Modeling of the Mechanical Behavior of 3D Bioplotted Scaffolds Considering the Penetration in Interlocked Strands
Abstract
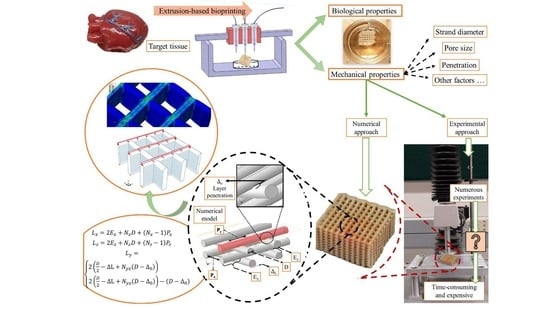
:1. Introduction
2. Materials and Methods
2.1. Material Preparation for Fabrication
2.2. Design and Fabrication of Scaffolds
2.3. Image Analyzing
2.4. Mechanical Testing
2.5. Finite Element Modeling
2.6. Statistical Analysis
3. Results and Discussion
3.1. Model Verification
3.2. Some More Simulation Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, M.G.; Tian, X.Y.; Chen, X.B. A brief review of dispensing-based rapid prototyping techniques in tissue scaffold fabrication: Role of modeling on scaffold properties prediction. Biofabrication 2009, 1, 032001. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Chen, X.B.; Schreyer, D.J. Experimental approaches to vascularisation within tissue engineering constructs. J. Biomater. Sci. Polym. Ed. 2015, 26, 683–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-B.B. Dispensed-Based Bio-Manufacturing Scaffolds for Tissue Engineering Applications. Int. J. Eng. Appl. 2014, 2, 1–31. [Google Scholar]
- Naghieh, S.; Badrossamay, M.; Foroozmehr, E.; Kharaziha, M. Combination of PLA Micro-fibers and PCL-Gelatin Nano-fibers for Development of Bone Tissue Engineering Scaffolds. Int. J. Swarm Intell. Evol. Comput. 2017, 6. [Google Scholar] [CrossRef]
- Naghieh, S.; Foroozmehr, E.; Badrossamay, M.; Kharaziha, M. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: Mechanical and biological assessment. Mater. Des. 2017, 133, 128–135. [Google Scholar] [CrossRef]
- Jin, T.; Stanciulescu, I. Numerical investigation of the influence of pattern topology on the mechanical behavior of PEGDA hydrogels. Acta Biomater. 2017, 49, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Reihany, A.; Haghighat, A.; Foroozmehr, E.; Badrossamay, M.; Forooghi, F. Fused Deposition Modeling and Fabrication of a Three-dimensional Model in Maxillofacial Reconstruction. Regen. Reconstr. Restor. 2016, 1, 139–144. [Google Scholar]
- Naghieh, S.; Karamooz Ravari, M.R.R.; Badrossamay, M.; Foroozmehr, E.; Kadkhodaei, M. Numerical investigation of the mechanical properties of the additive manufactured bone scaffolds fabricated by FDM: The effect of layer penetration and post-heating. J. Mech. Behav. Biomed. Mater. 2016, 59, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, M.M.; Naghieh, S.; Mcinnes, A.D.A.D.; Schreyer, D.J.D.J.; Chen, X. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol. J. 2018, 1700635. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Sarker, M.; Izadifar, M.; Chen, X. Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications—A brief review. J. Mech. Behav. Biomed. Mater. 2018, 78, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Ariful Islam Sarker, M.; Izadifar, M.; Schreyer, D.; Chen, X. Influence of ionic cross linkers (Ca2+/Ba2+/Zn2+) on the Mechanical and Biological Properties of 3D Bioplotted Hydrogel Scaffolds. J. Biomater. Sci. Polym. Ed. 2018, 29, 1126–1154. [Google Scholar] [CrossRef] [PubMed]
- Basu, B. Fundamentals of Scaffolds Fabrication Using Low Temperature Additive Manufacturing. In Biomaterials for Musculoskeletal Regeneration; Springer: Berlin, Germany, 2017; pp. 127–173. [Google Scholar]
- Das, S.; Pati, F.; Choi, Y.-J.; Rijal, G.; Shim, J.-H.; Kim, S.W.; Ray, A.R.; Cho, D.-W.; Ghosh, S. Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Sirohi, R.; Archana, D.; Jain, A.; Dutta, P.K. Porous chitosan scaffolds: A systematic study for choice of crosslinker and growth factor incorporation. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 242–252. [Google Scholar] [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.-J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadifar, Z.; Chang, T.; Kulyk, W.M.; Chen, D.; Eames, B.F. Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng. Part C Methods 2016, 22, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2013, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Wu, X.; Chen, X. 3D Printing of Porous Alginate/gelatin Hydrogel Scaffolds and Their Mechanical Property Characterization. J. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 299–306. [Google Scholar] [CrossRef]
- Bracaglia, L.G.; Smith, B.T.; Watson, E.; Arumugasaamy, N.; Mikos, A.G.; Fisher, J.P. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017, 56, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Olubamiji, A.D.; Izadifar, Z.; Si, J.L.; Cooper, D.M.L.; Eames, B.F.; Chen, D.X. Modulating mechanical behaviour of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight and pore geometry. Biofabrication 2016, 8, 025020. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Ravari, M.R.K.; Badrossamay, M.; Foroozmehr, E.; Kadkhodaei, M. Finite element analysis for predicting the mechanical properties of bone scaffolds fabricated by fused deposition modeling (FDM). In Modares Mechanical Engineering, Proceedings of the Advanced Machining and Machine Tools Conference, Tehran, Iran, 4–5 November 2015; Tarbiat Modares University Press: Tehran, Iran, 2015; Volume 15, pp. 450–454. [Google Scholar]
- Bawolin, N.K. Modeling Material-Degradation-Induced Elastic Property of Tissue Engineering Scaffolds. J. Biomech. Eng. 2010, 132, 111001. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Chen, X.B. Modeling the flow behavior and flow rate of medium viscosity alginate for scaffold fabrication with a 3D bioplotter. J. Manuf. Sci. Eng. 2017, 139, 081002. [Google Scholar] [CrossRef]
- ASTM: D 695 15, Standard Test Method for Compressive Properties of Rigid Plastics; ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM: F1635, Standard Guide for Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products; ASTM International: West Conshohocken, PA, USA, 2013.
- Nguyen, V.B.; Wang, C.X.; Thomas, C.R.; Zhang, Z. Mechanical properties of single alginate microspheres determined by microcompression and finite element modelling. Chem. Eng. Sci. 2009, 64, 821–829. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Chen, H.; Ozbolat, I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013, 5, 025004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Module: 2 Finite Element Formulation Techniques Lecture 3: Finite Element Method: Displacement Approach. Available online: https://nptel.ac.in/courses/105105041/m2l7.pdf (accessed on 20 July 2018).
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, F.; Eames, B.F.; Chen, X. Application of Extrusion-Based Hydrogel Bioprinting for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 1597. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Karamooz-Ravari, M.R.; Sarker, M.; Karki, E.; Chen, X. Influence of crosslinking on the mechanical behavior of 3D printed alginate scaffolds: Experimental and numerical approaches. J. Mech. Behav. Biomed. Mater. 2018, 80, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamooz Ravari, M.R.; Kadkhodaei, M.; Badrossamay, M.; Rezaei, R. Numerical investigation on mechanical properties of cellular lattice structures fabricated by fused deposition modeling. Int. J. Mech. Sci. 2014, 88, 154–161. [Google Scholar] [CrossRef]
- Karamooz Ravari, M.R.; Kadkhodaei, M.; Ghaei, A. A Unit Cell Model for Simulating the Stress-Strain Response of Porous Shape Memory Alloys. J. Mater. Eng. Perform. 2015, 24, 4096–4105. [Google Scholar] [CrossRef]
- Ravari, M.R.K.; Kadkhodaei, M.; Ghaei, A. Effects of asymmetric material response on the mechanical behavior of porous shape memory alloys. J. Intell. Mater. Syst. Struct. 2016, 27, 1687–1701. [Google Scholar] [CrossRef]
- Karamooz Ravari, M.R.; Kadkhodaei, M. A Computationally Efficient Modeling Approach for Predicting Mechanical Behavior of Cellular Lattice Structures. J. Mater. Eng. Perform. 2015, 24, 245–252. [Google Scholar] [CrossRef]
- Singh, R.; Lee, P.D.; Lindley, T.C.; Kohlhauser, C.; Hellmich, C.; Bram, M.; Imwinkelried, T.; Dashwood, R.J. Characterization of the deformation behavior of intermediate porosity interconnected Ti foams using micro-computed tomography and direct finite element modeling. Acta Biomater. 2010, 6, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K.; Chen, X. Synchrotron-Based in Situ Characterization of the Scaffold Mass Loss from Erosion Degradation. J. Funct. Biomater. 2016, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K.; Dolovich, A.T.; Chen, D.X.B.; Zhang, C.W.J. Characterization of mechanical properties of tissue scaffolds by phase contrast imaging and finite element modeling. J. Biomech. Eng. 2015, 137, 81004. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K.; Chen, X.B. Remote Determination of Time-Dependent Stiffness of Surface-Degrading-Polymer Scaffolds via Synchrotron-Based Imaging. J. Biomech. Eng. 2017, 139, 41004. [Google Scholar] [CrossRef] [PubMed]








| Concentration | Needle Diameter (µm) | Head Speed (mm/s) | Pressure (bar) | Temperature (°C) | Crosslinker |
|---|---|---|---|---|---|
| 3% (w/v) | 200 | 8 | 0.2 | 18–20 | CaCl2 (50 mM) |
| Parameters | Values (µm) |
|---|---|
| Nx | 7 |
| Nyx | 15 |
| Nyz | 16 |
| Nz | 7 |
| D | 580 |
| 0 | 510 |
| L | 10 |
| Px | 470 |
| Pz | 390 |
| Ex | 10 |
| Ez | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naghieh, S.; Sarker, M.D.; Karamooz-Ravari, M.R.; McInnes, A.D.; Chen, X. Modeling of the Mechanical Behavior of 3D Bioplotted Scaffolds Considering the Penetration in Interlocked Strands. Appl. Sci. 2018, 8, 1422. https://doi.org/10.3390/app8091422
Naghieh S, Sarker MD, Karamooz-Ravari MR, McInnes AD, Chen X. Modeling of the Mechanical Behavior of 3D Bioplotted Scaffolds Considering the Penetration in Interlocked Strands. Applied Sciences. 2018; 8(9):1422. https://doi.org/10.3390/app8091422
Chicago/Turabian StyleNaghieh, Saman, M. D. Sarker, Mohammad Reza Karamooz-Ravari, Adam D. McInnes, and Xiongbiao Chen. 2018. "Modeling of the Mechanical Behavior of 3D Bioplotted Scaffolds Considering the Penetration in Interlocked Strands" Applied Sciences 8, no. 9: 1422. https://doi.org/10.3390/app8091422