Numerical Investigations on Melting Behavior of Phase Change Material in a Rectangular Cavity at Different Inclination Angles
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Setup
2.1. Thermophysical Properties of Paraffin
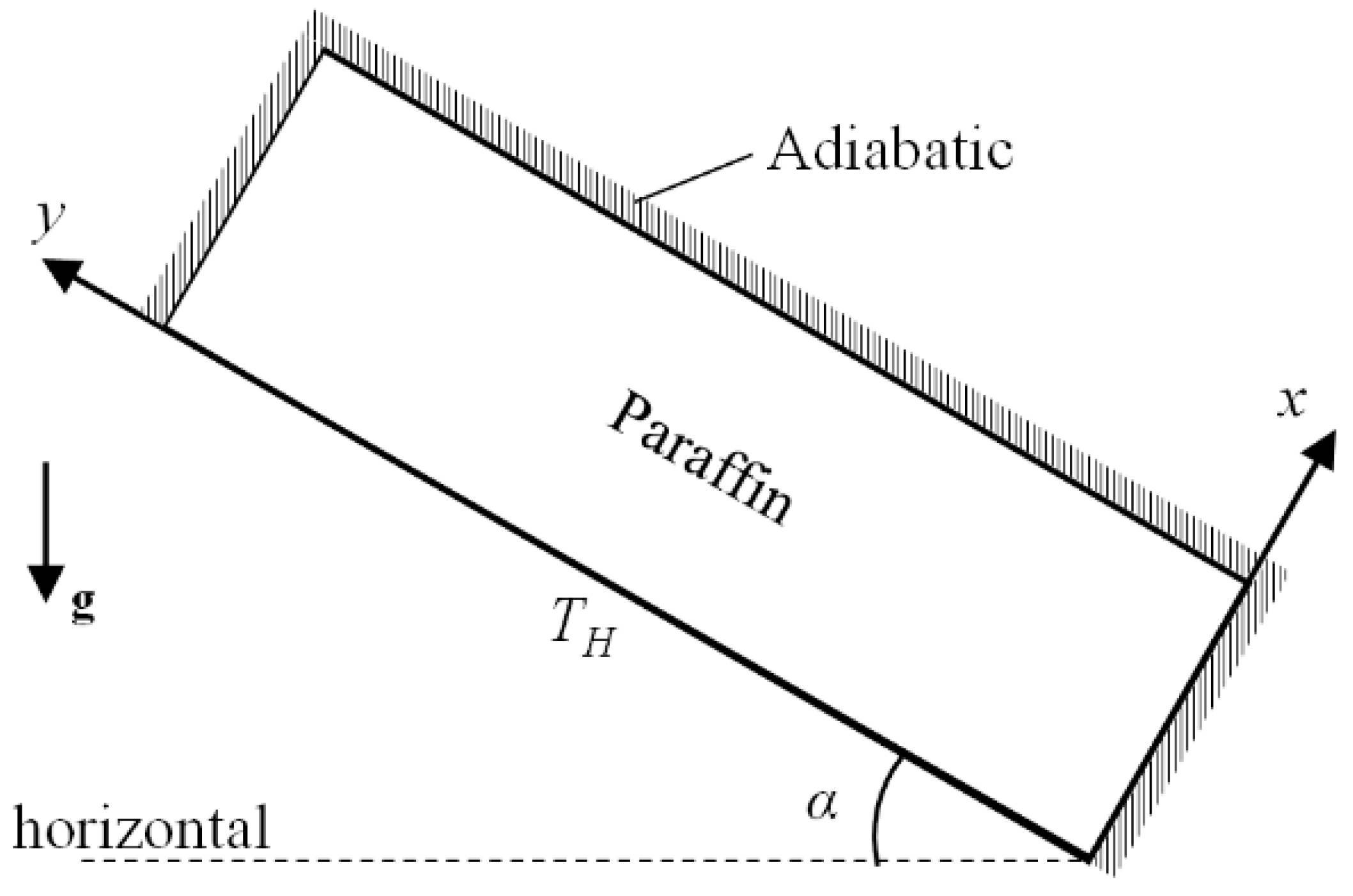
2.2. Physical Model
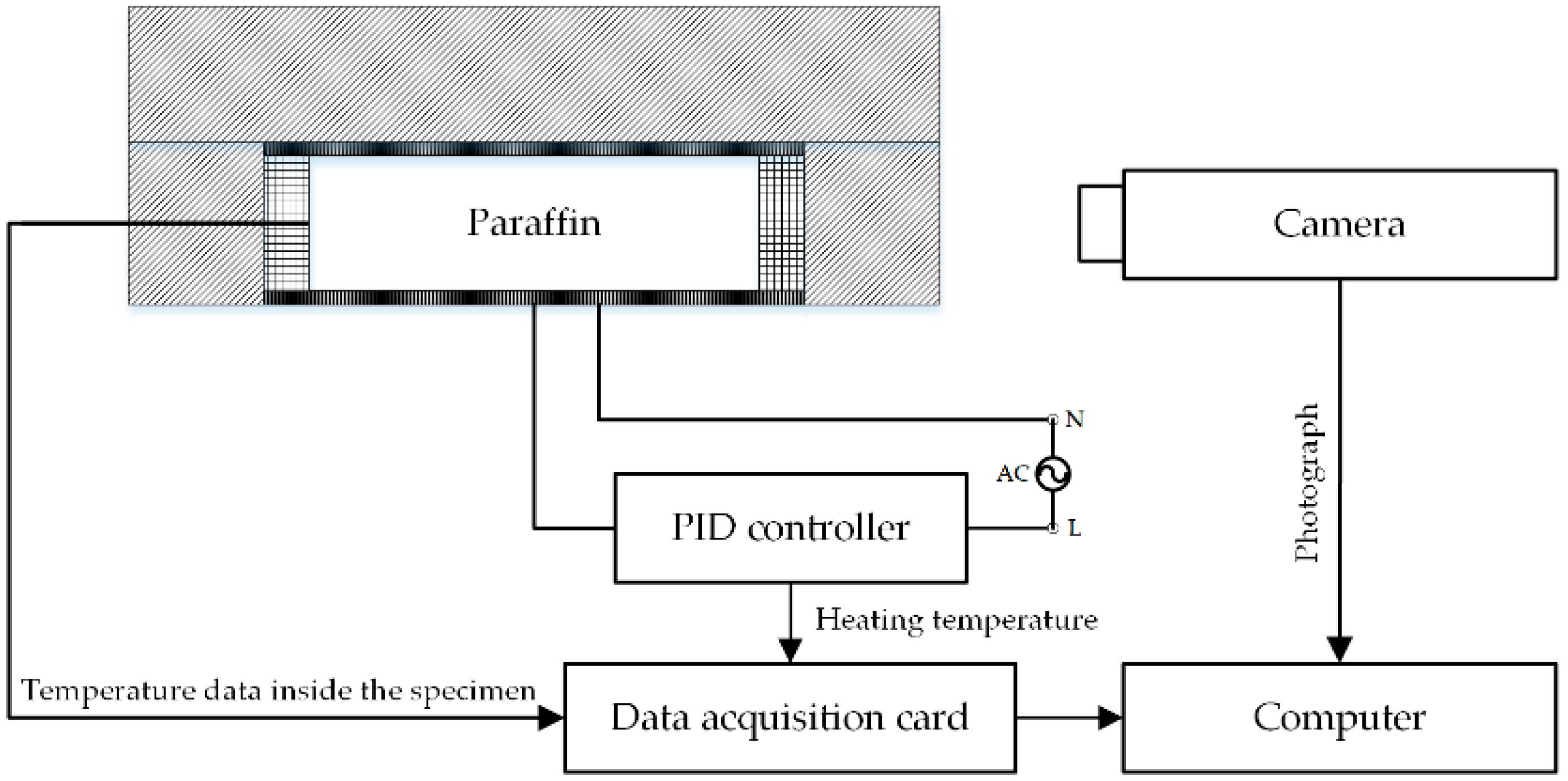
2.3. Experimental Apparatus
3. Mathematical Model
3.1. Governing Equations
- (1)
- The liquid paraffin is regarded as an incompressible Newtonian fluid, and its density is subjected to the Boussinesq approximation.
- (2)
- The paraffin is assumed isotropic and homogeneous, and the flow of liquid paraffin is laminar flow. The volume change of paraffin during phase change is not taken into account.
- (3)
- The thermal properties of paraffin are constant in the solid or liquid region. In the phase change region, these parameters vary linearly with temperature.
- (4)
- Because the specimen is surrounded by thermal insulation tiles, the walls are regarded as adiabatic, except for the heating wall.
3.2. Initial and Boundary Conditions
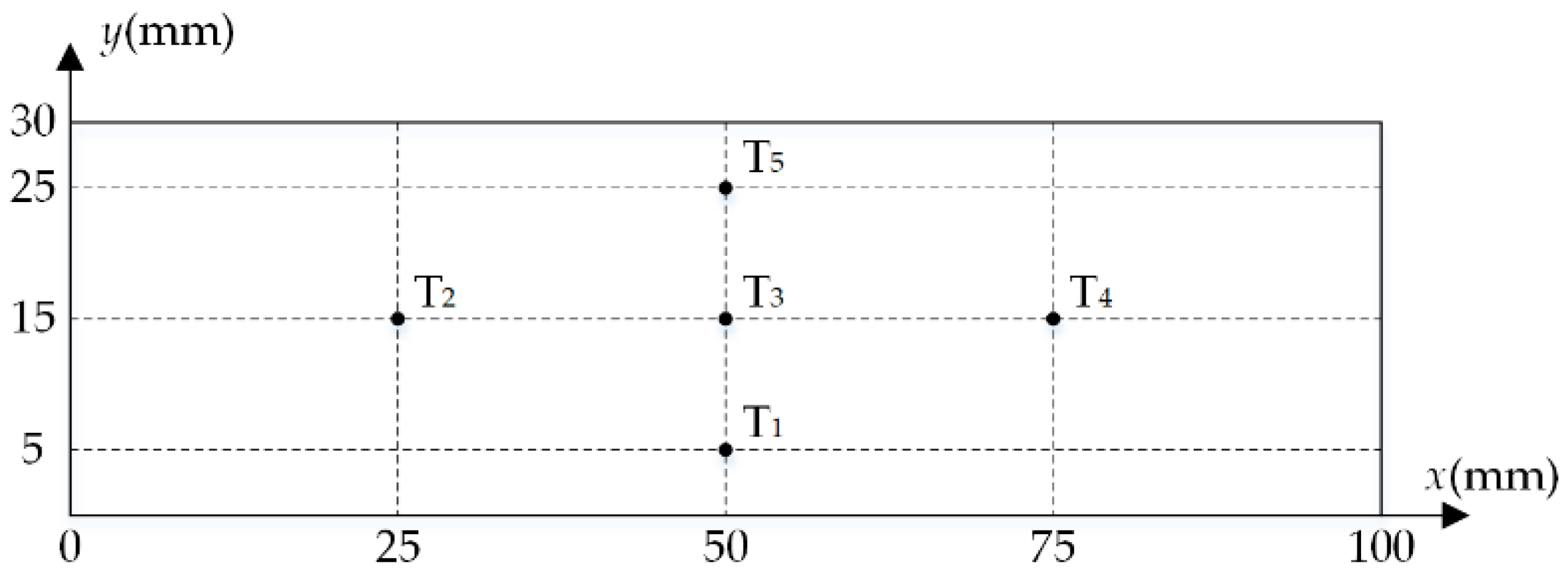
3.3. Numerical Procedure
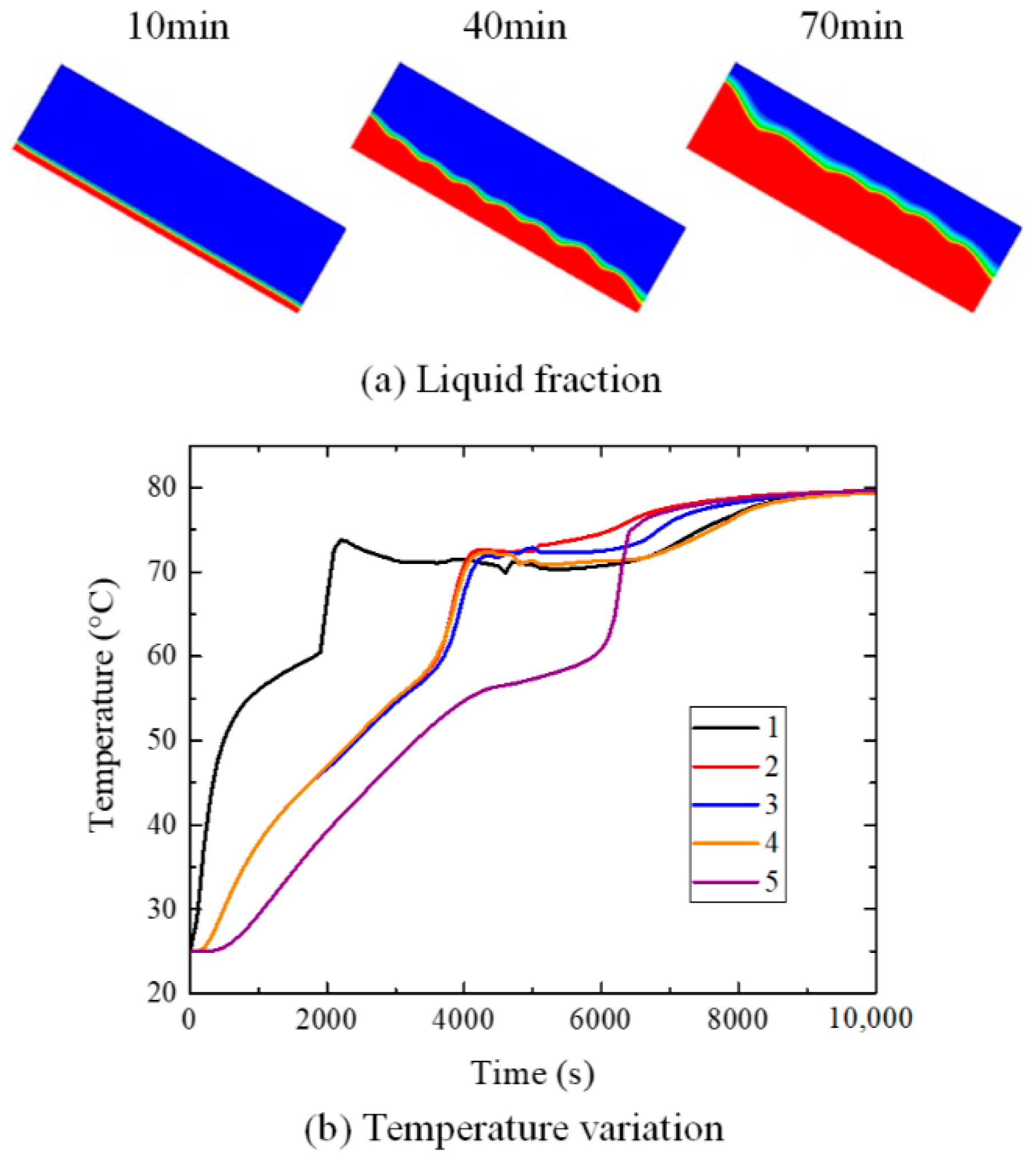
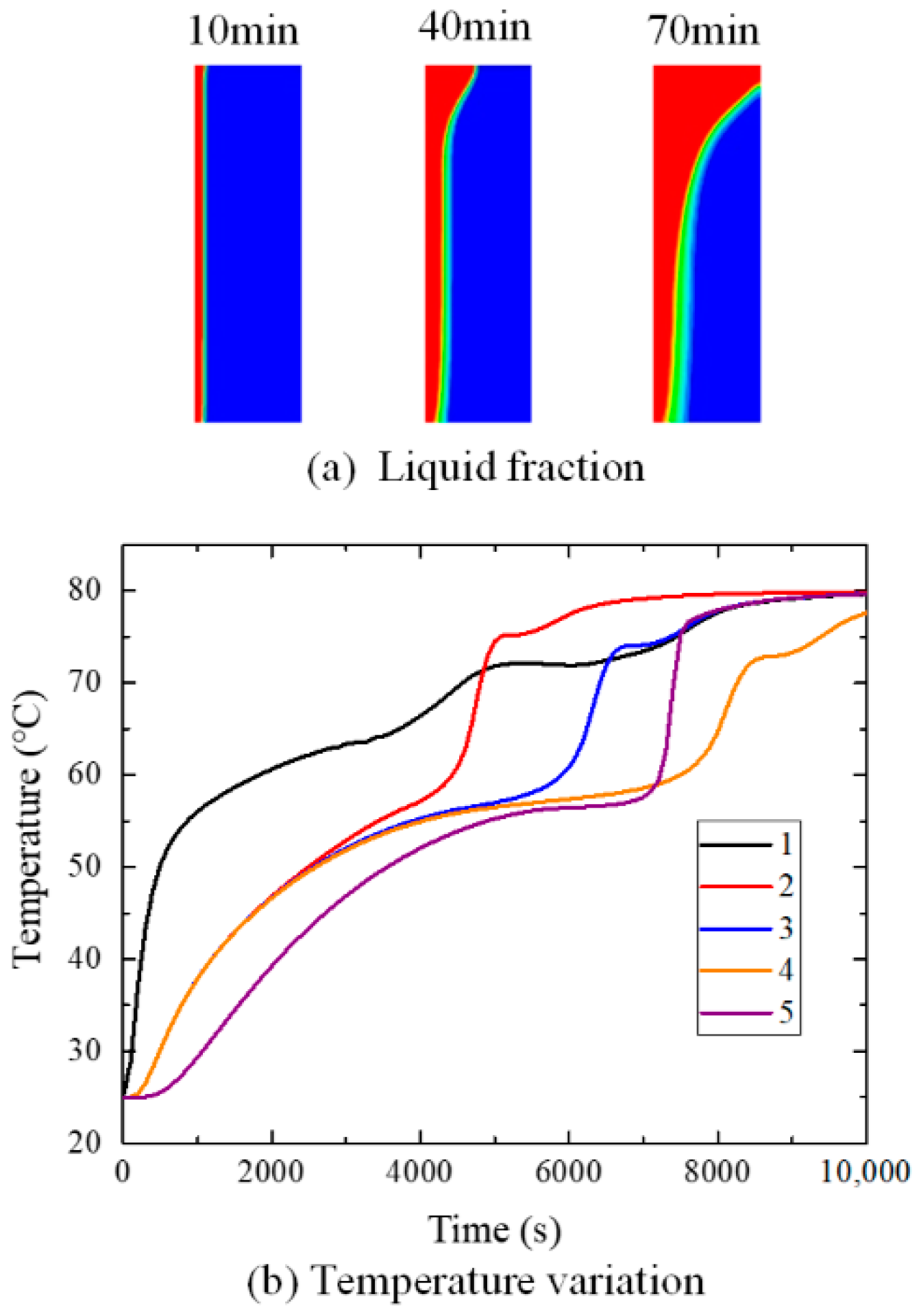
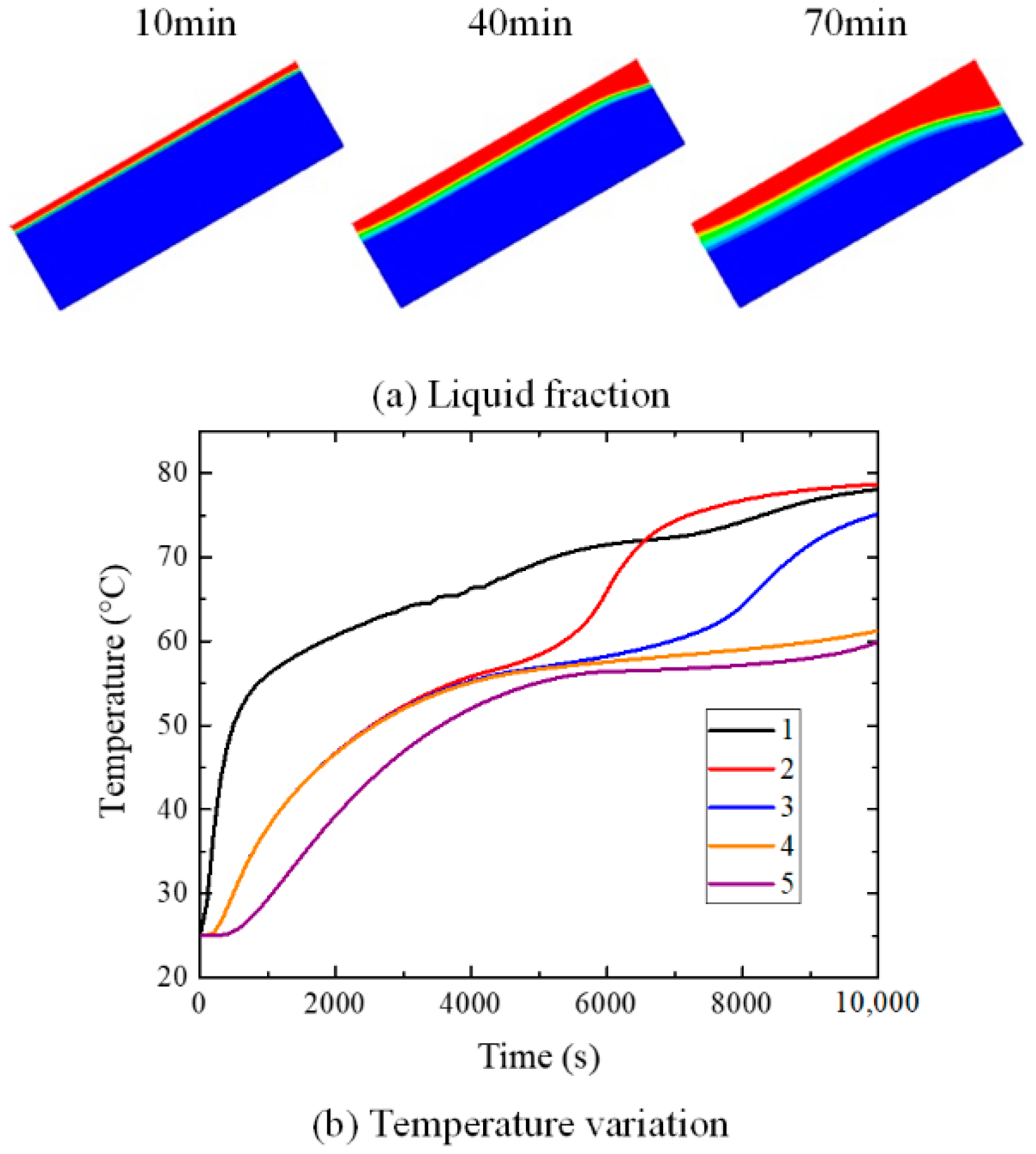
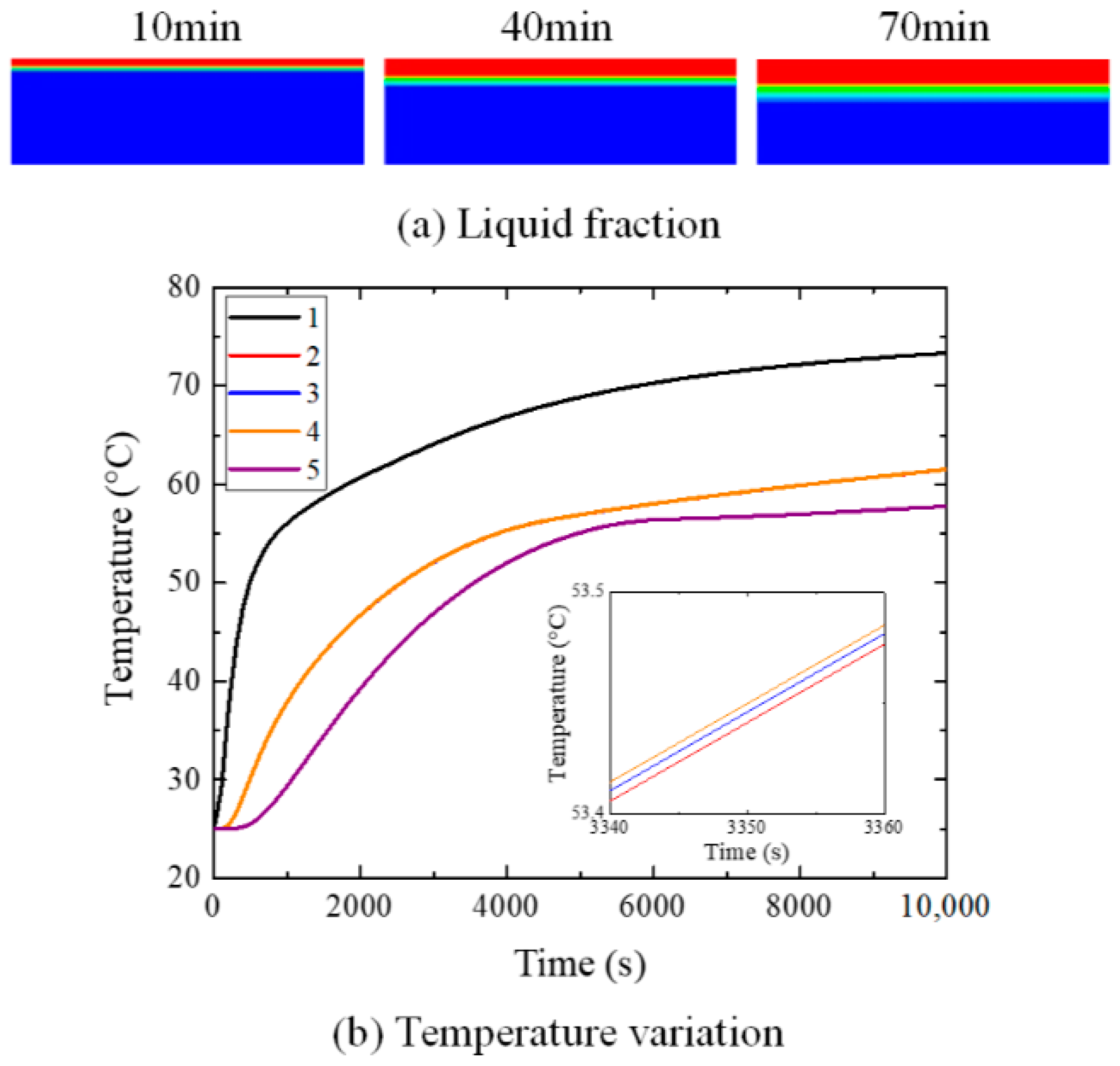
4. Results and Discussions
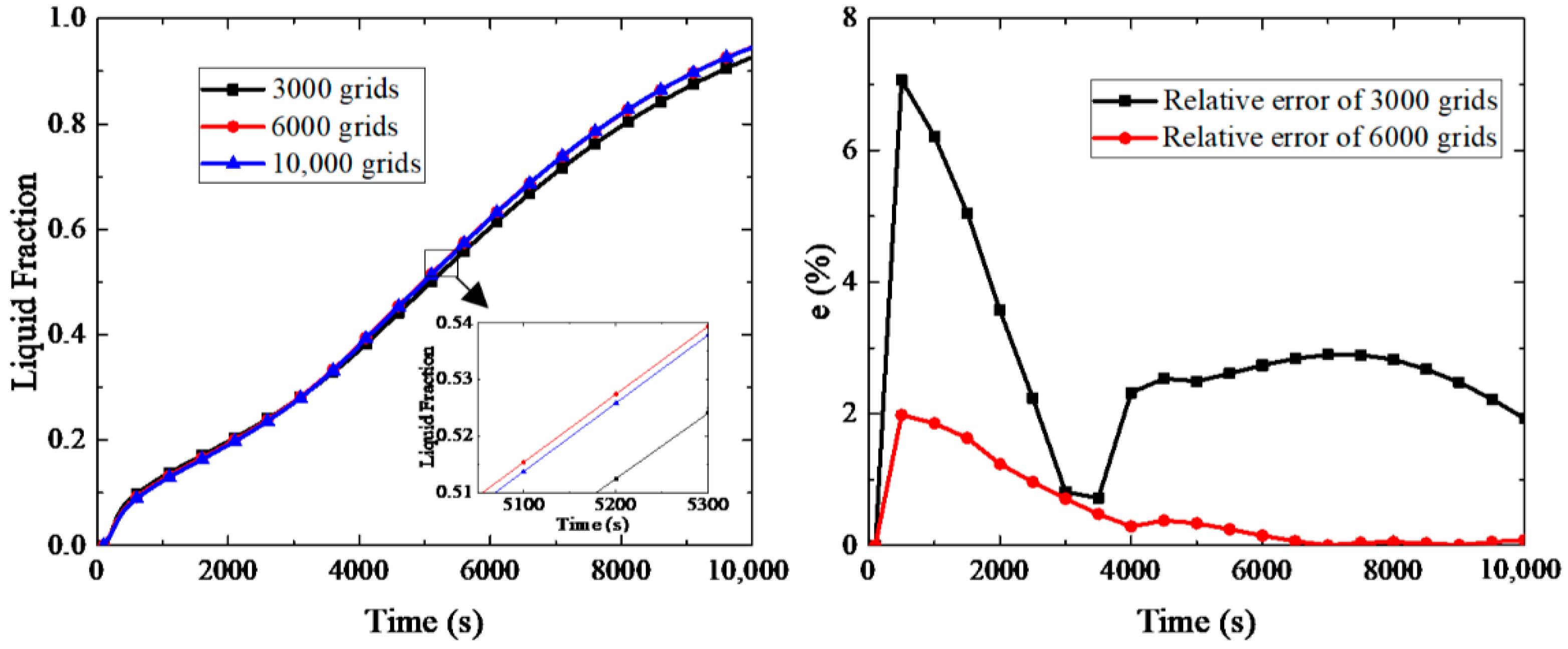
4.1. Model Validation
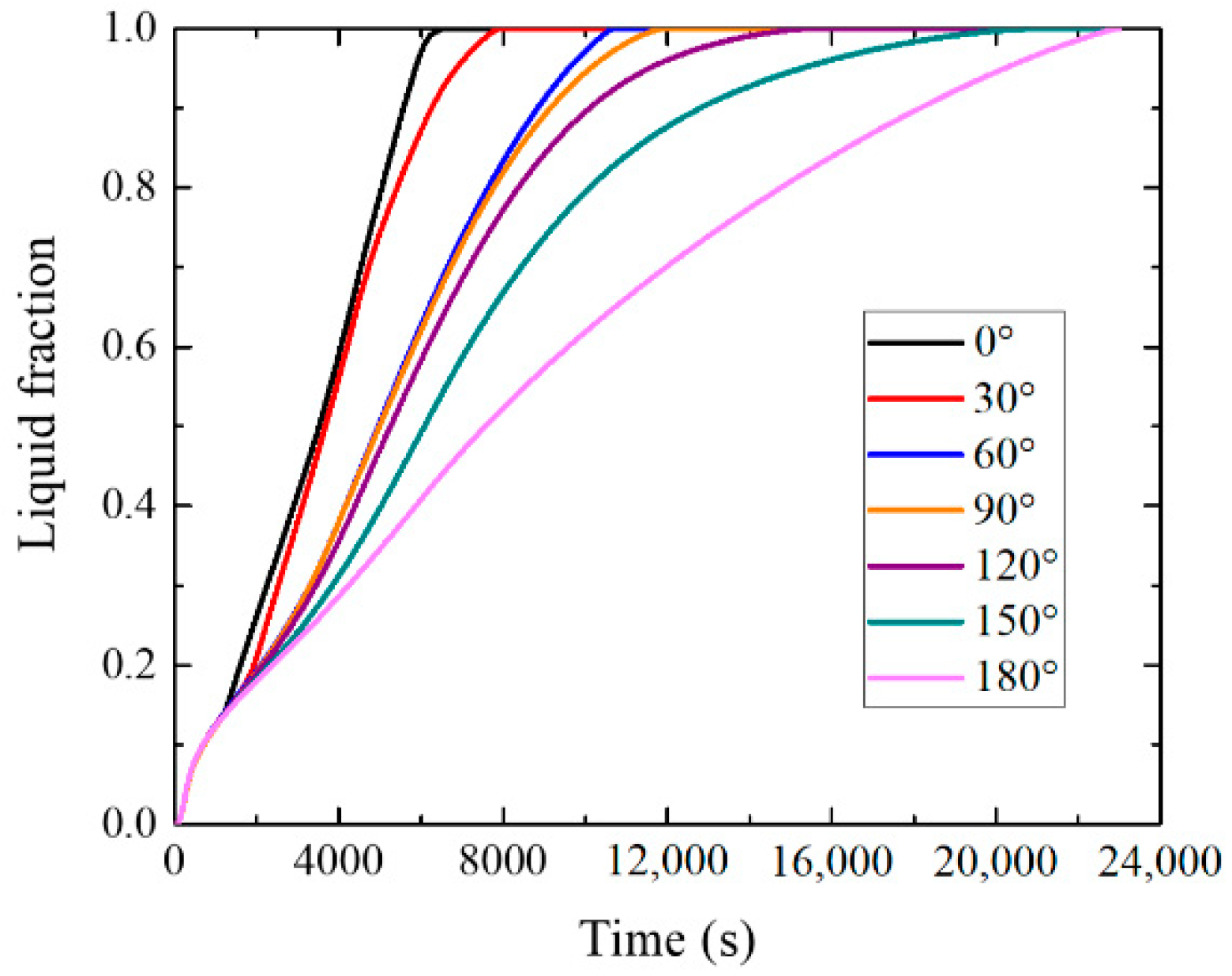
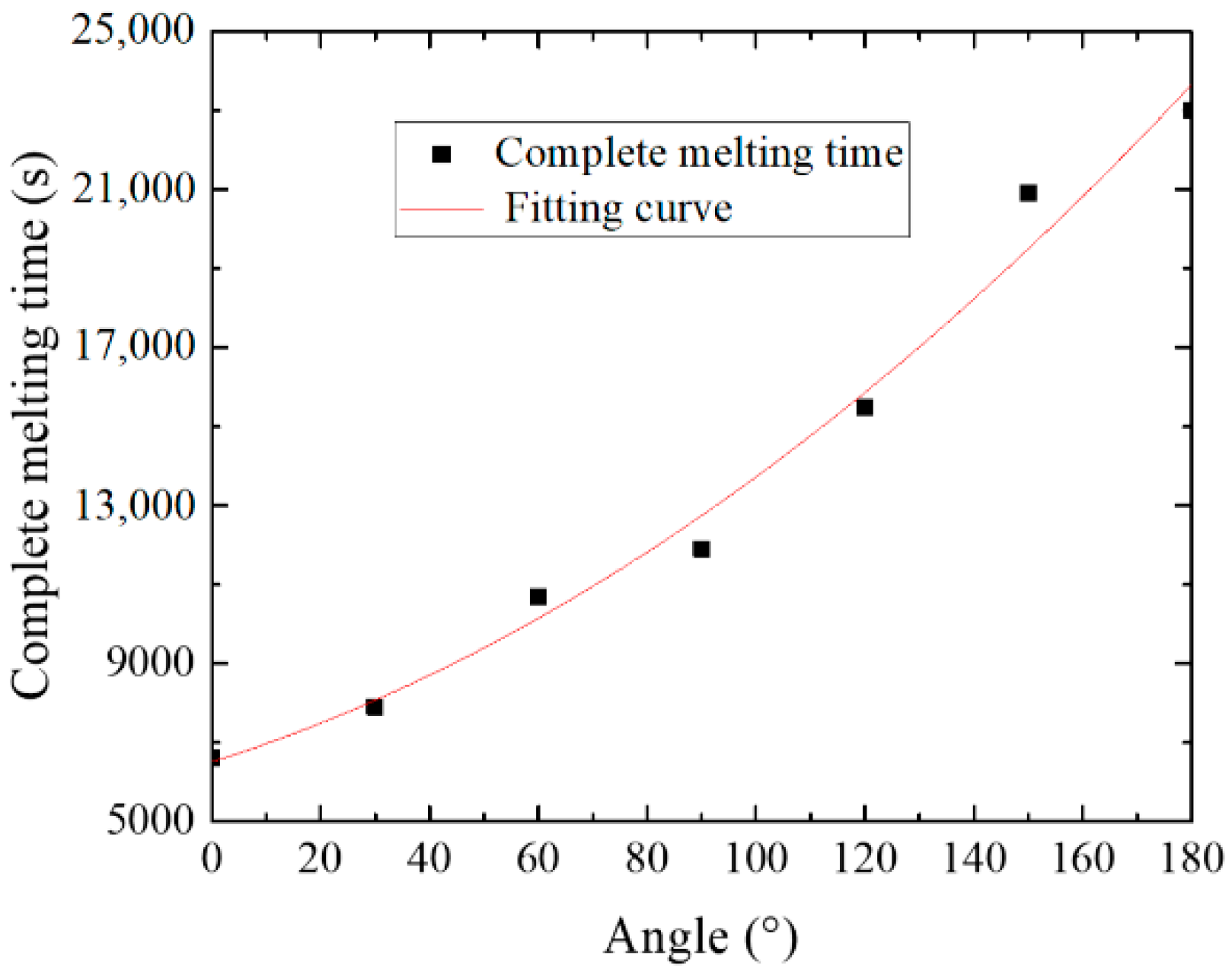
4.2. Influence of Inclination Angle on Melting Process
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, W.; Liang, F.; Lin, S.; Jin, X.Y. Experimental studies of phase change and microencapsulated phase change materials in a cold storage/transportation system with solar driven cooling cycle. Energies 2017, 10, 1867. [Google Scholar] [CrossRef]
- Mahavar, S.; Sengar, N.; Rajawat, P.; Verma, M.; Dashora, P. Design development and performance studies of a novel single family solar cooker. Renew. Energy 2012, 47, 67–76. [Google Scholar] [CrossRef]
- Yin, H.; Gao, X.; Ding, J. Thermal management of electronic components with thermal adaptation composite material. Appl. Energy 2010, 87, 3784–3791. [Google Scholar] [CrossRef]
- Hasan, A.; Hejase, H.; Abdelbaqi, S.; Assi, A.; Hamdan, M.O. Comparative effectiveness of different phase change materials to improve cooling performance of heat sinks for electronic devices. Appl. Sci. 2016, 6, 226. [Google Scholar] [CrossRef]
- Sqanson, T.D.; Birur, G.C. NASA thermal control technologies for robotic spacecraft. Appl. Therm. Eng. 2003, 23, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Bamonte, P.; Caverzan, A.; Kalaba, N.; Lamperti Tornaghi, M. Lightweight concrete containing phase change materials (PCMs): A numerical investigation on the thermal behavior of cladding panels. Buildings 2017, 7, 35. [Google Scholar] [CrossRef]
- Zhang, Q.N.; Huo, Y.T.; Rao, Z.H. Numerical study on solid-liquid phase change in paraffin as phase change material for battery thermal management. Sci. Bull. 2016, 61, 391–400. [Google Scholar] [CrossRef]
- Sankar, M.; Do, Y. Numerical simulation of free convection heat transfer in a vertical annular cavity with discrete heating. Int. Commun. Heat Mass. 2010, 37, 600–606. [Google Scholar] [CrossRef]
- Bohoosh, R.; Mohamadi, F.; Karimi, M. Numerical investigation of natural convection in a square cavity with tilting walls. J. Thermophys. Heat Transf. 2015, 29, 725–731. [Google Scholar] [CrossRef]
- Akgun, M.; Aydm, O.; Kaygusuz, K. Thermal energy storage performance of paraffin in a novel tube-in-shell system. Appl. Therm. Eng. 2008, 28, 405–413. [Google Scholar] [CrossRef]
- Li, S.W.; Chen, Y.; Sun, Z.Q. Numerical simulation and optimization of melting process of phase change material inside horizontal annulus. Energies 2017, 10, 1249. [Google Scholar]
- Hale, N.W.; Viskanta, R. Solid liquid phase change heat transfer and interface motion in materials cooled or heated form above or below. Int. J. Heat Mass Transf. 1980, 23, 283–292. [Google Scholar] [CrossRef]
- Ho, C.J.; Viskanta, R. Heat Transfer During Melting from an Isothermal Vertical Wall. J. Heat Mass Transf. 1984, 106, 12–19. [Google Scholar] [CrossRef]
- Pal, D.; Joshi, Y.K. Melting in a side heated tall enclosure by a uniformly dissipating heat source. Int. J. Heat Mass Transf. 2001, 44, 375–387. [Google Scholar] [CrossRef]
- Shokouhand, H.; Kamkari, B. Experimental investigation on melting heat transfer characteristics of lauric acid in a rectangular thermal storage unit. Exp. Therm. Fluid Sci. 2013, 50, 201–212. [Google Scholar] [CrossRef]
- Wang, C.H.; Lin, T.; Li, N.; Zheng, H.P. Heat transfer enhancement of phase change composite material: Copper foam/paraffin. Renew. Energy 2016, 96, 960–965. [Google Scholar] [CrossRef]
- Webb, B.W.; Viskanta, R. Natural convection dominated melting heat transfer in an inclined rectangular enclosure. Int. J. Heat Mass Transf. 1986, 29, 183–192. [Google Scholar] [CrossRef]
- Akgun, M.; Kaygusuz, K. Experimental study on melting/solidification characteristics of a paraffin as PCM. Energy Convers. Manag. 2007, 48, 669–678. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Gu, M.W.; Peng, D.H. Heat transfer performance analysis of a solar flat-plate collector with an integrated metal foam porous structure filled with paraffin. Appl. Therm. Eng. 2010, 30, 1967–1973. [Google Scholar] [CrossRef]
- Libeer, W.; Romos, F.; Newton, C.; Alipanahrostami, M.; Depcik, C.; Li, X.L. Two-phase heat and mass transfer of phase change materials in thermal management systems. Int. J. Heat Mass Transf. 2016, 100, 215–223. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, P.; Dai, J.M. Design and construction of a new steady-state apparatus for medium thermal conductivity measurement at high temperature. Rev. Sci. Instrum. 2017, 88, 104903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, Z.N.; Zhu, H.; Wang, Y.L.; Peng, S.P. Melting heat transfer characteristics of a composite phase change material fabricated by paraffin and metal foam. Appl. Energy 2017, 185, 1971–1983. [Google Scholar] [CrossRef]
- Diaz, L.A.; Viskanta, R. Visualization of the solid-liquid interface morphology formed by natural convection during melting of a solid from below. Int. Commun. Heat Mass. 1984, 11, 35–43. [Google Scholar] [CrossRef]
- Yang, J.L.; Yang, L.J.; Xu, C.; Du, X.Z. Numerical analysis on thermal behavior of solid-liquid phase change within copper foam with varying porosity. Int J. Heat Mass Transf. 2015, 84, 1008–1018. [Google Scholar] [CrossRef]







| Parameters | Value |
|---|---|
| Density of solid (kg·m−3) | 805.90 |
| Latent heat (J·g−1) | 110.28 |
| Melting temperature (K) | 56.4–64.3 °C |
| Thermal conductivity (W·m−1·K−1) | 0.20 (solid); 0.11 (liquid) |
| Specific heat (J·kg−1·K−1) | 2.10 × 103 |
| Kinematic viscosity (m2·s−1) | 4.464 × 10−6 |
| Thermal expansion coefficient (K−1) | 1.10 × 10−4 |
| Definite Conditions | Locations | Velocity | Conditions |
|---|---|---|---|
| Initial condition | 0 ≤ x ≤ 100, 0 ≤ y ≤ 30 | U = v = 0 | T = Tinit = 25 °C |
| Boundary conditions | 0 ≤ x ≤ 100, y = 0 | U = v = 0 | UDF from 25 to 80 °C |
| 0 ≤ x ≤ 100, y = 30 | U = v = 0 | ||
| x = 0, 0 ≤ y ≤ 30 | U = v = 0 | ||
| x = 100, 0 ≤ y ≤ 30 | U = v = 0 |
| Angle | 30 min | 60 min | 90 min |
|---|---|---|---|
| 0° |  |  |  |
| 30° |  |  |  |
| 60° |  |  |  |
| 90° |  |  |  |
| 120° |  |  |  |
| 150° |  |  |  |
| 180° |  |  |  |
| Legend |  | ||
| Angle | 0° | 30° | 60° | 90° |
| Numerical results |  |  |  |  |
| Experimental photos |  |  |  |  |
| Angle | 120° | 150° | 180° | numerical legend |
| Numerical results |  |  |  |  |
| Experimental photos |  |  |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dai, J.; An, D. Numerical Investigations on Melting Behavior of Phase Change Material in a Rectangular Cavity at Different Inclination Angles. Appl. Sci. 2018, 8, 1627. https://doi.org/10.3390/app8091627
Wang Y, Dai J, An D. Numerical Investigations on Melting Behavior of Phase Change Material in a Rectangular Cavity at Different Inclination Angles. Applied Sciences. 2018; 8(9):1627. https://doi.org/10.3390/app8091627
Chicago/Turabian StyleWang, Yong, Jingmin Dai, and Dongyang An. 2018. "Numerical Investigations on Melting Behavior of Phase Change Material in a Rectangular Cavity at Different Inclination Angles" Applied Sciences 8, no. 9: 1627. https://doi.org/10.3390/app8091627





