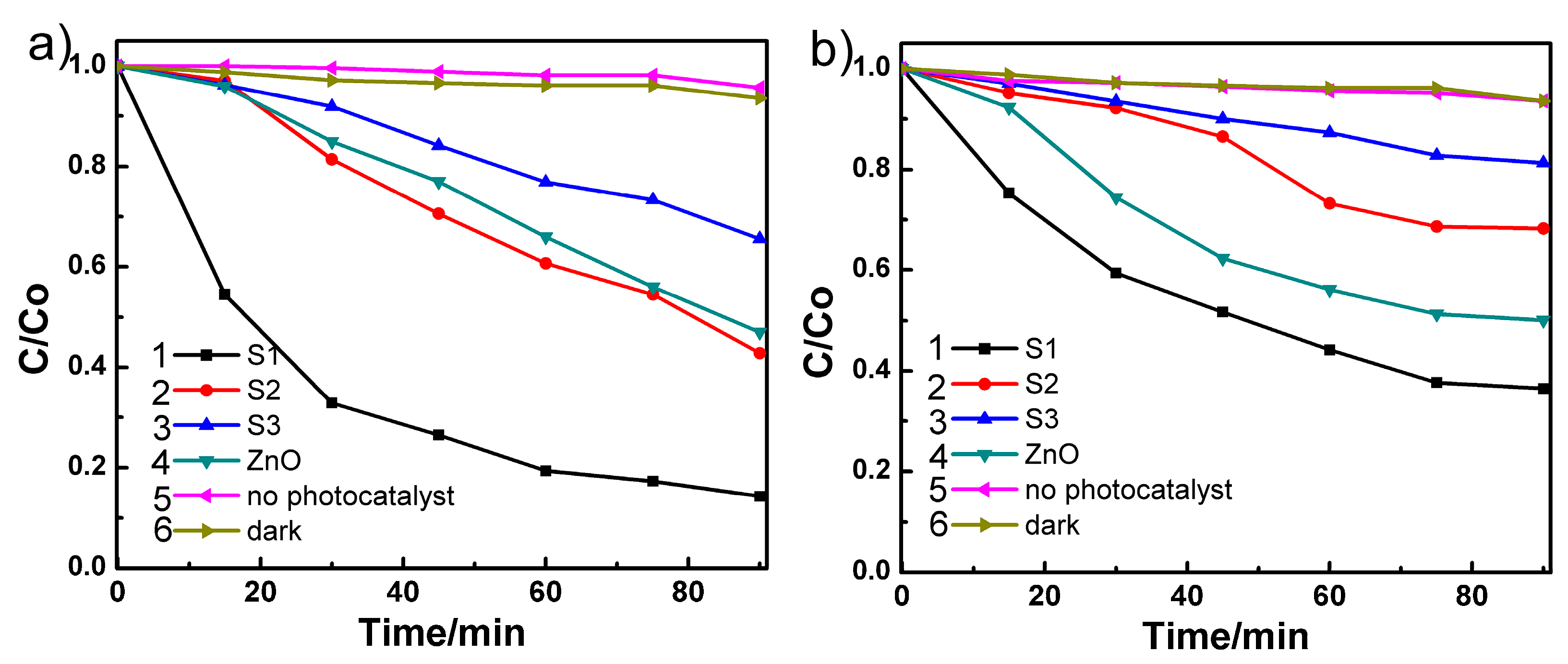
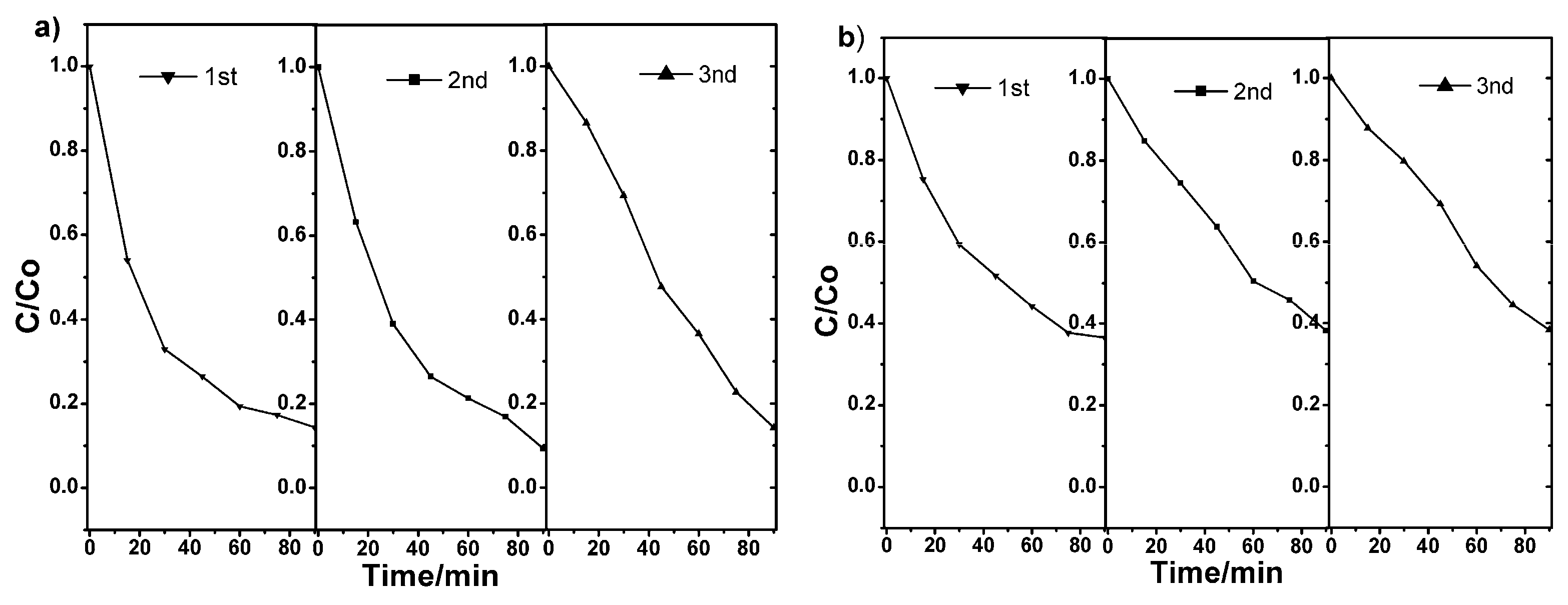
3.3. Photocatalytic Testing
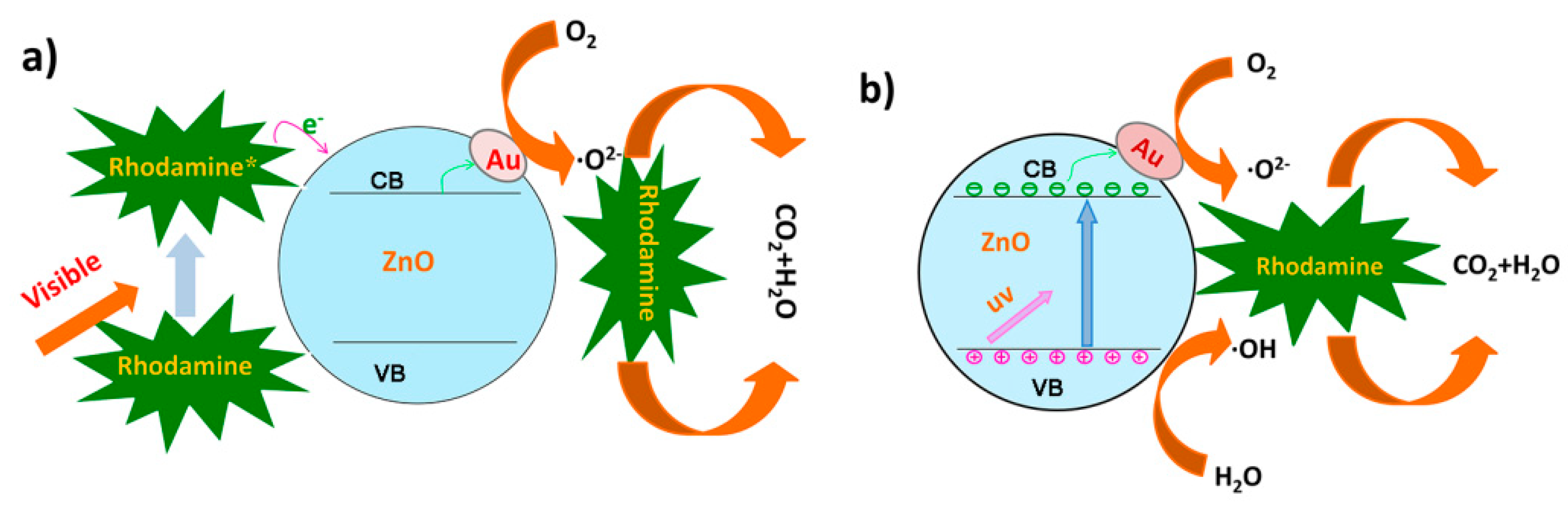
3.4. Mechanisms in Enhancing Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dhatshanamurthi, P.; Shanthi, M. Enhanced photocatalytic degradation of azo dye in aqueous solutions using Ba@Ag@ZnO nanocomposite for self-sensitized under sunshine irradiation. Int. J. Hydrogen Energy 2017, 42, 5523–5536. [Google Scholar] [CrossRef]
- Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Coagulation-flocculation-decantation of dye house effluents: Concentrated effluents. J. Hazard. Mater. 2004, 116, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Golob, V.; Vinder, A.; Simoniè, M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dyes Pigments 2005, 67, 93–97. [Google Scholar] [CrossRef]
- Alinsafi, M.; Khemis, M.N.; Pons, J.P.; Leclerc, A.; Yaacoubi, A.; Benhammou, A.; Nejmeddine, A. Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Process. 2005, 44, 461–470. [Google Scholar] [CrossRef]
- Papiæ, S.; Koprivanac, N.; Božiæ, A.L.; Meteš, A. Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigments 2004, 62, 291–298. [Google Scholar]
- Meshko, V.; Markovska, L.; Mincheva, M.; Rodrigues, A.E. Adsorption of basic dyes on granular acivated carbon and natural zeolite. Water Res. 2001, 35, 3357–3366. [Google Scholar] [CrossRef]
- Oakes, J.; Gratton, P. Kinetic investigations of the oxidation of arylazonaphthol dyes in hypochlorite solutions as a function of pH. J. Chem. Soc. Perkin 1998, 2, 2201–2206. [Google Scholar] [CrossRef]
- Lin, S.H.; Lo, C.C. Treatment of textile wastewater by foam flotation. Environ. Technol. 1996, 17, 841–849. [Google Scholar] [CrossRef]
- McClung, S.M.; Lemley, A.T. Electrochemical treatment and HPLC analysis of wastewater containing acid dyes. Text. Chem. Color. 1994, 26, 17–22. [Google Scholar]
- Yeh, R.Y.L.; Thomas, A. Color difference measurement and color removal from dye wastewaters using different adsorbents. J. Chem. Technol. Biot. 1995, 63, 55–59. [Google Scholar] [CrossRef]
- Bousher, A.; Shen, X.; Edyvean, R.G.J. Removal of coloured organic matter by adsorption onto low-cost waste materials. Water Res. 1997, 31, 2084–2092. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and photoinduced hydrophilicity of various metal oxide thin films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Liu, W.; Yang, D. Facile preparation of well-combined lignin-based carbon/ZnO hybrid composite with excellent photocatalytic activity. Appl. Surf. Sci. 2017, 426, 206–216. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Yang, M. TiO2 nanoparticles supported on PMMA nanofibers for photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2017, 508, 500–507. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Ying, Z.; Albadarin, A.B.; Walker, G. Enhanced photocatalytic degradation of acetaminophen from wastewater using WO3/TiO2/SiO2 composite under UV-Vis irradiation. J. Mol. Liquids 2017, 243, 761–770. [Google Scholar] [CrossRef]
- Wang, X.; Tian, H.; Yang, Y.; Wang, H.; Wang, S.; Zheng, W.; Liu, Y. Reduced graphene oxide/CdS for efficiently photocatalystic degradation of methylene blue. J. Alloys Compd. 2012, 524, 5–12. [Google Scholar] [CrossRef]
- Bian, X.; Hong, K.; Liu, L.; Xu, M. Magnetically separable hybrid CdS-TiO2-Fe3O4 nanomaterial: Enhanced photocatalystic activity under UV and visible irradiation. Appl. Surf. Sci. 2013, 280, 349–353. [Google Scholar] [CrossRef]
- Fan, J.; Li, T.; Heng, H. Hydrothermal growth of ZnO nanoflowers and their photocatalyst application. Bull. Mater. Sci. 2016, 39, 19–26. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Liu, T.; Du, S.; Qiang, Q.; Wang, Y.; Sato, T. Comparison of photocatalytic reaction-induced selective corrosion with photocorrosion: Impact on morphology and stability of Ag-ZnO. Appl. Catal. B Environ. 2017, 201, 348–358. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Zhang, S.; Qiu, C. High photocatalytic performance of high concentration Al-doped ZnO nanoparticles. Sep. Purif. Technol. 2017, 172, 236–241. [Google Scholar] [CrossRef]
- Moradi, M.; Haghighi, M.; Allahyari, S. Precipitation dispersion of Ag-ZnO nanocatalyst over functionalized multiwall carbon nanotube used in degradation of Acid Orange from wastewater. Process Saf. Environ. 2017, 107, 414–427. [Google Scholar] [CrossRef]
- Güy, N.; Özacar, M. The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int. J. Hydrogen Energy 2016, 41, 20100–20112. [Google Scholar] [CrossRef]
- He, W.; Wu, H.; Wamer, W.G.; Kim, H.K.; Zheng, J.; Jia, H.; Yin, J.J. Unraveling the enhanced photocatalytic activity and phototoxicity of ZnO/metal hybrid nanostructures from generation of reactive oxygen species and charge carriers. ACS Appl. Mater. Interfaces 2014, 6, 15527–15535. [Google Scholar] [CrossRef]
- Misra, M.; Kapur, P.; Singla, M.L. Surface plasmon quenched of near band edge emission and enhanced visible photocatalytic activity of Au@ZnO core-shell nanostructure. Appl. Catal. B. Environ. 2014, 150, 605–611. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhang, X.Y.; Cheng, W.Z.; Shao, H.Q.; Liu, X.; Li, X.M.; Liu, H.L.; Wu, J.H. Facile synthesis and optical properties of polymer-laced ZnO-Au hybrid nanoparticles. Nanoscale Res. Lett. 2014, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction. 2001. Available online: http://lib.hpu.edu.vn/handle/123456789/29008 (accessed on 29 December 2018).
- Lupan, O.; Emelchenko, G.A.; Ursaki, V.V.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Heinrich, H. Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Morozov, I.G.; Belousova, O.V.; Ortega, D.; Mafina, M.K.; Kuznetcov, M.V. Structural, optical, XPS and magnetic properties of Zn particles capped by ZnO nanoparticles. J. Alloys Compd. 2015, 633, 237–245. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, Y.; Kim, K. Dodecanethiol-derivatized Au/Ag bimetallic nanoparticles: TEM, UV/VIS, XPS, and FTIR analysis. J. Colloid Interface Sci. 1998, 208, 272–278. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, J.H.; Min, J.H.; Hou, P.; Song, A.Y.; Kim, Y.K. Non-aqueous synthesis of water-dispersible Fe3O4-Ca3(PO4)2 core-shell nanoparticles. Nanotechnology 2010, 22, 055701. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Liu, H.L.; Zhang, W.X.; Cheng, W.Z.; Liu, X.; Li, X.M.; Wu, J.H. Synthesis and characterization of polymer-coated AgZnO nanoparticles with enhanced photocatalytic activity. RSC Adv. 2014, 4, 44011–44017. [Google Scholar] [CrossRef]
- Liu, H.L.; Hou, P.; Zhang, W.X.; Kim, Y.K.; Wu, J.H. The synthesis and characterization of polymer-coated FeAu multifunctional nanoparticles. Nanotechnology 2010, 21, 335602. [Google Scholar] [CrossRef] [PubMed]
- Pawinrat, P.; Mekasuwandumrong, O.; Panpranot, J. Synthesis of Au-ZnO and Pt-ZnO nanocomposites by one-step flame spray pyrolysis and its application for photocatalytic degradation of dyes. Catal. Commun. 2009, 10, 1380–1385. [Google Scholar] [CrossRef]
- Tarwal, N.L.; Patil, P.S. Enhanced photoelectrochemical performance of Ag-ZnO thin films synthesized by spray pyrolysis technique. Electrochim. Acta 2011, 56, 6510–6516. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, Y.H.; Gu, H.C. Synthesis of Ag-Fe3O4 heterodimeric nanoparticles. J. Colloid Interface Sci. 2006, 297, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation and stabilization of silver nanoparticles through reduction by N,N-dimethylformamide. Langmuir 1999, 15, 948–951. [Google Scholar] [CrossRef]
- Tong, Y.H.; Liu, Y.C.; Lu, S.X.; Dong, L.; Chen, S.J.; Xiao, Z.Y. The optical properties of ZnO nanoparticles capped with polyvinyl butyral. J. Sol-Gel. Sci. Technol. 2004, 30, 157–161. [Google Scholar] [CrossRef]
- Singh, A.K.; Viswanath, V.; Janu, V.C. Synthesis, effect of capping agents, structural, optical and photoluminescence properties of ZnO nanoparticles. J. Luminescence 2009, 129, 874–878. [Google Scholar] [CrossRef]
- Wu, X.L.; Siu, G.G.; Fu, C.L.; Ong, H.C. Photoluminescence and cathodoluminescence studies of stoichiometric and oxygen-deficient ZnO films. Appl. Phys. Lett. 2001, 78, 2285–2287. [Google Scholar] [CrossRef]
- Wang, X.; Kong, X.; Yu, Y.; Zhang, H. Synthesis and characterization of water-soluble and bifunctional ZnO-Au nanocomposites. J. Phys. Chem. C 2007, 111, 3836–3841. [Google Scholar] [CrossRef]
- Li, M.; Lv, X.; Ma, X.; Sun, F.; Tang, L.; Wang, Z. Direct synthesis of monodispersed ZnO nanoparticles in an aqueous solution. Mater. Lett. 2007, 61, 690–693. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Xiao, G.; Liu, H.; Wang, M. Synthesis and morphology control of ZnO nanostructures in microemulsions. J. Colloid Interface Sci. 2009, 333, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Maensiri, S.; Laokul, P.; Promarak, V. Synthesis and optical properties of nanocrystalline ZnO powders by a simple method using zinc acetate dihydrate and poly (vinyl pyrrolidone). J. Cryst. Growth 2006, 289, 102–106. [Google Scholar] [CrossRef]
- Chen, P.K.; Lee, G.J.; Davies, S.H.; Masten, S.J.; Amutha, R.; Wu, J.J. Hydrothermal synthesis of coral-like Au/ZnO catalyst and photocatalytic degradation of Orange II dye. Mater. Res. Bull. 2013, 48, 2375–2382. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, T.; Wu, K.; Oikawa, K.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. 3. Degradation of the cationic dye rhodamine B in aqueous anionic surfactant/TiO2 dispersions under visible light irradiation: Evidence for the need of substrate adsorption on TiO2 particles. Environ. Sci. Technol. 1998, 32, 2394–2400. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Wang, X.; Zhan, S.; Li, X.; Liu, X.; Chai, Y.; Xing, R.; Liu, H. Photocatalytic Activity of Monosized AuZnO Composite Nanoparticles. Appl. Sci. 2019, 9, 111. https://doi.org/10.3390/app9010111
Ma C, Wang X, Zhan S, Li X, Liu X, Chai Y, Xing R, Liu H. Photocatalytic Activity of Monosized AuZnO Composite Nanoparticles. Applied Sciences. 2019; 9(1):111. https://doi.org/10.3390/app9010111
Chicago/Turabian StyleMa, Chenguang, Xianhong Wang, Shixia Zhan, Xuemei Li, Xiao Liu, Yun Chai, Ruimin Xing, and Hongling Liu. 2019. "Photocatalytic Activity of Monosized AuZnO Composite Nanoparticles" Applied Sciences 9, no. 1: 111. https://doi.org/10.3390/app9010111



