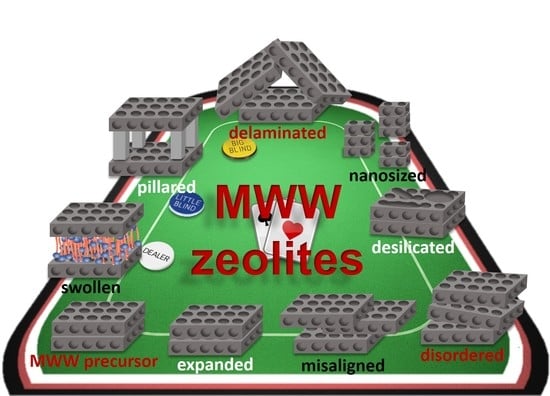
Lamellar MWW-Type Zeolites: Toward Elegant Nanoporous Materials
Abstract
:Featured Application
Abstract
1. Introduction
2. The Precursor (P)MCM-22 and MCM-22 Zeolites
Structure Directing Agent (SDA)
3. MWW-Type Materials by Post-Synthesis Modifications
4. MWW-Type Materials by Direct Synthesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanabe, K.; Hölderich, W.F. Industrial application of solid acid–base catalysts. Appl. Catal. A Gen. 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Vartuli, J.C.; Degnan, T.F., Jr. Applications of mesoporous molecular sieves in catalysis and separations. In Studies in Surface Science and Catalysis; Jiří Čejka, H.v.B.A.C., Ferdi, S., Eds.; Elsevier: New York, NY, USA, 2007; Volume 168, pp. 837–854. [Google Scholar]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Schuth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610–626. [Google Scholar] [CrossRef]
- International Zeolite Association (IZA). Available online: http://www.iza-structure.org/ (accessed on 20 June 2018).
- Roth, W.J.; Gil, B.; Makowski, W.; Marszalek, B.; Eliasova, P. Layer like porous materials with hierarchical structure. Chem. Soc. Rev. 2016, 45, 3400–3438. [Google Scholar] [CrossRef] [PubMed]
- Diaz, U.; Corma, A. Layered zeolitic materials: An approach to designing versatile functional solids. Dalton Trans. 2014, 43, 10292–10316. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.J.; Cejka, J. Two-dimensional zeolites: Dream or reality? Catal. Sci. Technol. 2011, 1, 43–53. [Google Scholar] [CrossRef]
- Ramos, F.S.O.; de Pietre, M.K.; Pastore, H.O. Lamellar zeolites: An oxymoron? RSC Adv. 2013, 3, 2084–2111. [Google Scholar] [CrossRef]
- Opanasenko, M.V.; Roth, W.J.; Cejka, J. Two-dimensional zeolites in catalysis: Current status and perspectives. Catal. Sci. Technol. 2016, 6, 2467–2484. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Pergher, S. Hierarchical MWW Zeolites by Soft and Hard Template Routes. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Berlin, Germany, 2017; pp. 1–23. [Google Scholar]
- Rubin, M.K.; Chu, P. Composition of Synthetic Porous Crystalline Material, Its Synthesis and Use. U.S. Patent 4954325A, 4 September 1990. [Google Scholar]
- Leonowicz, M.E.; Lawton, J.A.; Lawton, S.L.; Rubin, M.K. MCM-22: A Molecular Sieve with Two Independent Multidimensional Channel Systems. Science 1994, 264, 1910–1913. [Google Scholar] [CrossRef] [PubMed]
- Camblor, M.A.; Corma, A.; Díaz-Cabañas, M.-J.; Baerlocher, C. Synthesis and Structural Characterization of MWW Type Zeolite ITQ-1, the Pure Silica Analog of MCM-22 and SSZ-25. J. Phys. Chem. B 1998, 102, 44–51. [Google Scholar] [CrossRef]
- Zhou, D.; Bao, Y.; Yang, M.; He, N.; Yang, G. DFT studies on the location and acid strength of Brönsted acid sites in MCM-22 zeolite. J. Mol. Catal. A Chem. 2006, 244, 11–19. [Google Scholar] [CrossRef]
- Lawton, S.L.; Fung, A.S.; Kennedy, G.J.; Alemany, L.B.; Chang, C.D.; Hatzikos, G.H.; Lissy, D.N.; Rubin, M.K.; Timken, H.-K.C.; Steuernagel, S.; et al. Zeolite MCM-49: A Three-Dimensional MCM-22 Analogue Synthesized by in Situ Crystallization. J. Phys. Chem. B 1996, 100, 3788–3798. [Google Scholar] [CrossRef]
- Bennett, J.M.; Lawton, C.D.C.S.L.; Leonowicz, M.E.; Lissy, D.N.; Rubin, M.K. Synthetic porous crystalline MCM-49, its synthesis and use. U.S. Patent 5236575, 17 August 1993. [Google Scholar]
- Fung, A.S.; Lawton, S.L.; Roth, W.J. Synthetic Layered MCM-56, Its Synthesis and Use. U.S. Patent 5362697A, 8 November 1994. [Google Scholar]
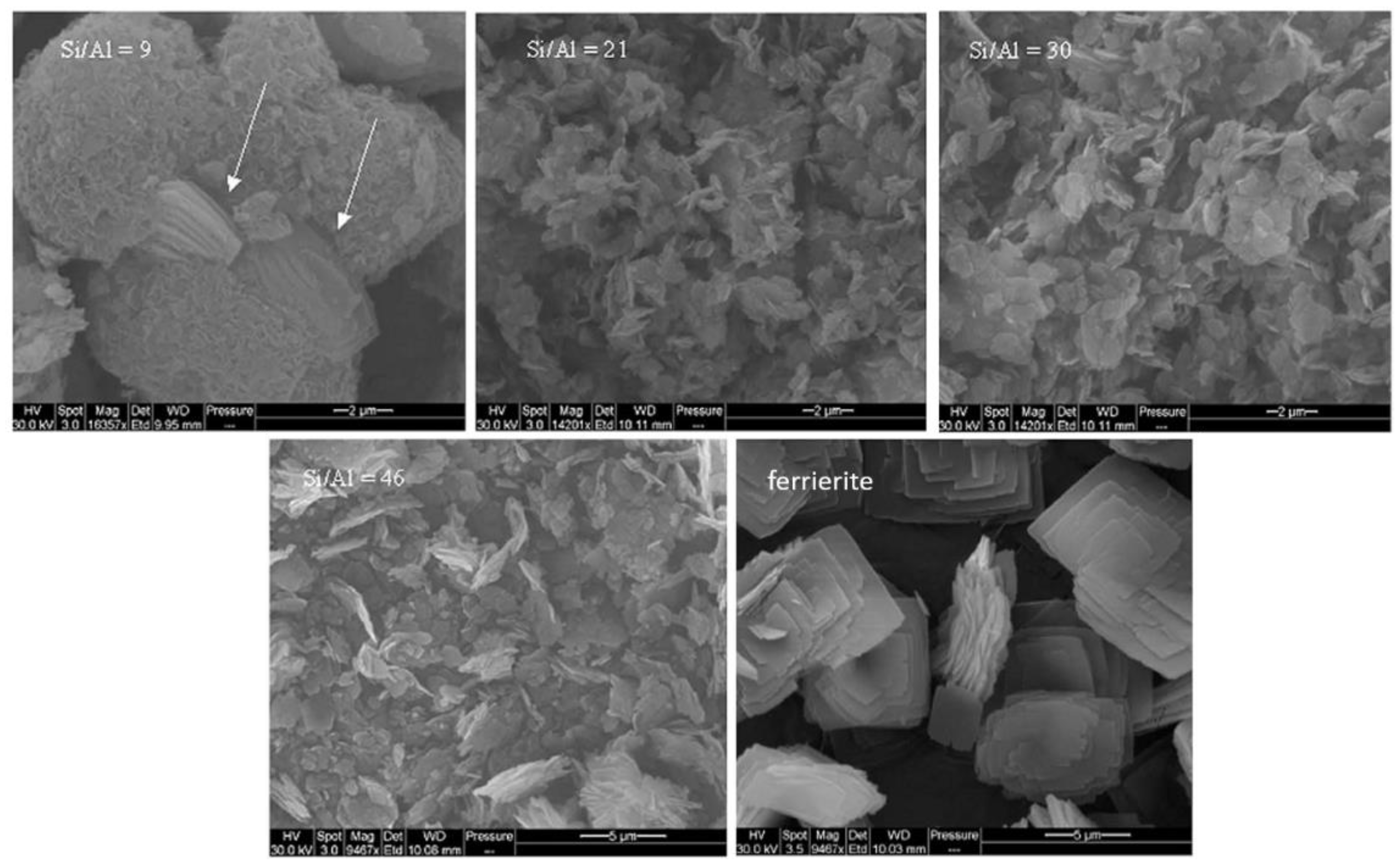
- Delitala, C.; Alba, M.D.; Becerro, A.I.; Delpiano, D.; Meloni, D.; Musu, E.; Ferino, I. Synthesis of MCM-22 zeolites of different Si/Al ratio and their structural, morphological and textural characterisation. Microporous Mesoporous Mater. 2009, 118, 1–10. [Google Scholar] [CrossRef]
- Ravishankar, R.; Sen, T.; Ramaswamy, V.; Soni, H.S.; Ganapathy, S.; Sivasanker, S. Synthesis, Characterization and Catalytic properties of Zeolite PSH-3/MCM-22. In Studies in Surface Science and Catalysis; Weitkamp, J., Karge, H.G., Pfeifer, H., Hölderich, W., Eds.; Elsevier: New York, NY, USA, 1994; Volume 84, pp. 331–338. [Google Scholar]
- Corma, A.; Corell, C.; Pérez-Pariente, J.; Guil, J.M.; Guil-López, R.; Nicolopoulos, S.; Calbet, J.G.; Vallet-Regi, M. Adsorption and catalytic properties of MCM-22: The influence of zeolite structure. Zeolites 1996, 16, 7–14. [Google Scholar] [CrossRef]
- Wang, Y.M.; Shu, X.T.; He, M.Y. 02-P-34—Static synthesis of zeolite MCM-22. In Studies in Surface Science and Catalysis; Galarneau, A., Fajula, F., Di Renzo, F., Vedrine, J., Eds.; Elsevier: New York, NY, USA, 2001; Volume 135, p. 194. [Google Scholar]
- Bevilacqua, M.; Meloni, D.; Sini, F.; Monaci, R.; Montanari, T.; Busca, G. A Study of the Nature, Strength, and Accessibility of Acid Sites of H-MCM-22 Zeolite. J. Phys. Chem. C 2008, 112, 9023–9033. [Google Scholar] [CrossRef]
- Meloni, D.; Laforge, S.; Martin, D.; Guisnet, M.; Rombi, E.; Solinas, V. Acidic and catalytic properties of H-MCM-22 zeolites: 1. Characterization of the acidity by pyridine adsorption. Appl. Catal. A 2001, 215, 55–66. [Google Scholar] [CrossRef]
- Onida, B.; Geobaldo, F.; Testa, F.; Aiello, R.; Garrone, E. H-Bond Formation and Proton Transfer in H-MCM-22 Zeolite as Compared to H-ZSM-5 and H-MOR: An FTIR Study. J. Phys. Chem. B 2002, 106, 1684–1690. [Google Scholar] [CrossRef]
- Güray, I.; Warzywoda, J.; Baç, N.; Sacco, A., Jr. Synthesis of zeolite MCM-22 under rotating and static conditions. Microporous Mesoporous Mater. 1999, 31, 241–251. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, X.; Wang, J. Facile synthesis and morphology control of zeolite MCM-22 via a two-step sol–gel route with tetraethyl orthosilicate as silica source. Mater. Chem. Phys. 2009, 113, 773–779. [Google Scholar] [CrossRef]
- Inagaki, S.; Kamino, K.; Hoshino, M.; Kikuchi, E.; Matsukata, M. Textural and catalytic properties of MCM-22 zeolite crystallized by the vapor-phase transport method. Bull. Chem. Soc. Jpn. 2004, 77, 1249–1254. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, X.; Lu, Y.; Wang, J. Crystallization and morphology of zeolite MCM-22 influenced by various conditions in the static hydrothermal synthesis. Microporous Mesoporous Mater. 2008, 112, 138–146. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, M.; Li, J.; Su, X.; Pan, S.; Jiao, C.; Feng, M. Synthesis of MCM-22 zeolite using rice husk as a silica source under varying-temperature conditions. J. Colloid Interface Sci. 2012, 369, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Zones, S.I. A crystalline zeolite SSZ-25 Is Prepared Using an Adamantane Quaternary Ammonium Ion as a Template. E.U. Patent 231860, 12 May 1987. [Google Scholar]
- Puppe, L.; Weisser, J. Crystalline Aluminosilicate PSH-3 and Its Process of Preparation. U.S. Patent 4439409A, 23 March 1984. [Google Scholar]
- Bellussi, G.; Perego, G.; Cierici, M.G.; Giusti, A. Bulletin of the Chemical Society of Japan. E.U. Patent 293032, 30 November 1988. [Google Scholar]
- Rohde, L.M.; Lewis, G.J.; Miller, M.A.; Moscoso, J.G.; Gisselquist, J.L.; Patton, R.L.; Wilson, S.T.; Jan, D.Y. Crystalline Aluminosilicate Zeolitic Composition: UZM-8. U.S. Patent 6756030B1, 21 March 2003. [Google Scholar]
- Corma, A.; Díaz-Cabanas, M.J.; Moliner, M.; Martínez, C. Discovery of a new catalytically active and selective zeolite (ITQ-30) by high-throughput synthesis techniques. J. Catal. 2006, 241, 312–318. [Google Scholar] [CrossRef]
- Roth, W.J.; Dorset, D.L.; Kennedy, G.J. Discovery of new MWW family zeolite EMM-10: Identification of EMM-10P as the missing MWW precursor with disordered layers. Microporous Mesoporous Mater. 2011, 142, 168–177. [Google Scholar] [CrossRef]
- Zones, S.I.; Davis, T.M. Zeolite SSZ-70 Having Enhanced External Surface Area. E.U. Patent 3027559B1, 30 July 2013. [Google Scholar]
- Xu, L.; Ji, X.; Jiang, J.-G.; Han, L.; Che, S.; Wu, P. Intergrown Zeolite MWW Polymorphs Prepared by the Rapid Dissolution–Recrystallization Route. Chem. Mater. 2015, 27, 7852–7860. [Google Scholar] [CrossRef]
- Xing, E.; Shi, Y.; Xie, W.; Zhang, F.; Mu, X.; Shu, X. Synthesis, characterization and application of MCM-22 zeolites via a conventional HMI route and temperature-controlled phase transfer hydrothermal synthesis. RSC Adv. 2015, 5, 8514–8522. [Google Scholar] [CrossRef]
- Xing, E.; Shi, Y.; Xie, W.; Zhang, F.; Mu, X.; Shu, X. Perspectives on the multi-functions of aniline: Cases from the temperature-controlled phase transfer hydrothermal synthesis of MWW zeolites. Microporous Mesoporous Mater. 2017, 254, 201–210. [Google Scholar] [CrossRef]
- Ji, P.; Shen, M.; Lu, K.; Hu, B.; Jiang, J.-G.; Xu, H.; Wu, P. ECNU-10 zeolite: A three-dimensional MWW-Type analogue. Microporous Mesoporous Mater. 2017, 253, 137–145. [Google Scholar] [CrossRef]
- Chu, W.; Li, X.; Liu, S.; Zhu, X.; Xie, S.; Chen, F.; Wang, Y.; Xin, W.; Xu, L. Direct synthesis of three-dimensional MWW zeolite with cyclohexylamine as an organic structure-directing agent. J. Mat. Chem. A 2018. [Google Scholar] [CrossRef]
- Wu, P.; Ruan, J.; Wang, L.; Wu, L.; Wang, Y.; Liu, Y.; Fan, W.; He, M.; Terasaki, O.; Tatsumi, T. Methodology for Synthesizing Crystalline Metallosilicates with Expanded Pore Windows Through Molecular Alkoxysilylation of Zeolitic Lamellar Precursors. J. Am. Chem. Soc. 2008, 130, 8178–8187. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.J. Cation Size Effects in Swelling of the Layered Zeolite Precursor MCM-22-P. Pol. J. Chem. 2006, 80, 703–708. [Google Scholar]
- Maheshwari, S.; Jordan, E.; Kumar, S.; Bates, F.S.; Penn, R.L.; Shantz, D.F.; Tsapatsis, M. Layer Structure Preservation during Swelling, Pillaring, and Exfoliation of a Zeolite Precursor. J. Am. Chem. Soc. 2008, 130, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Schwanke, A.J.; Pergher, S.; Díaz, U.; Corma, A. The influence of swelling agents molecular dimensions on lamellar morphology of MWW-type zeolites active for fructose conversion. Microporous Mesoporous Mater. 2017, 254, 17–27. [Google Scholar] [CrossRef]
- Chlubná, P.; Roth, W.J.; Zukal, A.; Kubů, M.; Pavlatová, J. Pillared MWW zeolites MCM-36 prepared by swelling MCM-22P in concentrated surfactant solutions. Catal. Today 2012, 179, 35–42. [Google Scholar] [CrossRef]
- Roth, W.J.; Chlubná, P.; Kubů, M.; Vitvarová, D. Swelling of MCM-56 and MCM-22P with a new medium—Surfactant-tetramethylammonium hydroxide mixtures. Catal. Today 2013, 204, 8–14. [Google Scholar] [CrossRef]
- Roth, W.J.; Čejka, J.; Millini, R.; Montanari, E.; Gil, B.; Kubu, M. Swelling and Interlayer Chemistry of Layered MWW Zeolites MCM-22 and MCM-56 with High Al Content. Chem. Mater. 2015, 27, 4620–4629. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Díaz, U.; Corma, A.; Pergher, S. Recyclable swelling solutions for friendly preparation of pillared MWW-type zeolites. Microporous Mesoporous Mater. 2017, 253, 91–95. [Google Scholar] [CrossRef]
- Kresge, C.T.; Roth, W.J.; Simmons, K.G.; Vartuli, J.C. Layered oxide materials and swollen and pillared forms thereof. WO Patent 1992011934A1, 23 July 1992. [Google Scholar]
- Roth, W.J.; Kresge, C.T.; Vartuli, J.C.; Leonowicz, M.E.; Fung, A.S.; McCullen, S.B. MCM-36: The first pillared molecular sieve with zeolite properties. In Studies in Surface Science and Catalysis; Beyer, H.K., Karge, H.G., Kiricsi, I., Nagy, J.B., Eds.; Elsevier: New York, NY, USA, 1995; Volume 94, pp. 301–308. [Google Scholar]
- Corma, A.; Díaz, U.; García, T.; Sastre, G.; Velty, A. Multifunctional Hybrid Organic−Inorganic Catalytic Materials with a Hierarchical System of Well-Defined Micro- and Mesopores. J. Am. Chem. Soc. 2010, 132, 15011–15021. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Fornes, V.; Pergher, S.B.C.; Maesen, T.L.M.; Buglass, J.G. Delaminated zeolite precursors as selective acidic catalysts. Nature 1998, 396, 353–356. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Fornés, V.; Guil, J.M.; Martínez-Triguero, J.; Creyghton, E.J. Characterization and Catalytic Activity of MCM-22 and MCM-56 Compared with ITQ-2. J. Catal. 2000, 191, 218–224. [Google Scholar] [CrossRef]
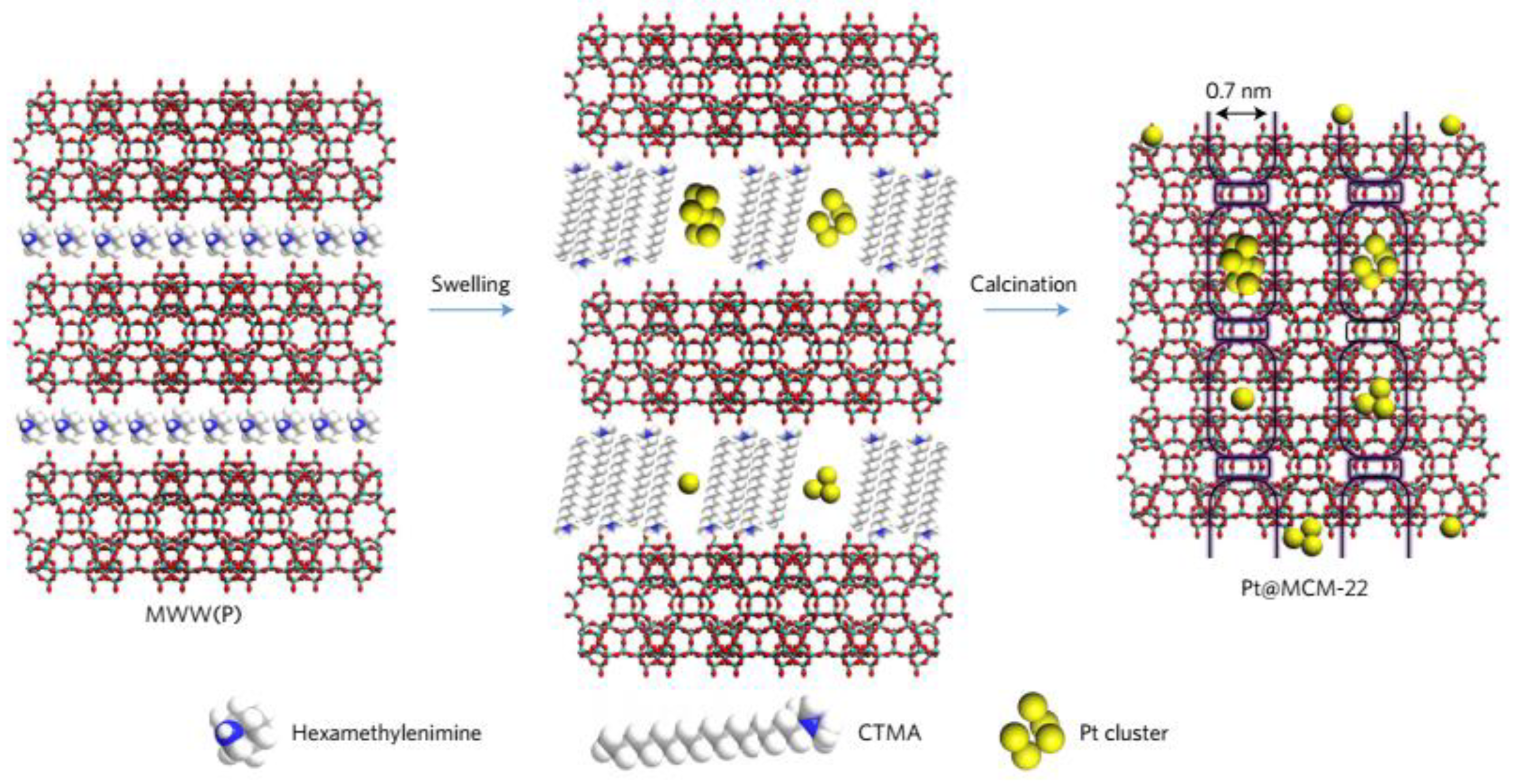
- Liu, L.; Díaz, U.; Arenal, R.; Agostini, G.; Concepción, P.; Corma, A. Corrigendum: Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mat. 2017, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
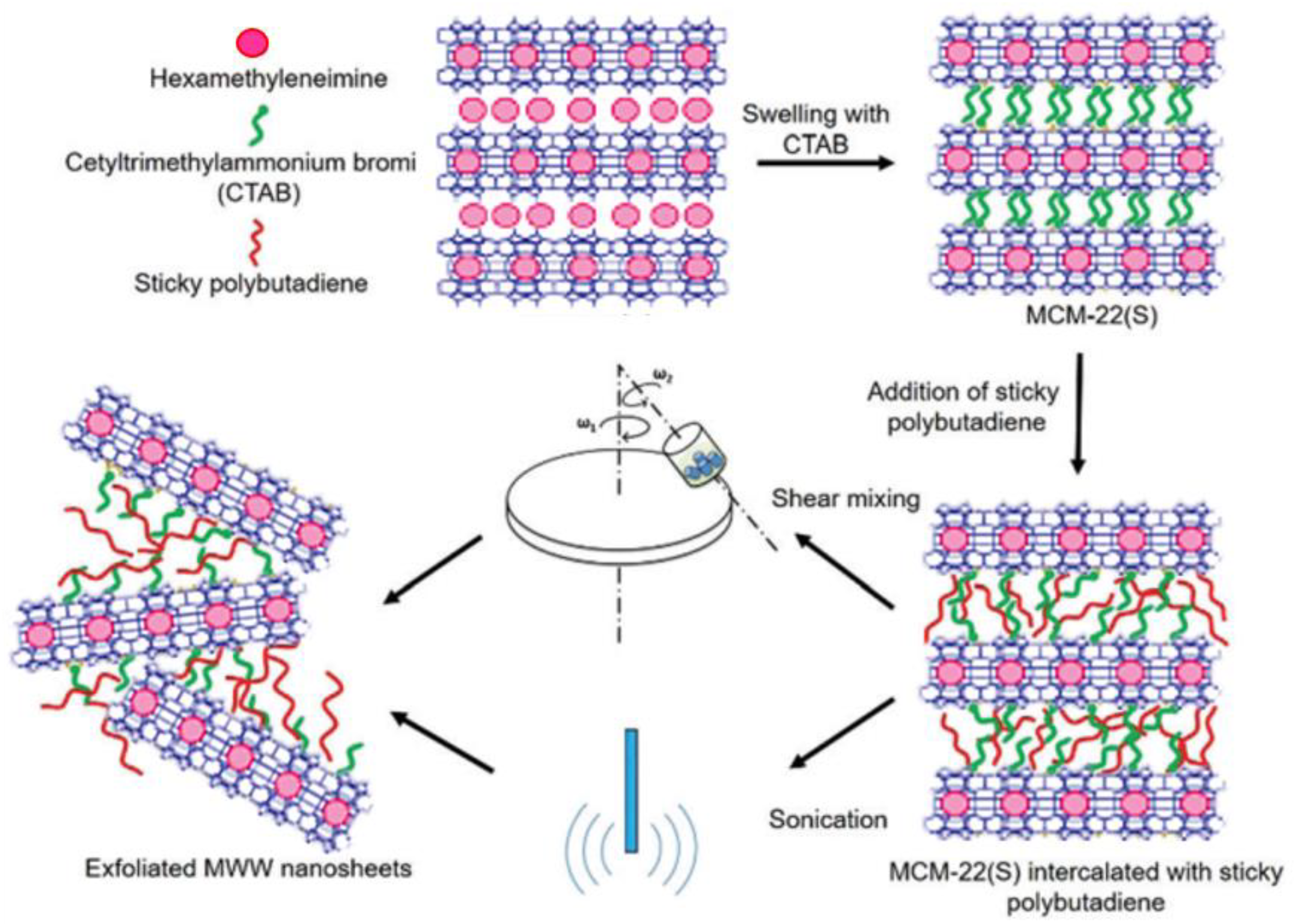
- Sabnis, S.; Tanna, V.A.; Li, C.; Zhu, J.; Vattipalli, V.; Nonnenmann, S.S.; Sheng, G.; Lai, Z.; Winter, H.H.; Fan, W. Exfoliation of two-dimensional zeolites in liquid polybutadienes. Chem. Commun. 2017, 53, 7011–7014. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Xie, S.; Liu, S.; Xin, W.; Gao, Y.; Li, X.; Wei, H.; Liu, H.; Xu, L. Development of hierarchical MCM-49 zeolite with intracrystalline mesopores and improved catalytic performance in liquid alkylation of benzene with ethylene. Microporous Mesoporous Mater. 2015, 212, 1–7. [Google Scholar] [CrossRef]
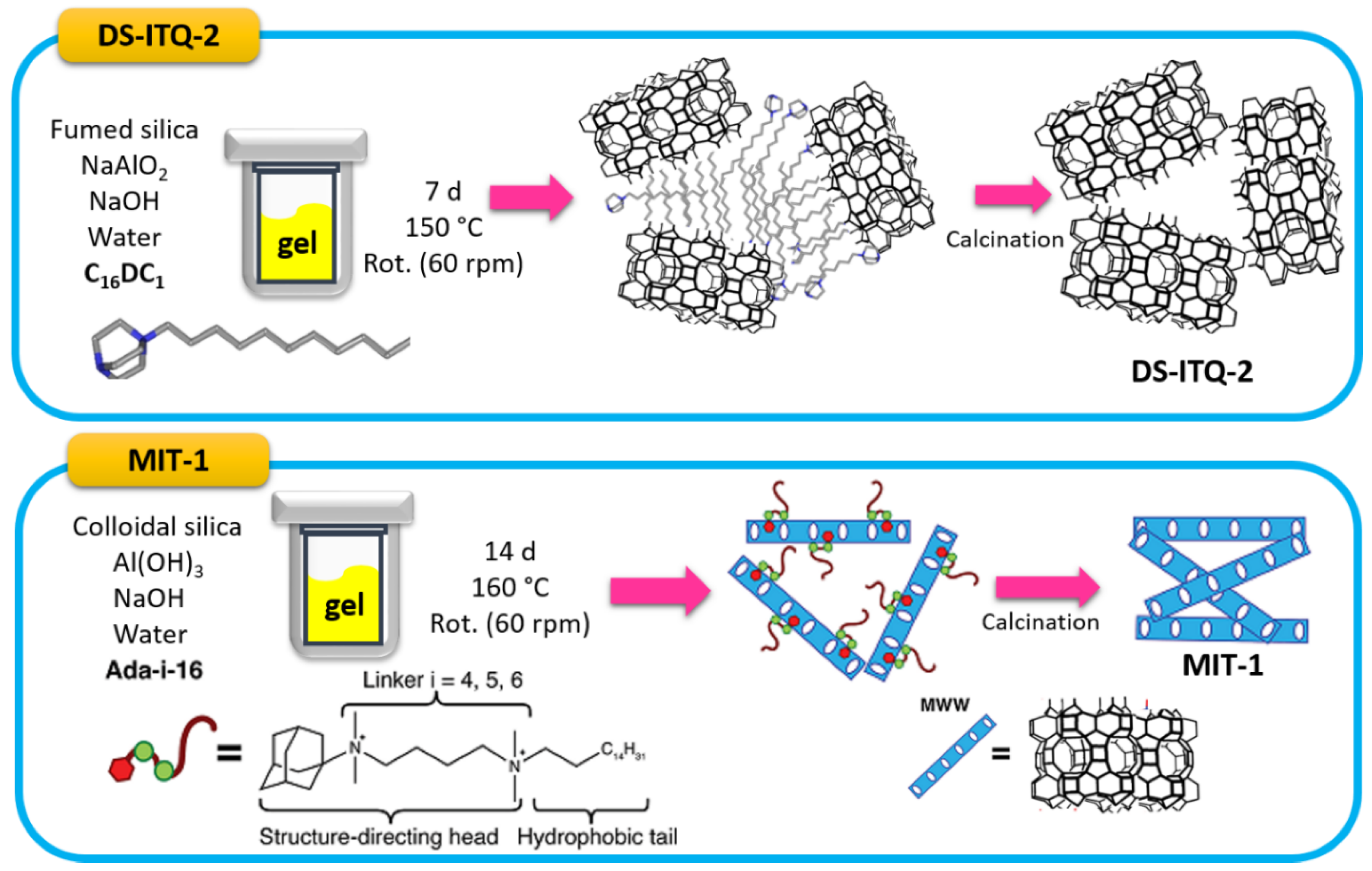
- Luo, H.Y.; Michaelis, V.K.; Hodges, S.; Griffin, R.G.; Roman-Leshkov, Y. One-pot synthesis of MWW zeolite nanosheets using a rationally designed organic structure-directing agent. Chem. Sci. 2015, 6, 6320–6324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Ji, X.; Li, S.; Zhou, Z.; Du, X.; Sun, J.; Deng, F.; Che, S.; Wu, P. Self-Assembly of Cetyltrimethylammonium Bromide and Lamellar Zeolite Precursor for the Preparation of Hierarchical MWW Zeolite. Chem. Mater. 2016, 28, 4512–4521. [Google Scholar] [CrossRef]
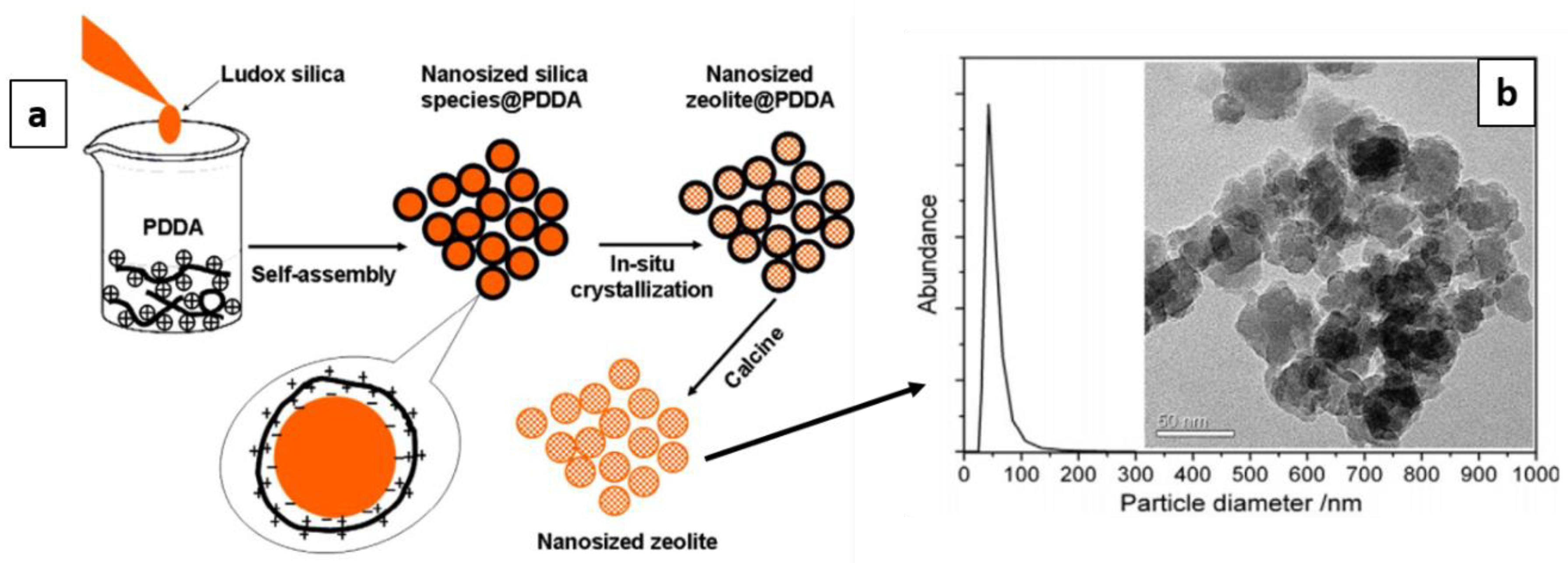
- Mintova, S.; Grand, J.; Valtchev, V. Nanosized zeolites: Quo Vadis? C. R. Chim. 2016, 19, 183–191. [Google Scholar] [CrossRef]
- Yin, X.; Chu, N.; Yang, J.; Wang, J.; Li, Z. Synthesis of the nanosized MCM-22 zeolite and its catalytic performance in methane dehydro-aromatization reaction. Catal. Commun. 2014, 43, 218–222. [Google Scholar] [CrossRef]
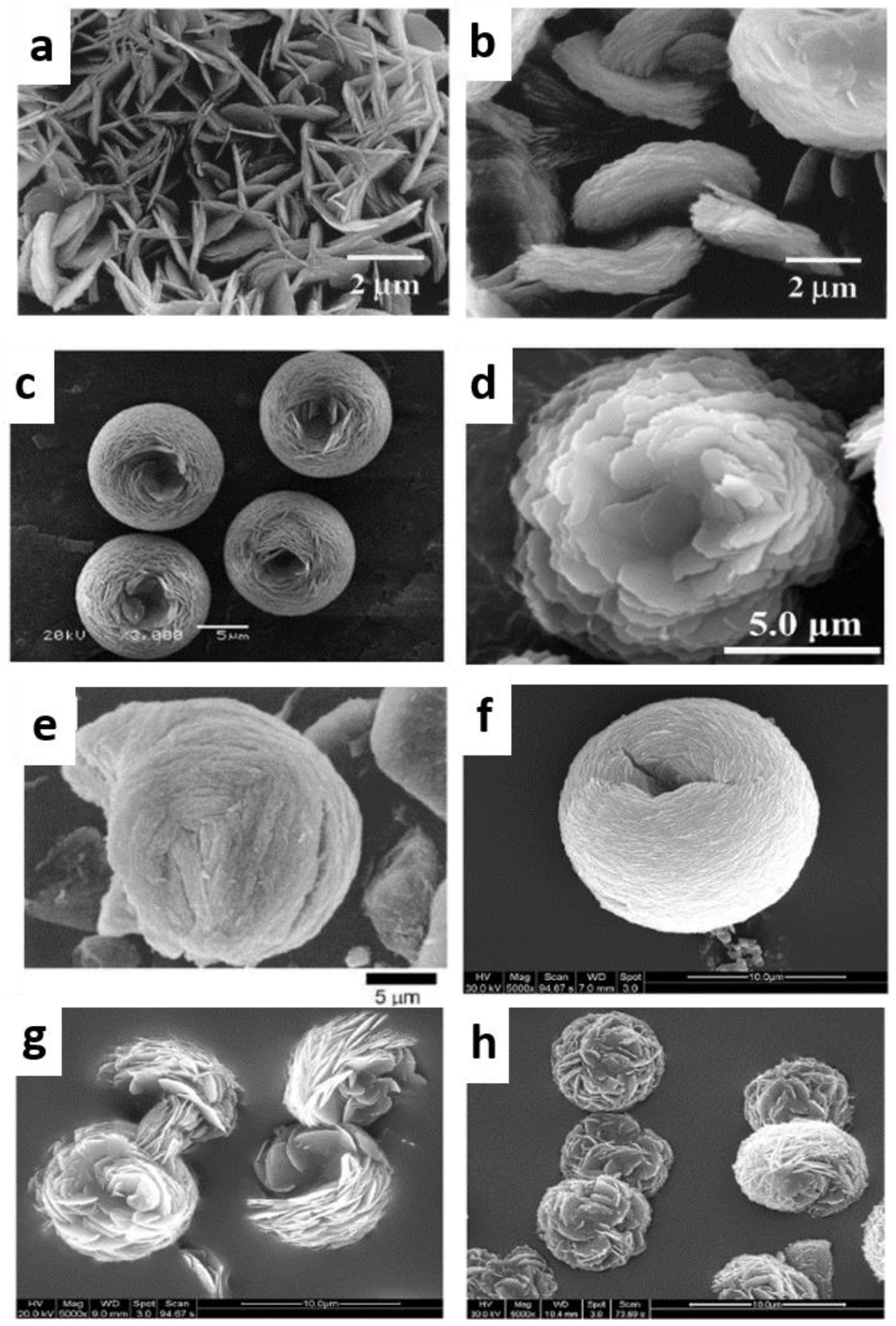
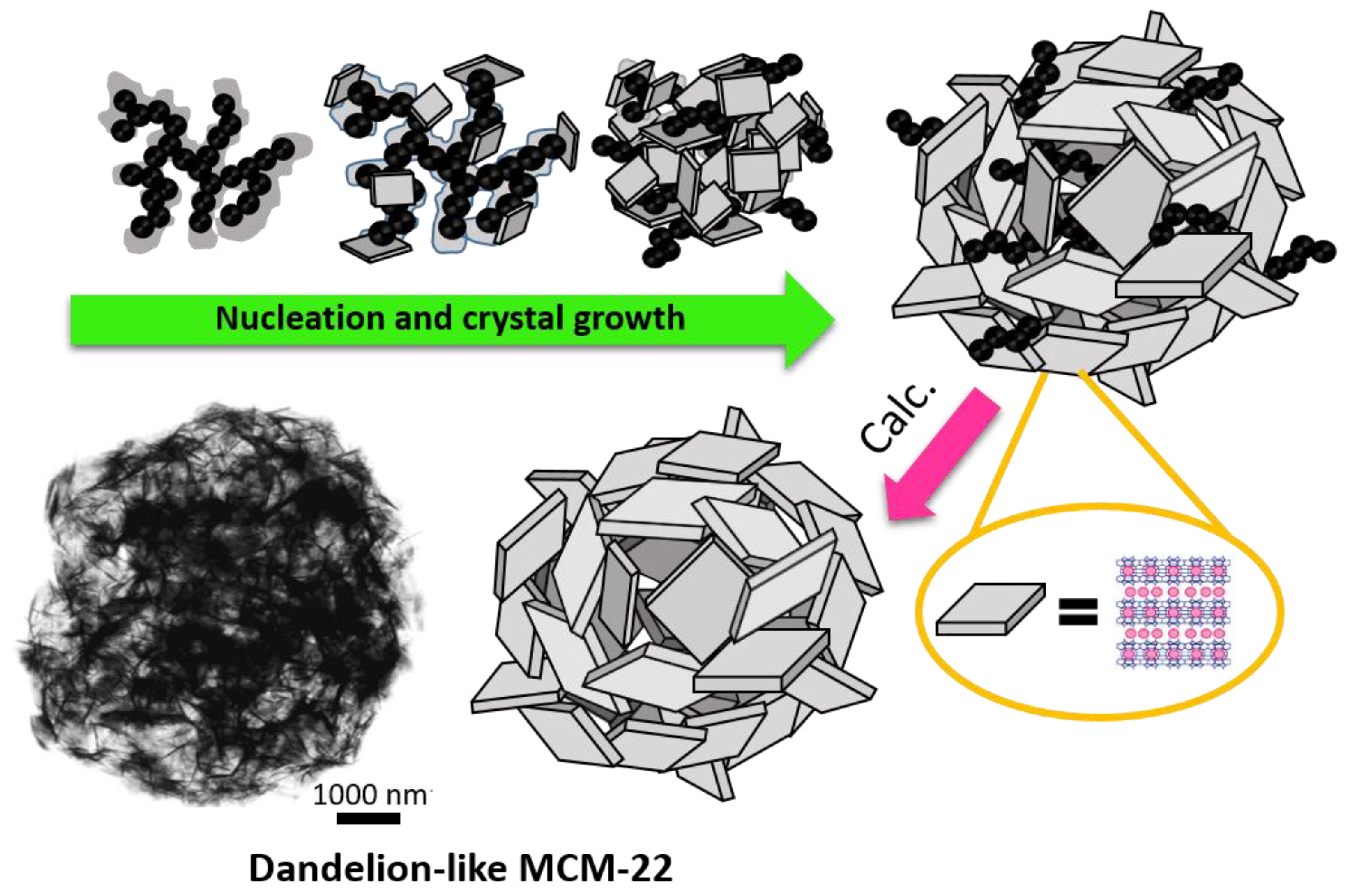
- Schwanke, A.; Villarroel-Rocha, J.; Sapag, K.; Díaz, U.; Corma, A.; Pergher, S. Dandelion-Like Microspherical MCM-22 Zeolite Using BP 2000 as a Hard Template. ACS Omega 2018, 3, 6217–6223. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwanke, A.; Pergher, S. Lamellar MWW-Type Zeolites: Toward Elegant Nanoporous Materials. Appl. Sci. 2018, 8, 1636. https://doi.org/10.3390/app8091636
Schwanke A, Pergher S. Lamellar MWW-Type Zeolites: Toward Elegant Nanoporous Materials. Applied Sciences. 2018; 8(9):1636. https://doi.org/10.3390/app8091636
Chicago/Turabian StyleSchwanke, Anderson, and Sibele Pergher. 2018. "Lamellar MWW-Type Zeolites: Toward Elegant Nanoporous Materials" Applied Sciences 8, no. 9: 1636. https://doi.org/10.3390/app8091636