Aqueous Garlic Extract as a Plant Biostimulant Enhances Physiology, Improves Crop Quality and Metabolite Abundance, and Primes the Defense Responses of Receiver Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Garlic Extracts and Acetyl Salicylic Acid Solution
2.2. Evaluation of AGE and ASA as Biostimulants to Improve Crop Quality, Growth Parameters, Metabolites Abundance, and Antioxidant Enzymes Activities
2.2.1. Plant Morphological Indices
2.2.2. Antioxidant Enzymes and Lipid Peroxidation
2.2.3. Chlorophyll and Carotenoid Content
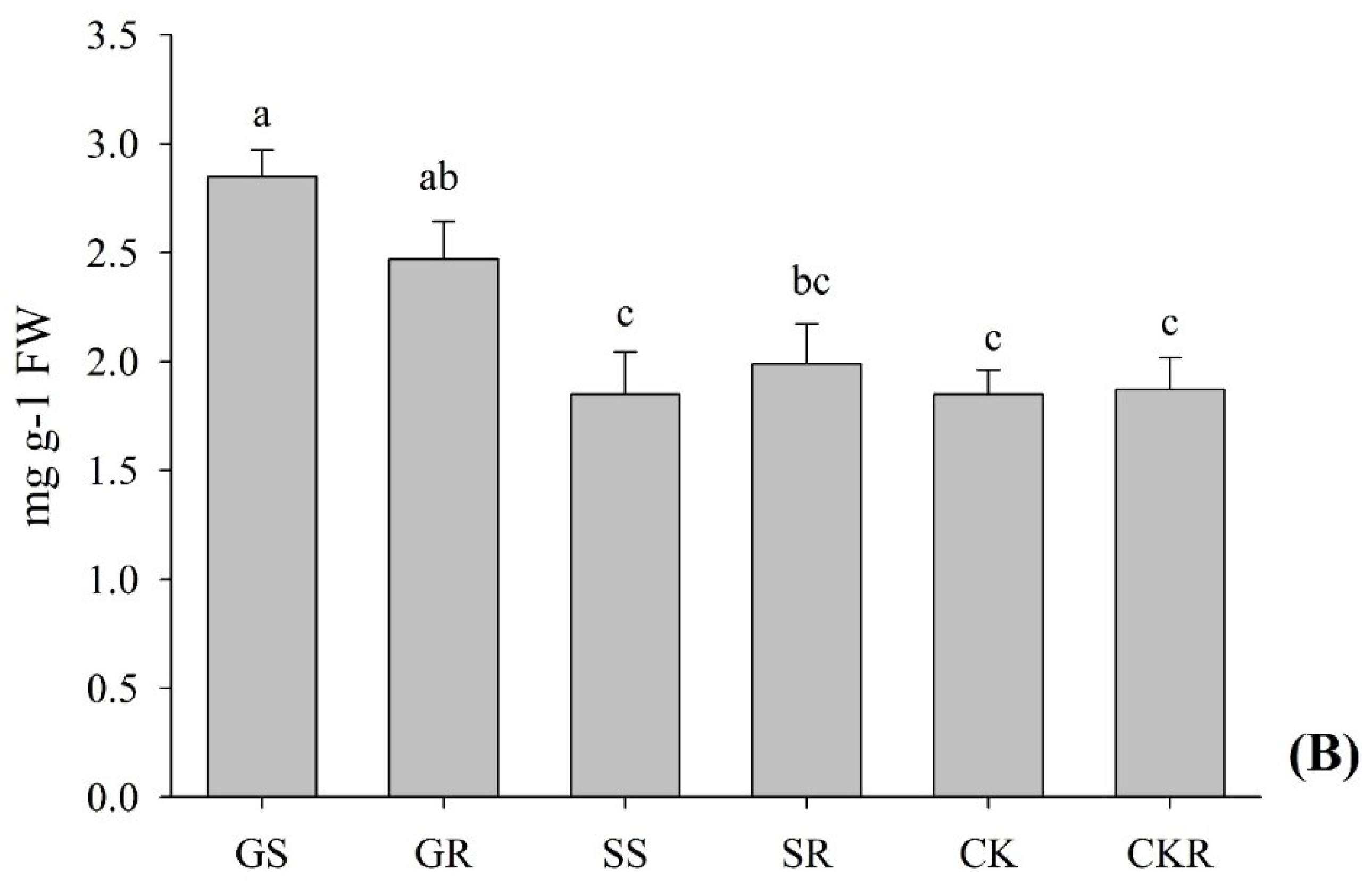
2.2.4. Soluble Sugars Content
2.2.5. Root Activity
2.3. Evaluation of AGE and ASA as Biostimulants for Priming the Defense Responses of Pepper Seedlings against Pathogenic Infection
2.3.1. H2O2 Measurement
2.3.2. Disease Incidence and Severity Index
2.4. Statistical Analysis
3. Results
3.1. Effect of AGE and ASA on the Growth and Development of Pepper
3.1.1. Morphology of Pepper Seedlings
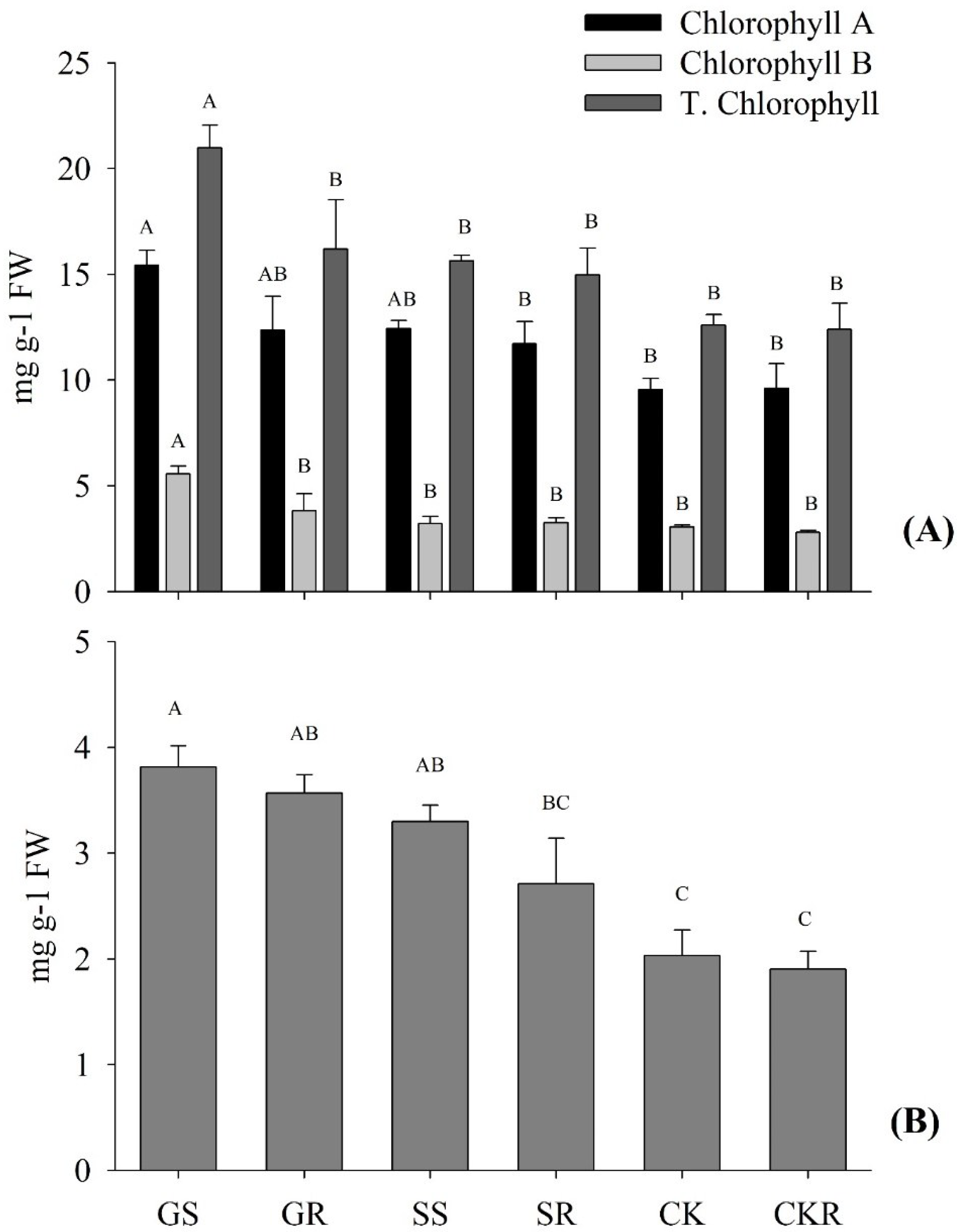
3.1.2. Chlorophyll and Carotenoid Contents of Pepper
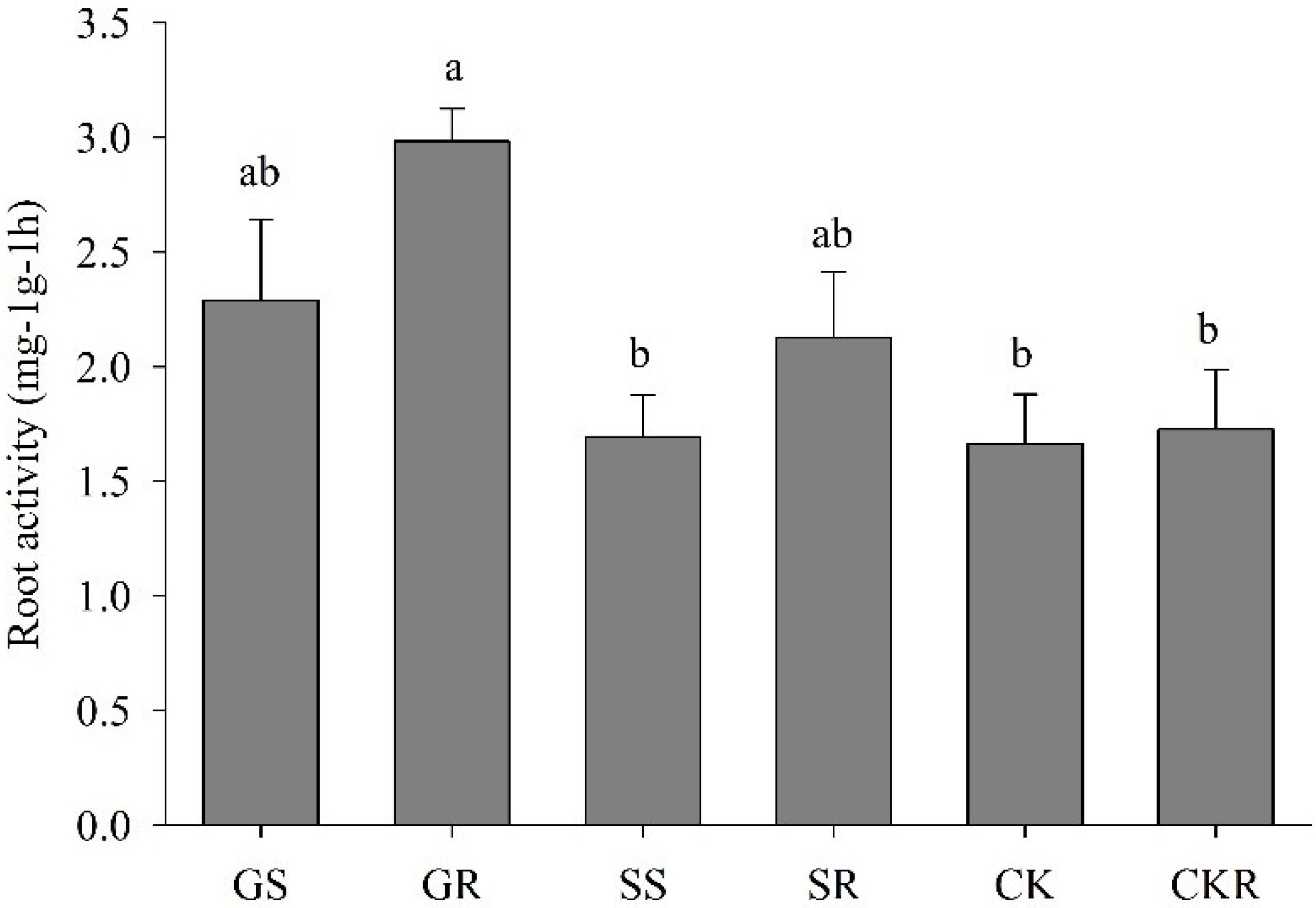
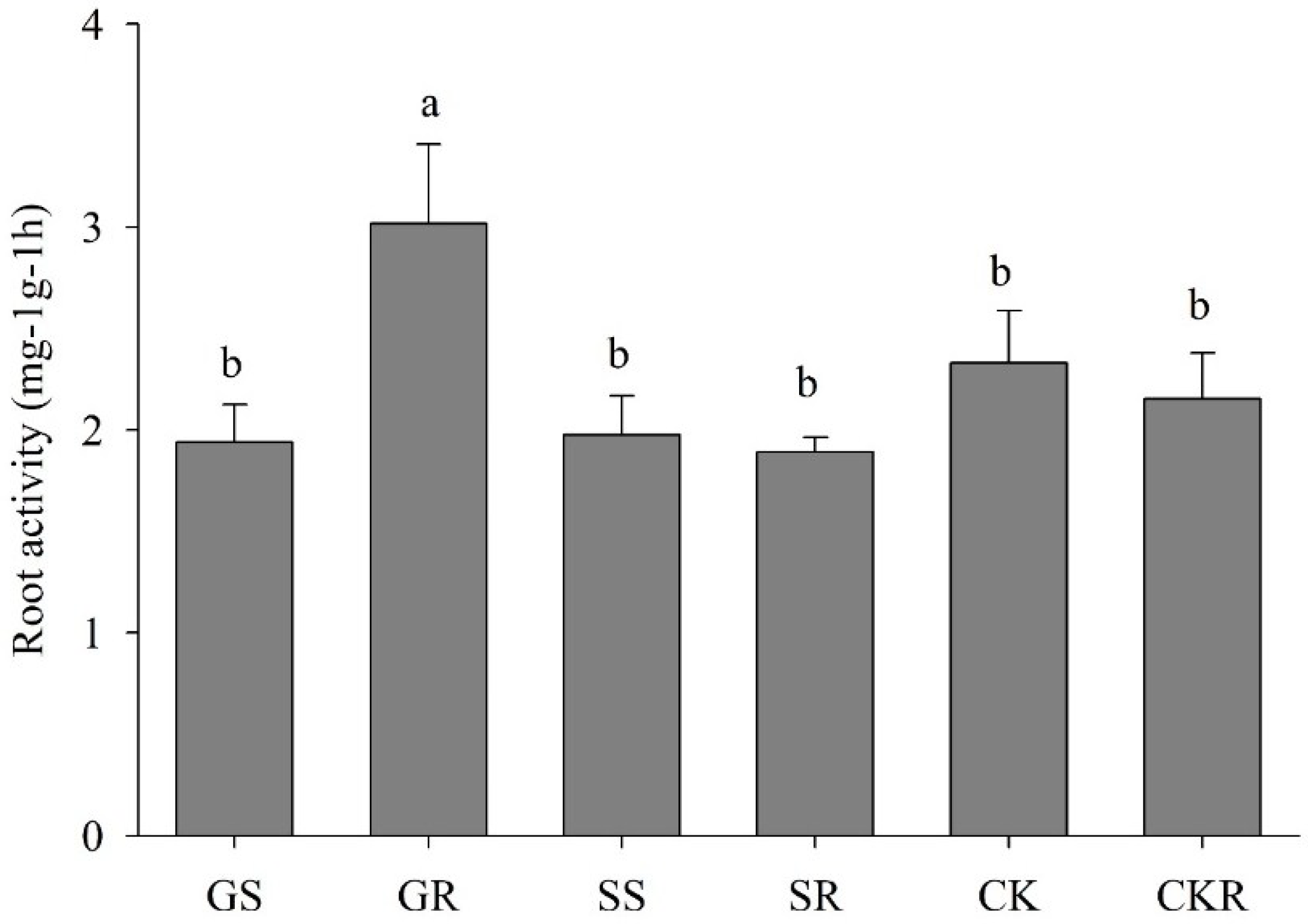
3.1.3. Root Activity of Pepper Plants Influenced by AGE and ASA Treatments
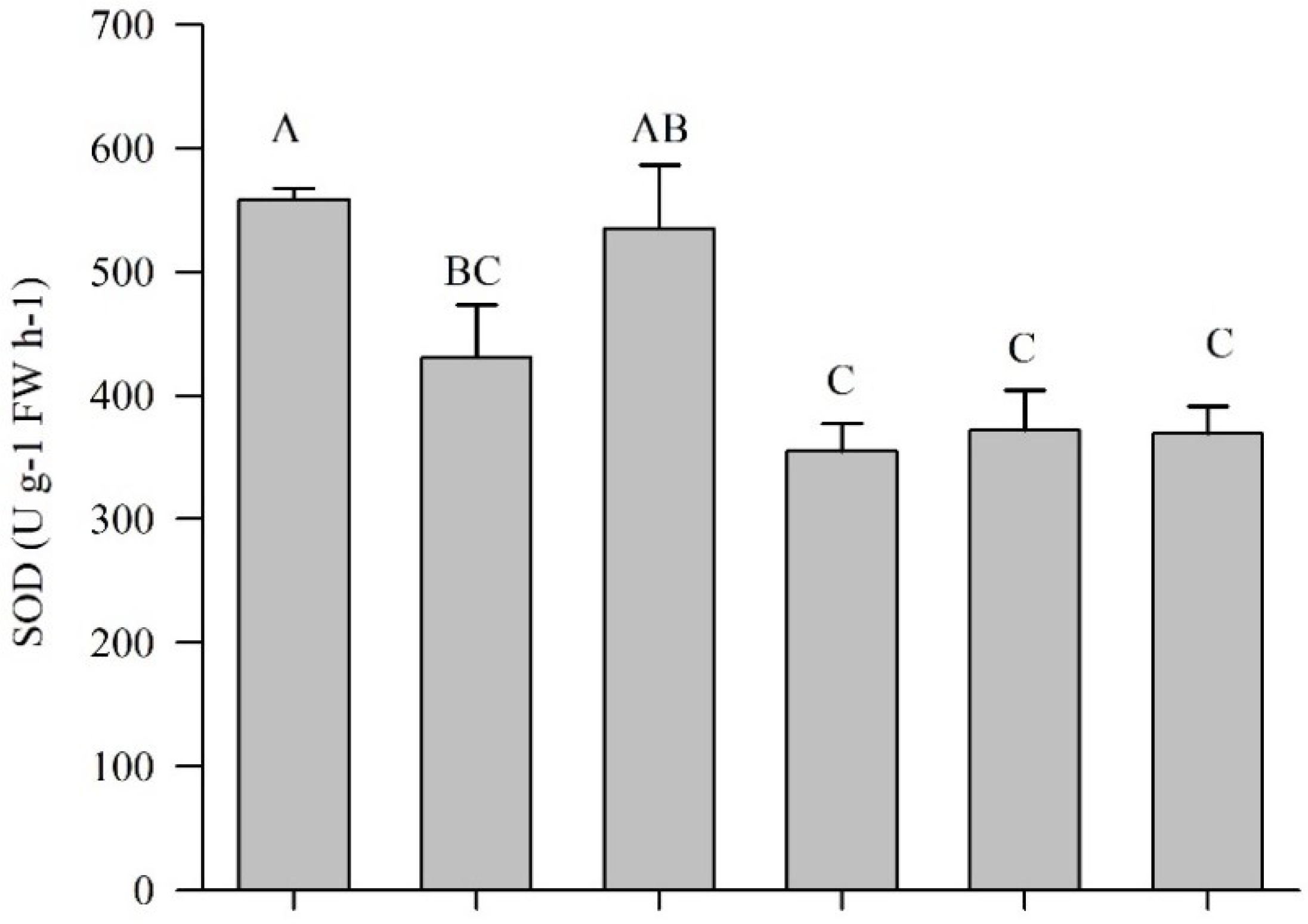
3.1.4. SOD, POD, and Lipid Peroxidation of Pepper Plants Applied with AGE and ASA
3.2. Response of Eggplant Growth to Foliar and Fertigation of AGE and ASA
3.2.1. Influence of AGE and ASA Applied as Foliar and Fertigation on the Morphology of Eggplant
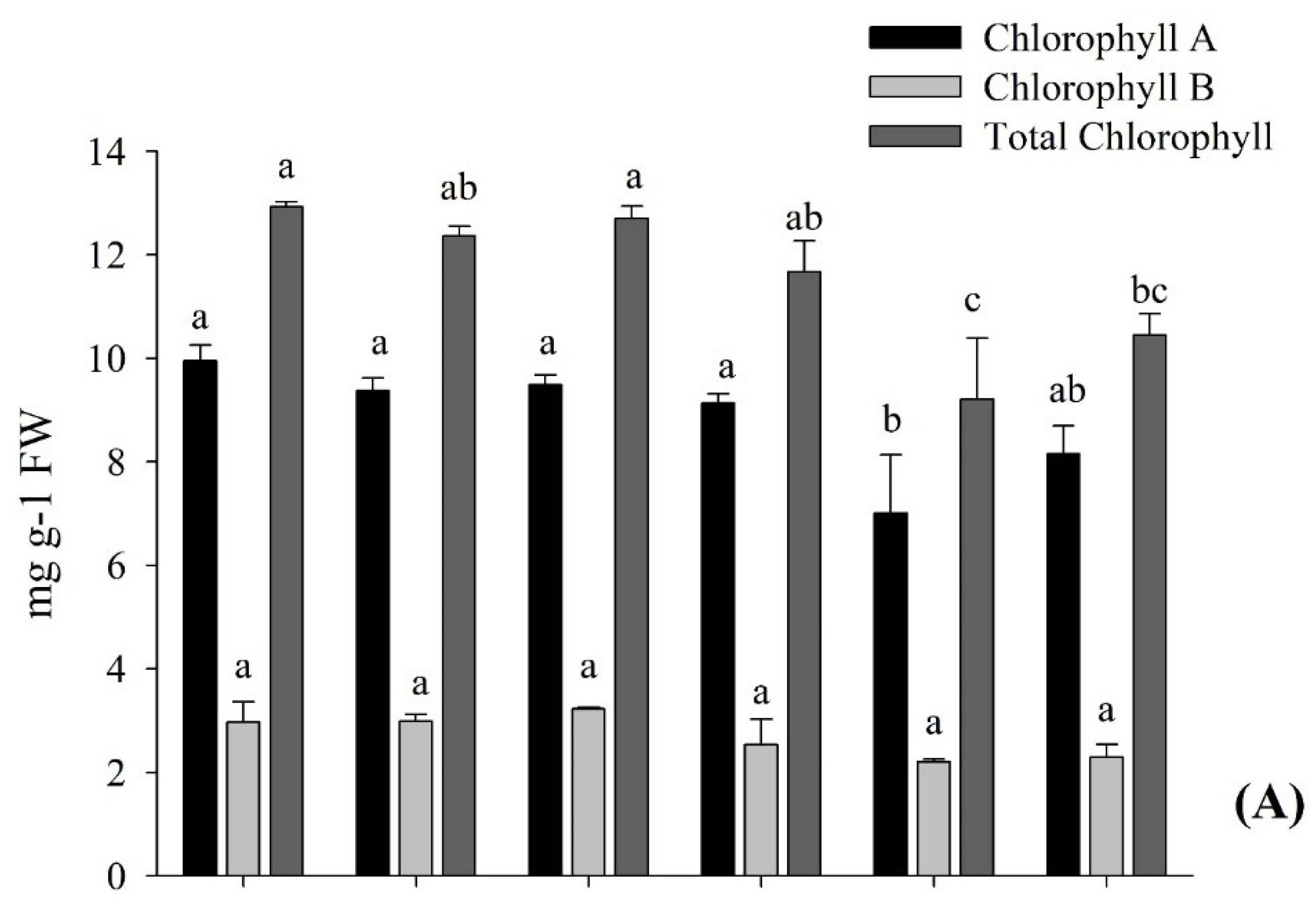
3.2.2. The Chlorophyll and Carotenoid Contents of the Eggplant Applied with AGE and ASA
3.2.3. Root Activity of Eggplants Treated with Various Treatments of AGE and ASA
3.2.4. The Antioxidative Response of Eggplant Applied with AGE and ASA Treatments
3.3. Priming the Defense Response of Pepper Against Phytopthora Capsici
3.3.1. Antioxidants and Lipid Peroxidation Levels of Pepper before and after Inoculation
3.3.2. Disease Incidence and Severity of Phytopthora capsici Observed in Pepper Seedlings
3.3.3. H2O2 Accumulation during Infection of the Seedlings with Phytopthora capsici
4. Discussion
4.1. Application of AGE-Improved Photosynthetic Pigments, Soluble Sugars Accumulation, and Incremented Root Development, Promoting Plant Growth
4.2. Biostimulation of Defense System Leads to Induced Resistance Against Pathogen Inoculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflict of Interest
References
- McMahon, M.; Kofranek, A.; Rubatzky, V. Hartmann’s Plant Science: Growth, Development and Utilization of Cultivated Plants, 4th ed.; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2007; ISBN 0131140752. [Google Scholar]
- New Methods for Organic Raspberry Production in Polyethylene Tunnels. Available online: http://orgprints.org/14970/1/Project_description_final.pdf (accessed on 10 August 2018).
- Bonanomi, G.; D’Ascoli, R.; Scotti, R.; Gaglione, S.A.; Caceres, M.G.; Sultana, S.; Scelza, R.; Rao, M.A.; Zoina, A. Soil quality recovery and crop yield enhancement by combined application of compost and wood to vegetables grown under plastic tunnels. Agric. Ecosyst. Environ. 2014, 192, 1–7. [Google Scholar] [CrossRef]
- Role and Hazards. Available online: https://www.researchgate.net/publication/283500340_Synthetic_Fertilizers_Role_and_Hazards (accessed on 10 August 2018).
- Aremu, A.O.; Masondo, N.A.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Bíba, O.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K.; et al. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta 2015, 241, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Malo, I.; De Bastiani, M.; Arevalo, P.; Bernacchia, G. Natural extracts from pepper, wild rue and clove can activate defenses against pathogens in tomato plants. Eur. J. Plant Patol. 2017, 149, 89–101. [Google Scholar] [CrossRef]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of Gelatin as a Biostimulant Seed Treatment to Improve Plant Performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Amirkhani, M.; Netravali, A.N.; Huang, W.; Taylor, A.G. Investigation of Soy Protein-based Biostimulant Seed Coating for Broccoli Seedling and Plant Growth Enhancement. HortScience 2016, 51, 1121–1126. [Google Scholar] [CrossRef]
- Michalak, I.; Dmytryk, A.; Schroeder, G.; Chojnacka, K. The Application of Homogenate and Filtrate from Baltic Seaweeds in Seedling Growth Tests. Appl. Sci. 2017, 7, 230. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Hopkins, H.; Huner, N. Introduction to Plant Physiology; John Wiley and Sons: Hoboken, NJ, USA, 2009; Volume 43, ISBN 9780470247662. [Google Scholar]
- Król, P.; Igielski, R.; Pollmann, S.; Kepczyńska, E. Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f.sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid, and flavonol accumulation. J. Plant Physiol. 2015, 179, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kennedy, R.; Greenshields, D.L.; Peng, G.; Forseille, L.; Selvaraj, G.; Wei, Y. Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol. Plant Microbe Interact. 2007, 20, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Van Der Ent, S.; Van Hulten, M.; Pozo, M.; van Oosten, V.; van Loon, L.C.; Mauch-Mani, B.; Turlings, T.C.J.; Pieterse, C.M.J. Priming as a mechanism behind induced resistance against pathogens, insects and abiotic stress. IOBC/Wprs Bull. 2009, 44, 3–13. [Google Scholar]
- Conrath, U. Chapter 9 Priming of Induced Plant Defense Responses, 1st ed.; Elsevier Ltd.: New York, NY, USA, 2009; Volume 51, ISBN 9780123748348. [Google Scholar]
- Munné-bosch, S.; Müller, M. Hormonal cross-talk in plant development and stress responses. Front Plant Sci. 2013, 4, 529. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Boil. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Imran, M.; Mahmood, A.; Römheld, V.; Neumann, G. Nutrient seed priming improves seedling development of maize exposed to low root zone temperatures during early growth. Eur. J. Agron. 2013, 49. [Google Scholar] [CrossRef]
- Waseem, M.; Kulkarni, S.; Bhanuje, T.; Haleshkumar, B. Different Priming Treatments on Germination and Viability of Cluster Bean Seeds. Int. J. Adv. Res. 2015, 3, 108–111. [Google Scholar]
- Nawaz, A.; Amjad, M.; Pervez, M.A.; Afzal, I. Effect of halopriming on germination and seedling vigor of tomato. Afr. J. Agric. Res. 2011, 6, 2099–2107. [Google Scholar] [CrossRef]
- Pill, W.G.; Frett, J.J.; Morneau, D.C. Germination and seedling emergence of primed tomato and asparagus seeds under adverse conditions. HortScience 1991, 26, 1160–1162. [Google Scholar]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C.; Thompson, N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Rahman, K.; Lowe, G.M. Significance of Garlic and Its Constituents in Cancer and Cardiovascular Disease Garlic and Cardiovascular Disease: A Critical Review. J. Nutr. 2006, 136, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Cheng, Z.; Meng, H.; Khan, M.A.; Li, H. Intercropping with garlic alleviated continuous cropping obstacle of cucumber in plastic tunnel. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 696–705. [Google Scholar] [CrossRef]
- Hayat, S.; Cheng, Z.; Ahmad, H.; Ali, M.; Chen, X.; Wang, M. Garlic, from Remedy to Stimulant: Evaluation of Antifungal Potential Reveals Diversity in Phytoalexin Allicin Content among Garlic Cultivars; Allicin Containing Aqueous Garlic Extracts Trigger Antioxidants in Cucumber. Front. Plant Sci. 2016, 7, 1235. [Google Scholar] [CrossRef] [PubMed]
- Gao, J. The Guidance of Plant Physiology Experiments; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Zhang, Z. The Guidance of Plant Physiology Experiments; Chinese Agricultural Science and Technology Press: Beijing, China, 2004. [Google Scholar]
- Bestwick, C.S.; Brown, I.R.; Mansfield, J.W. Localized Changes in Peroxidase Activity Accompany Hydrogen Peroxide Generation during the Development of a Nonhost Hypersensitive Reaction in Lettuce. Plant Physiol. 1998, 118, 1067–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dere, Ş.; Güneş, T.; Sivaci, R. Spectrophotometric determination of chlorophyll—A, B and total caretenoid contents of some algae species using different solvents. Turk. J. Bot. 1998, 22, 13–16. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Onanuga, A.O.; Jiang, P.; Adl, S. Effect of Phytohormones, Phosphorus and Potassium on Cotton Varieties (Gossypium hirsutum) Root Growth and Root Activity Grown in Hydroponic Nutrient Solution. J. Agric. Sci. 2012, 4, 342–345. [Google Scholar] [CrossRef]
- Lariguet, P.; Ranocha, P.; de Meyer, M.; Barbier, O.; Penel, C.; Dunand, C. Identification of a hydrogen peroxide signalling pathway in the control of light-dependent germination in Arabidopsis. Planta 2013, 238, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; La Malfa, S.; Vitale, A.; Lorito, M.; Deng, Z.; Gentile, A. Defence-related gene expression in transgenic lemon plants producing an antimicrobial Trichoderma harzianum endochitinase during fungal infection. Transgenic Res. 2008, 17, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Ali, M.; Thomson, M.; Armstrong, D. Garlic and its medicinal potential. Inflammopharmacology 2000, 8, 123–148. [Google Scholar] [CrossRef]
- Otunola, G.A.; Oloyede, O.B.; Oladiji, A.T.; Afolayan, A.J. Comparative analysis of the chemical composition of three spices—Allium sativum L. Zingiber officinale Rosc. and Capsicum frutescens L. commonly consumed in Nigeria. Afr. J. Biotechnol. 2010, 9, 6927–6931. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sheren, A.; Abd, E.; El-Amary, E.I. Improving Growth and Productivity of “Pear” Trees Using Some Natural Plants Extracts under North Sinai Conditions. IOSR J. Agric. Vet. Sci. 2015, 8, 01–09. [Google Scholar] [CrossRef]
- Puvača, N.; Ljubojević, D.; Lukač, D.; Beuković, M.; Kostadinović, L.; Teodosin, S.; Stanaćev, V. Bioactive Compounds of Garlic, Black Pepper and Hot Red Pepper. In Proceedings of the XVI International Symposium, “Feed Technology”, Novi Sad, Serbia, 28–30 October 2014; pp. 116–122. [Google Scholar]
- Ding, H.; Cheng, Z.; Liu, M.; Hayat, S.; Feng, H. Garlic exerts allelopathic effects on pepper physiology in a hydroponic co-culture system. Boil. Open 2016, 5, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molazem, D.; Bashirzadeh, A.; Ardabili, M.F. Effect of exogenous application of salicylic acid on the growth, photosynthesis and proline content on maize in salt stress. J. Biodivers. Environ. Sci. 2014, 4, 46–52. [Google Scholar]
- Mohamed, H.I.; Akladious, S.A. Influence of Garlic Extract On Enzymatic and Non Enzymatic Antioxidants in Soybean Plants. Life Sci. J. 2014, 11, 46–58. [Google Scholar]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Slattery, R.A.; VanLoocke, A.; Bernacchi, C.J.; Zhu, X.G.; Ort, D.R. Photosynthesis, Light Use Efficiency, and Yield of Reduced-Chlorophyll Soybean Mutants in Field Conditions. Front. Plant Sci. 2017, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Puga-Freitas, R.; Blouin, M. A review of the effects of soil organisms on plant hormone signalling pathways. Environ. Exp. Bot. 2015, 114, 104–116. [Google Scholar] [CrossRef]
- Candan, N.; Tarhan, L. Relationship among chlorophyll-carotenoid content, antioxidant enzyme activities and lipid peroxidation levels by Mg2+ deficiency in the Mentha pulegium leaves. Plant Physiol. Biochem. 2003, 41, 35–40. [Google Scholar] [CrossRef]
- Fracheboud, Y. Using Chlorophyll Fluorescence to Study Photosynthesis; Institute of Plant Sciences ETH, Universitätstrasse: Zürich, Switzerland, 2006. [Google Scholar]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids in Adipose Tissue Biology and Obesity. Subcell Biochem. 2016, 79, 377–414. [Google Scholar] [PubMed]
- Karppinen, K.; Zoratti, L.; Sarala, M.; Carvalho, E.; Hirsimäki, J.; Mentula, H.; Martens, S.; Häggman, H.; Jaakola, L. Carotenoid metabolism during bilberry (Vaccinium myrtillus L.) fruit development under different light conditions is regulated by biosynthesis and degradation. BMC Plant Boil. 2016, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Candan, N.; Tarhan, L. Changes in chlorophyll-carotenoid contents, antioxidant enzyme activities and lipid peroxidation levels in Zn-stressed Mentha pulegium. Turk. J. Chem. 2003, 27, 21–30. [Google Scholar]
- Bartley, G.E.; Scolnik, P.A. Plant Carotenoids: Pigments for Photoprotection, Visual Attraction, and Human Health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhi, C.; Huan, M.; Ho, G. Allelopathy of garlic root aqueous extract s and root exudates. J. Northwest A F Univ. 2007, 35, 87–92. [Google Scholar]
- Bhandari, S.R.; Yoon, M.K.; Kwak, J.H. Contents of phytochemical constituents and antioxidant activity of 19 garlic (Allium sativum L.) parental lines and cultivars. Hortic. Environ. Biotechnol. 2014, 55, 138–147. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M. Antioxidant Defenses in Plants with Attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Eiberger, M.; Haefs, R.; Noga, G. Calcium deficiency—Influence on the antioxidative defense system in tomato plants. J. Plant Physiol. 2002, 159, 733–742. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Boil. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Oxidative stress in plants. Acta Physiol. Plant. 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Gokul, A.; Roode, E.; Klein, A.; Keyster, M. Exogenous 3,3’-diindolylmethane increases Brassica napus L. seedling shoot growth through modulation of superoxide and hydrogen peroxide content. J. Plant Physiol. 2016, 196–197, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Gapper, C.; Dolan, L. Control of Plant Development by Reactive Oxygen Species 1. Plant Physiol. 2006, 141, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Carol, R.J.; Dolan, L. The role of reactive oxygen species in cell growth: Lessons from root hairs. J. Exp. Bot. 2006, 57, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Heribert, H.; Kazuo, S. Plant Responses to Abiotic Stress. In Topics in Current Genetics; Hirt, H., Shinozaki, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 4, p. 320. ISBN 3540200371. [Google Scholar]
- Navarro, J.M.; Pérez-Tornero, O.; Morte, A. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 2014, 171, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wang, F.; Zhang, W.; Liu, C.; Zhao, X. Antioxidant mechanism and lipid peroxidation patterns in leaves and petals of marigold in response to drought stress. Hortic. Environ. Biotechnol. 2012, 53, 183–192. [Google Scholar] [CrossRef]
- Mauch-mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense Priming: An Adaptive Part of Induced Resistance. Annu. Rev. Plant Biol. 2017, 68, 482–512. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, S.J. Synthetic Plant Activators for Crop Disease Management—A Review. Int. J. Thesis Proj. Diss. 2014, 2, 19–28. [Google Scholar]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of Priming with Silicon on the Growth and Tolerance of Maize Plants to Alkaline Stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Boil. 1998, 1, 316–323. [Google Scholar] [CrossRef]
- Hutcheson, S.W. Current concepts of active defense in plants. Annu. Rev. Phytopathol. 1998, 36, 59–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Moyne, A.L.; Reddy, M.S.; Kloepper, J.W. The role of salicylic acid in induced systemic resistance elicited by plant growth-promoting rhizobacteria against blue mold of tobacco. Boil. Control 2002, 25, 288–296. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Poelman, E.H.; Van Wees, S.C.M.; Dicke, M. Induced plant responses to microbes and insects. Front. Plant Sci. 2013, 4, 475. [Google Scholar] [CrossRef] [PubMed]
- Usman Ghazanfar, M.; Wakil, W.; Talib Sahi, S. Induction of resistance in chickpea (Cicer arietinum L.) against Ascochyta rabiei by applying chemicals and plant extracts. Chil. J. Agric. Res. 2011, 71, 52–62. [Google Scholar] [CrossRef]
- Chemicals, R. Salicylic Acid. Br. Med. J. 2000, 841, 178. [Google Scholar]
- Szczepaniec, A.; Raupp, M.J.; Parker, R.D.; Kerns, D.; Eubanks, M.D. Neonicotinoid Insecticides Alter Induced Defenses and Increase Susceptibility to Spider Mites in Distantly Related Crop Plants. PLoS ONE 2013, 8, e62620. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wen, Z.; Zhang, J.; Chen, X.; Cui, J.; Xu, W.; Liu, H.Y. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Wan, R.; Hou, X.; Wang, X.; Qu, J.; Singer, S.D.; Wang, Y.; Wang, X. Resistance evaluation of Chinese wild Vitis genotypes against Botrytis cinerea and different responses of resistant and susceptible hosts to the infection. Front. Plant Sci. 2015, 6, 854. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, L.; Zhu, J.; Chen, T.; Wang, Y.; Xu, Y. Histological responses to downy mildew in resistant and susceptible grapevines. Protoplasma 2014, 252, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, C.; Cheng, Z.; Meng, H. Growth and physiological changes in continuously cropped eggplant (Solanum melongena L.) upon relay intercropping with garlic (Allium sativum L.). Front. Plant Sci. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yin, C.; Strasser, R.J.; Govindjee; Yang, C.; Qiang, S. Reactive oxygen species from chloroplasts contribute to 3-acetyl-5-isopropyltetramic acid-induced leaf necrosis of Arabidopsis thaliana. Plant Physiol. Biochem. 2012, 52, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.M.; Hussein, M.I.; Gadel, H.S.H.; Hammad, M.A. Effect Of Two Plant Extracts and Four Aromatic Oils on Tuta Absoluta population and productivity of tomato cultivar gold stone. Nat. Sci. 2014, 12, 108–118. [Google Scholar]
- Mukerji, K. Allelochemicals: Biological Control of Plant Pathogens and Diseases; Inderjit, Mukerji, K.G., Eds.; Springer: Berlin, Germany, 2006; ISBN 9781402044458. [Google Scholar]
- Chen, C.; Belanger, R.R.; Benhamou, N.; Paulitz, T.C. Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol. Mol. Plant Pathol. 2000, 56, 13–23. [Google Scholar] [CrossRef]
- Stangarlin, J.R.; Kuhn, O.J.; Assi, L. Control of plant diseases using extracts from medicinal plants and fungi. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1033–1042. [Google Scholar]
- Inagaki, H.; Yamaguchi, A.; Kato, K.; Kageyama, C.; Iyozumi, H. Induction of systemic resistance to anthracnose in cucumber by natural components of Allium vegetables and shiitake mushrooms. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, Spain, 2011; pp. 728–735. ISBN 978-84-939843-1-1. [Google Scholar]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Boil. Med. 2011, 3, 232–249. [Google Scholar]






| Treatment | Method of Application | Plant Height (cm) | Stem Diameter (mm) | No. of Leaves | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Root/Shoot Ratio | Soluble Sugars |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AGE | Foliar | 12.1 ± 0.87a | 2.4 ± 0.04a | 7.5 ± 0.29b | 26.5 ± 0.29a | 3.5 ± 0.06a | 3.1 ± 0.05a | 0.559 ± 0.01a | 0.278 ± 0.01a | 0.88 ± 0.02a | 3.75 ± 0.09a |
| Root | 11.7 ± 0.15ab | 2.4 ± 0.02a | 9.8 ± 0.44a | 24.0 ± 1.53ab | 3.4 ± 0.12a | 2.7 ± 0.08b | 0.531 ± 0.01a | 0.262 ± 0.02a | 0.78 ± 0.03ab | 3.25 ± 0.29ab | |
| ASA | Foliar | 9.7 ± 0.6bc | 2.4 ± 0.09a | 9.7 ± 0.60a | 20.7 ± 0.33b | 2.8 ± 0.02b | 1.34 ± 0.06e | 0.393 ± 0.01b | 0.148 ± 0.01b | 0.48 ± 0.02c | 3.12 ± 0.11b |
| Root | 8.7 ± 1.01c | 2.6 ± 0.03a | 7.8 ± 0.33b | 24.3 ± 2.19ab | 2.9 ± 0.30ab | 1.7 ± 0.07de | 0.374 ± 0.0b | 0.162 ± 0.01b | 0.56 ± 0.03c | 2.90 ± 0.08b | |
| Control | Foliar | 8.3 ± 0.63c | 1.9 ± 0.13b | 4.8 ± 0.17c | 19.3 ± 1.20b | 2.5 ± 0.08b | 1.9 ± 0.11c | 0.362 ± 0.02b | 0.144 ± 0.01b | 0.78 ± 0.05ab | 2.93 ± 0.05b |
| Root | 7.7 ± 0.44c | 1.8± 0.22b | 5.7 ± 0.33c | 18.9 ± 1.94b | 2.4 ± 0.12b | 1.7 ± 0.08cd | 0.359 ± 0.01b | 0.149 ± 0.01b | 0.71 ± 0.06b | 2.87 ± 0.04b |
| Treatment | Method of Application | Plant Height (cm) | Stem Diameter (mm) | No. of Leaves | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Root Shoot Ratio | Soluble Sugar Content |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AGE | Foliar | 4.7 ± 0.15a | 2.6 ± 0.09a | 3.6 ± 0.05a | 21.7 ± 1.20a | 4.0 ± 0.34a | 1.9 ± 0.03a | 0.514 ± 0.02a | 0.225 ± 0.01a | 0.48 ± 0.05ab | 7.27 ± 1.05a |
| Root | 4.4 ± 0.24ab | 2.6 ± 0.06a | 3.5 ± 0.12ab | 19.7 ± 2.61ab | 3.3 ± 0.19abc | 1.9 ± 0.07a | 0.457 ± 0.04a | 0.197 ± 0.04ab | 0.57 ± 0.03a | 7.30 ± 1.02a | |
| ASA | Foliar | 4.0 ± 0.14bc | 2.4 ± 0.14a | 3.5 ± 0.08ab | 20.3 ± 2.19ab | 3.2 ± 0.21abc | 1.4 ± 0.17b | 0.447 ± 0.01a | 0.134 ± 0.00d | 0.43 ± 0.07b | 7.34 ± 0.65a |
| Root | 3.8 ± 0.06cd | 2.4 ± 0.04a | 3.3 ± 0.05ab | 18.3 ± 0.67ab | 3.8 ± 0.27ab | 1.4 ± 0.01bc | 0.44 ± 0.01ab | 0.174 ± 0.01abc | 0.38 ± 0.03b | 7.24 ± 0.09a | |
| Control | Foliar | 3.4 ± 0.10de | 2.1 ± 0.06b | 3.3 ± 0.05ab | 14.3 ± 1.86b | 3.1 ± 0.22bc | 1.2 ± 0.09bc | 0.372 ± 0.02bc | 0.144 ± 0.01bcd | 0.39 ± 0.03b | 6.76 ± 0.92b |
| Root | 3.3 ± 0.13e | 2.1 ± 0.05b | 3.2 ± 0.05b | 14.5 ± 0.76b | 2.9 ± 0.05c | 1.1 ± 0.05c | 0.362 ± 0.03c | 0.138 ± 0.01cd | 0.39 ± 0.02b | 6.83 ± 0.07b |
| Treatment | Method of Application | Disease Incidence (%) | Control Effect (%) | Disease Severity (%) | Control Effect (%) |
|---|---|---|---|---|---|
| Control | Root | 76.7a | 0b | 82.4a | 0b |
| Foliage | 70ab | 0b | 79.3a | 0b | |
| AGE | Root | 43.3c | 42.3a | 21b | 73a |
| Foliage | 53.3bc | 23.4a | 22b | 71.4a | |
| ASA | Root | 46.7c | 38.6a | 22.6b | 72.4a |
| Foliage | 53.3bc | 23.4a | 30b | 59.7a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, S.; Ahmad, H.; Ali, M.; Hayat, K.; Khan, M.A.; Cheng, Z. Aqueous Garlic Extract as a Plant Biostimulant Enhances Physiology, Improves Crop Quality and Metabolite Abundance, and Primes the Defense Responses of Receiver Plants. Appl. Sci. 2018, 8, 1505. https://doi.org/10.3390/app8091505
Hayat S, Ahmad H, Ali M, Hayat K, Khan MA, Cheng Z. Aqueous Garlic Extract as a Plant Biostimulant Enhances Physiology, Improves Crop Quality and Metabolite Abundance, and Primes the Defense Responses of Receiver Plants. Applied Sciences. 2018; 8(9):1505. https://doi.org/10.3390/app8091505
Chicago/Turabian StyleHayat, Sikandar, Husain Ahmad, Muhammad Ali, Kashif Hayat, Muhammad Ali Khan, and Zhihui Cheng. 2018. "Aqueous Garlic Extract as a Plant Biostimulant Enhances Physiology, Improves Crop Quality and Metabolite Abundance, and Primes the Defense Responses of Receiver Plants" Applied Sciences 8, no. 9: 1505. https://doi.org/10.3390/app8091505





