1. Introduction
Evaporation processes are of interest in numerous areas such as agricultural [
1], industrial [
2], and academic [
3] communities, as well as for individuals [
4]. When evaporating at room temperature, the substrate material plays an important role in the evaporation process, as does the ambient air relative humidity [
5]. However, the ambient air relative humidity is less important when droplets evaporate on substrates at elevated temperatures [
6]. In a recent study of a wide range of water droplets sizes in the transition regime of a boiling crisis, Misyura [
7] demonstrated that several material parameters are important when water droplets hit objects, wet the surface in different ways, and then start evaporating. Droplet contact angles play a role [
8], and may depend on impurities, additives, etc. Additives may therefore be used to reduce surface tension and thus improve performance [
9,
10]. Some researchers have studied the behavior of different droplet parameters such as size, impinging velocity [
11], wall material [
12,
13], temperature below the Leidenfrost point [
14], and the influence of solid–liquid contact time [
15]. A review concerning droplets impinging onto hot metals was conducted by Liang and Mudawar [
16] for all of the involved boiling regimes for water droplets on metal surfaces. A literature review on the Leidenfrost temperature for water on different heated materials is given by Bernardin and Mudawar [
17]. Water droplet cooling efficiency for droplets hitting Teflon-covered copper was recorded by Sawyer et al. [
18], who reported a cooling efficiency in the range of 50% to 90% at temperatures up to the temperatures associated with a boiling crisis. However, no studies were identified as covering the whole range from below the boiling point and all the way into the film-boiling regime.
In the process industry, fire-exposed pressurized pipes and vessels may rupture violently and release their combustible or poisonous contents if weakened by overheating. Considerable resources are therefore used for designing and maintaining active fire protection systems, i.e., deluge systems, fire monitors, etc. The NORSOK standard S-001 [
19] devotes multiple pages to active fire protection. For process areas, the design criteria is 10 L/min·m
2. However, studies of the performance of these systems are scarce, and would need to cover all of the involved boiling regimes. Full-scale experiments were performed by Kazemi [
20] and Drange [
21] in standardized 0.3 kg/s jet fire scenarios. Deluge and fire monitors in large-scale jet fires were studied by Opstad [
22]. None of these studies reported water droplet cooling efficiency.
The purpose of the present study was to develop a method for measuring the cooling efficiency of water droplets impinging onto hot stainless steel and aluminum in the temperature range of 80–400 °C. This temperature range covers the different boiling regimes that are associated with water droplets on metal substrates. These water droplet boiling regimes are presented in
Section 2. The apparatus that was developed for the study is presented in
Section 3. The theory and calculative method to reveal the cooling efficiency based on temperature versus time measurements with and without water cooling is outlined in
Section 4. Statistical analysis in order to reveal the differences in cooling efficiency as a factor of surface roughness is described in
Section 5, and the overall results are discussed in
Section 6. The possibility for using the test rig for teaching purposes regarding water droplet boiling regimes and water droplet efficiency is also discussed.
5. Results for Stainless Steel and Aluminum with a Surface Roughness of Ra 0.4 and Ra 3
Water droplets impinging onto stainless steel and aluminum discs with a surface roughness of Ra 0.4 and Ra 3 were studied in the present work to demonstrate the method for recording water droplet cooling efficiency. A series of five tests with water droplet application, which were sandwiched by two reference measurements as explained earlier, were needed due to the stochastic variations observed, especially close to the temperatures associated with critical boiling.
5.1. Results of the Stainless Steel Surface Roughness Measurements
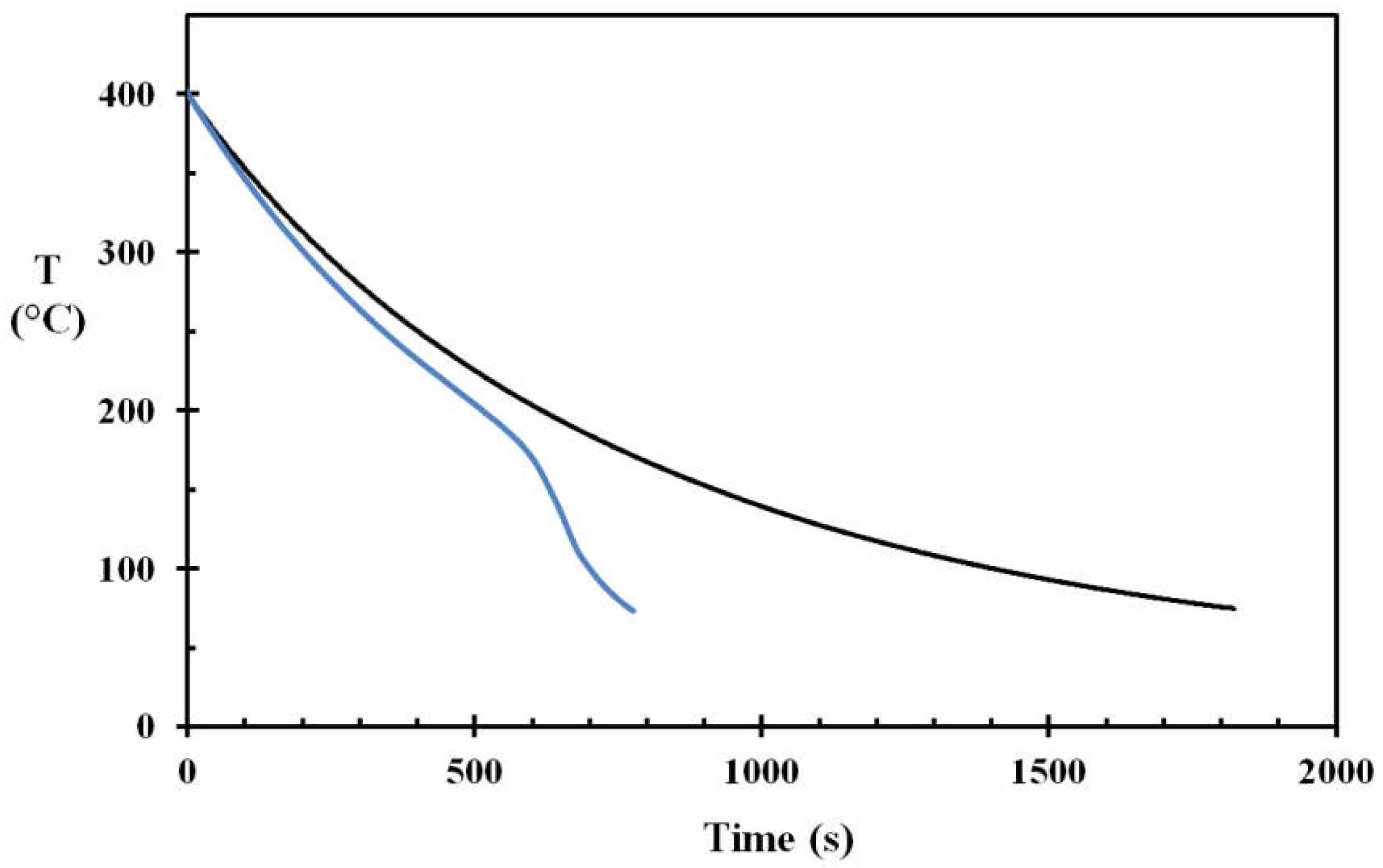
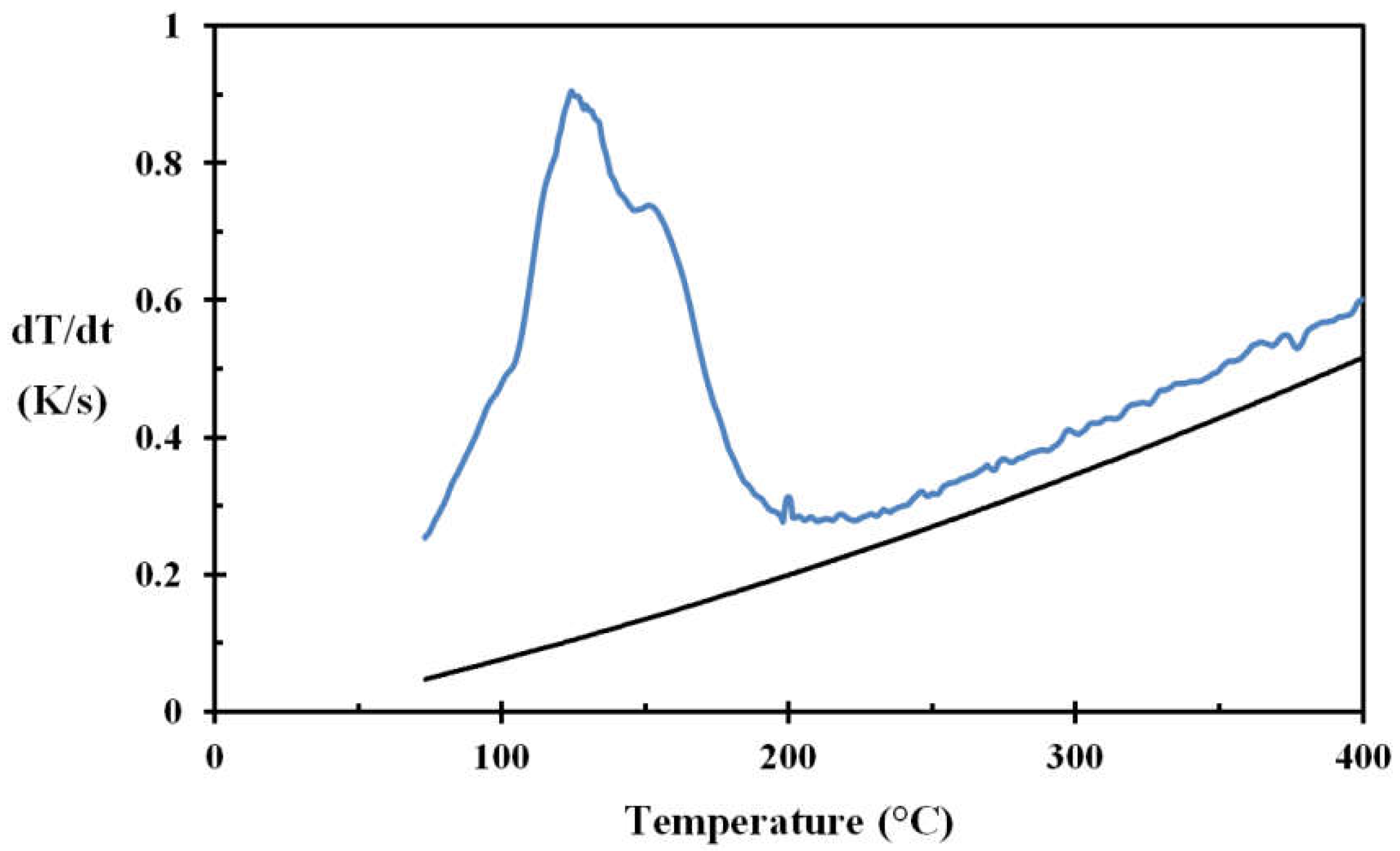
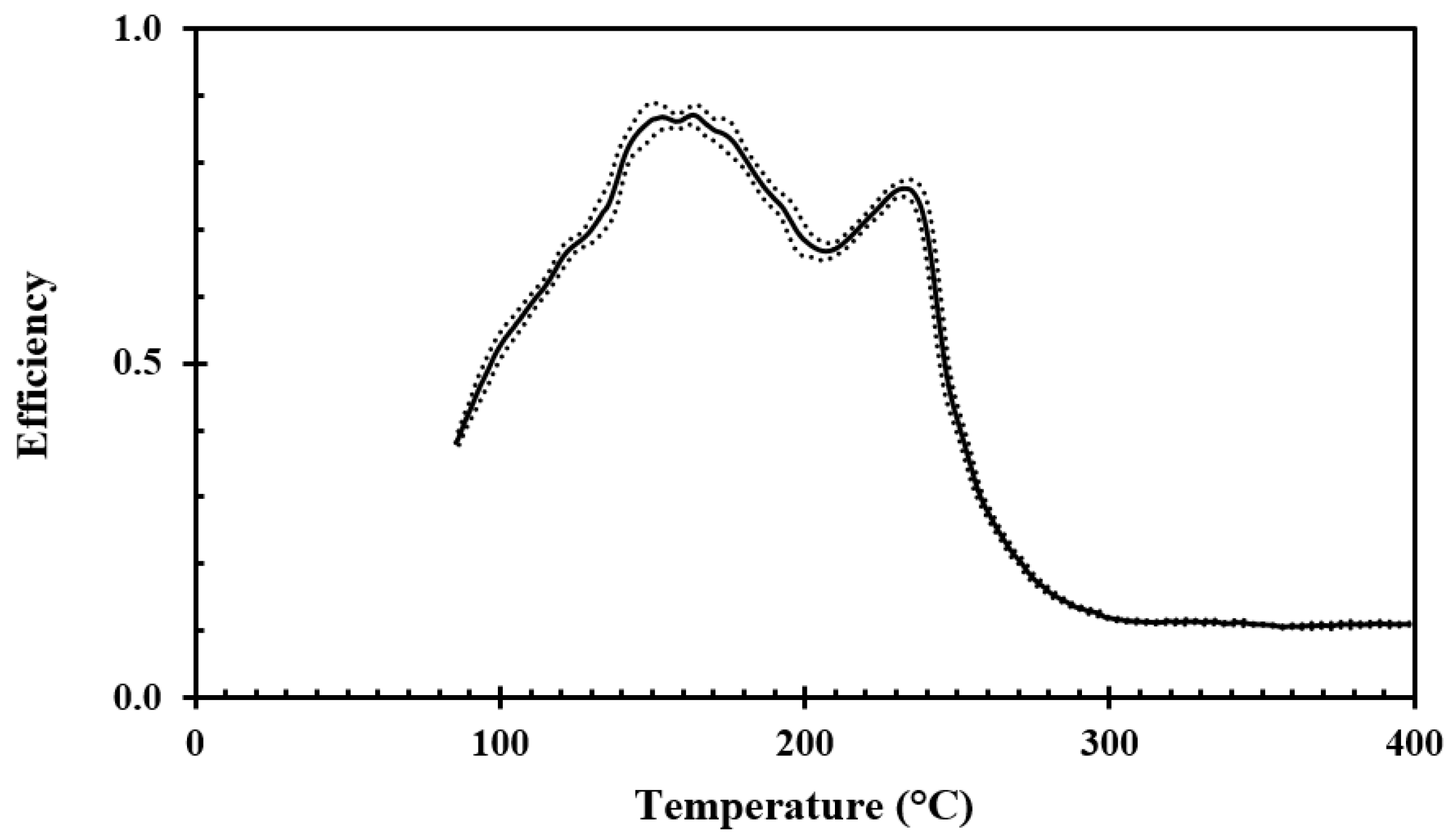
For testing the droplet cooling efficiency for the stainless steel disc, a water application rate of 0.02 g/s and droplet size of 2.4 mm, giving a droplet frequency of 3 s
−1, were targeted. The droplet speed, which was calculated through the fall height and acceleration of gravity, was 2.2 m/s. According to Pasandideh-Fard et al. [
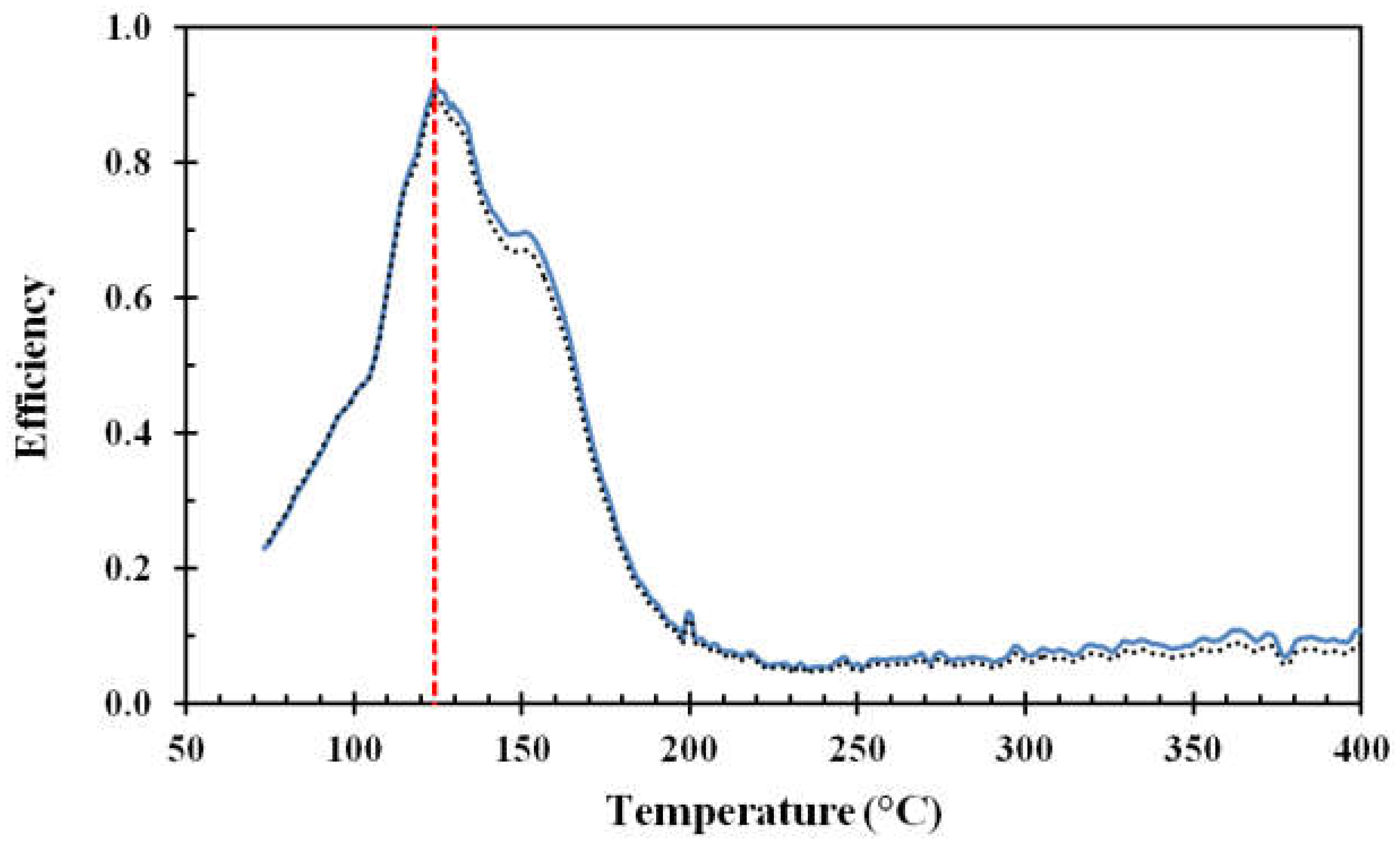
14], this corresponds well to the droplet speed obtained by photographic methods for similar sized droplets and similar fall heights. The results obtained for surface roughnesses of Ra 0.4 and Ra 3, are shown in
Figure 8 and
Figure 9, respectively.
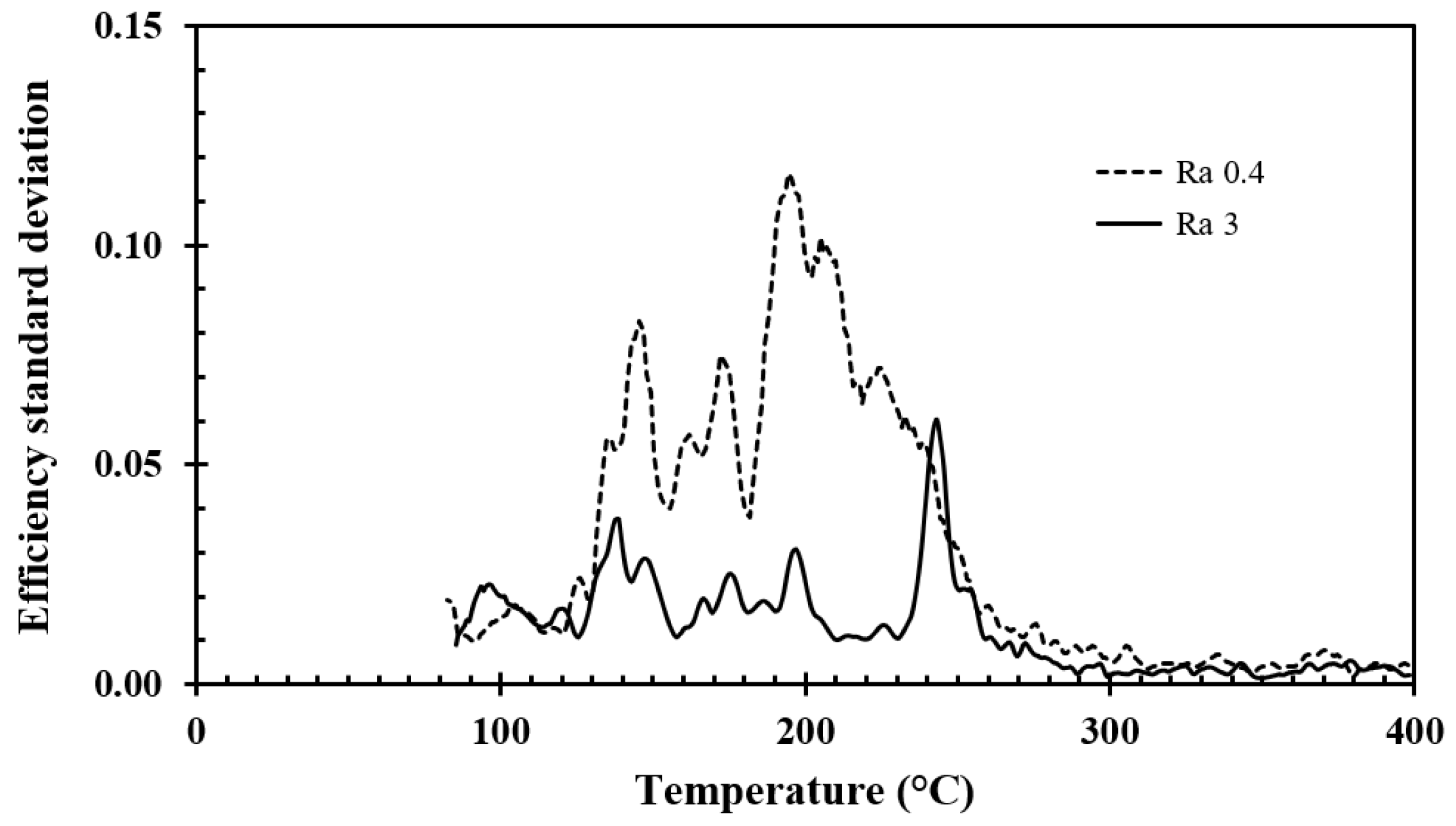
The standard deviation of the efficiency recorded for the surface roughnesses studied, i.e., Ra 0.4 and Ra 3, is shown in
Figure 10 as a function of temperature. The average results for the stainless steel discs with surface roughnesses of Ra 0.4 and Ra 3 are compared in
Figure 11.
A Student’s
t-test was used to evaluate whether the efficiency recorded for the stainless steel discs with surface roughnesses of Ra 0.4 and Ra 0.3 were significantly different. Checking through the whole temperature range from 85 °C to 400 °C and requiring an alpha <0.05 indicated that the difference in efficiency as presented in
Figure 11 was not statistically valid.
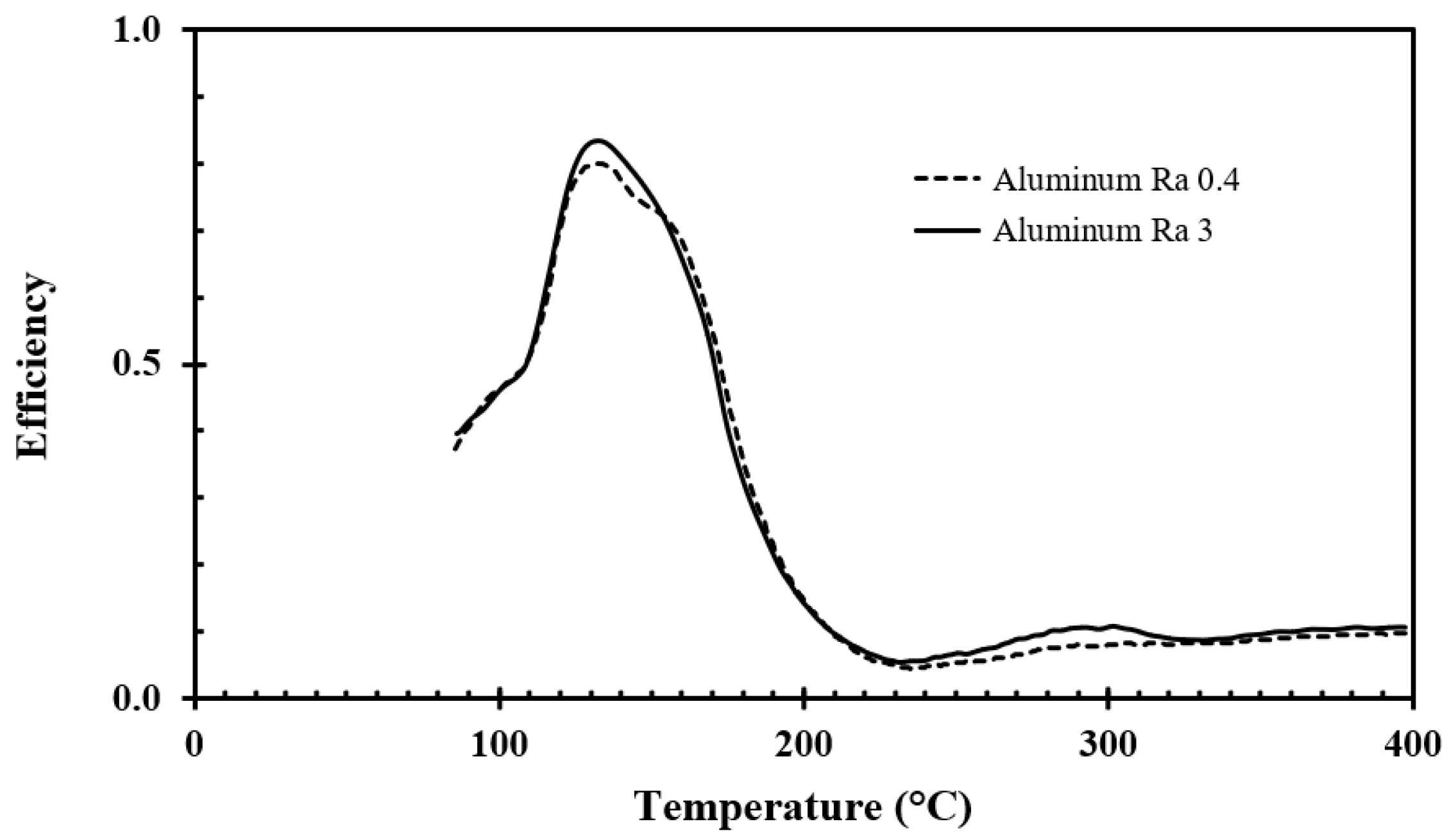
5.2. Results of the Aluminum Surface Roughness Measurements
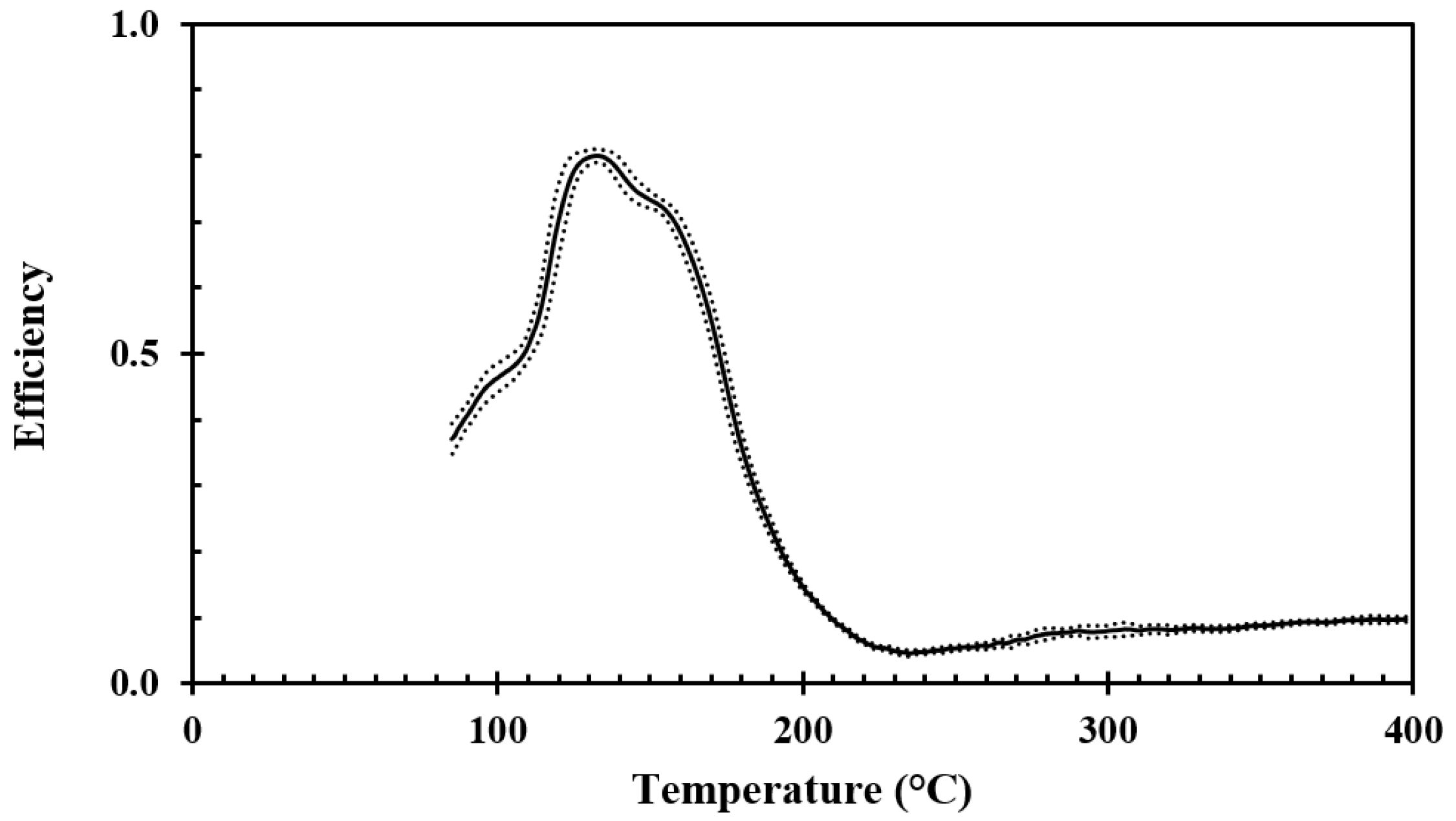
For recording the droplet cooling efficiency of the aluminum discs, similar water application rates, droplet sizes, droplet frequencies, and droplet speeds as for the stainless steel tests were used, i.e., respectively 0.02 g/s, 2.4 mm, 3 s
−1, and 2.2 m/s. The results obtained for surface roughnesses of Ra 0.4 and Ra 3 are shown in
Figure 12 and
Figure 13, respectively.
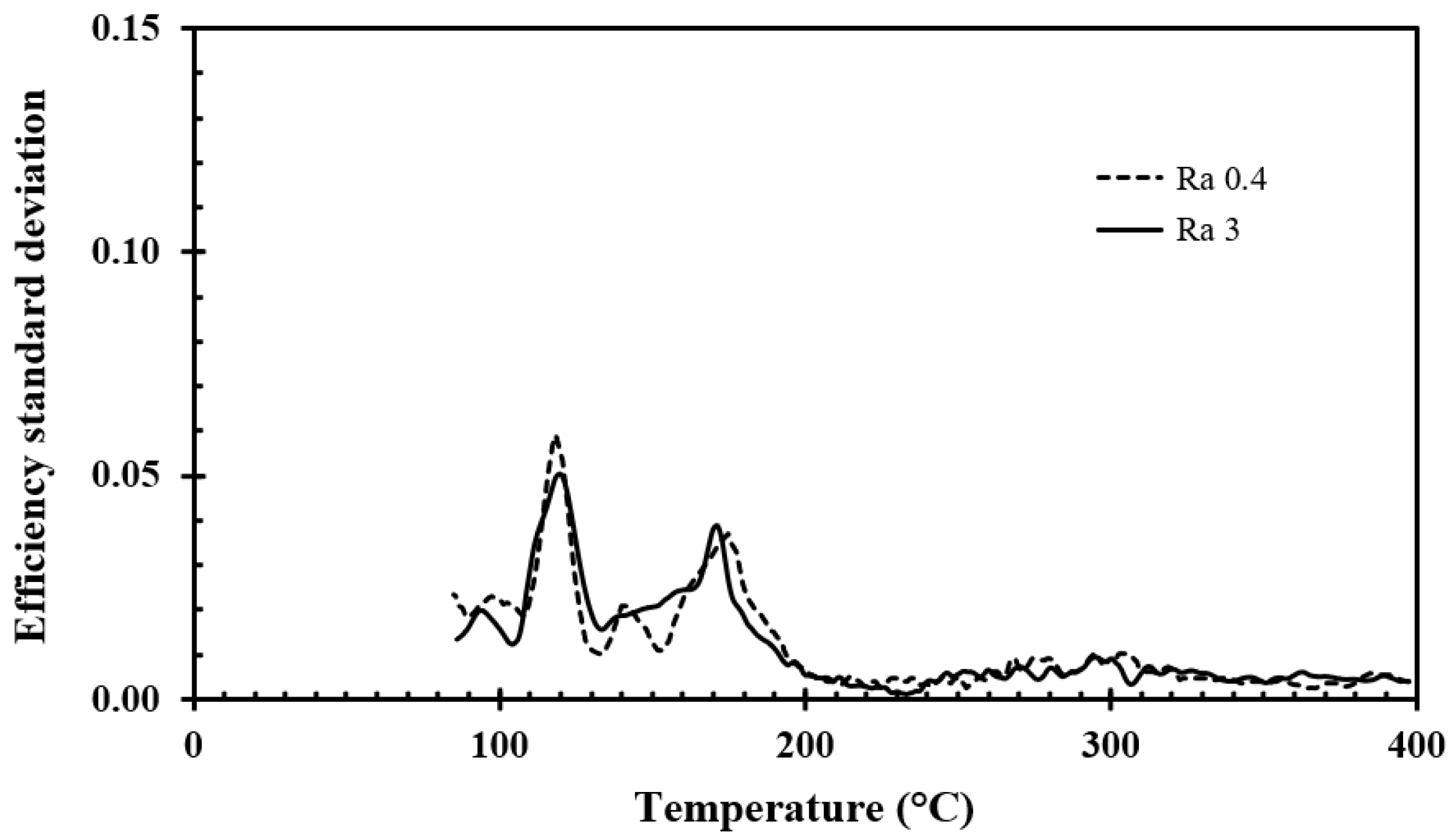
The standard deviation of the efficiency recorded for the surface roughnesses studied, i.e., Ra 0.4 and Ra 3, is shown in
Figure 14 as a function of temperature. The average results for the aluminum discs with surface roughnesses of Ra 0.4 and Ra 3 are compared in
Figure 15.
The average results for the aluminum discs with surface roughnesses of Ra 0.4 and Ra 3 are compared in
Figure 15.
For aluminum, the Student’s t-test (alpha <0.05) showed that the recorded difference in efficiency for surface roughnesses of Ra 0.4 and Ra 3 was significant only for temperatures between 220–310 °C.
5.3. Comparing the Results for Stainless Steel and Aluminum
The results for droplets impinging onto the stainless steel and aluminum discs with surface roughnesses of Ra 0.4 and Ra 3 are compared in
Figure 16 and
Figure 17, respectively. It is clearly seen from these figures that there are quite significant differences in the water droplet efficiency between the respective metals. However, for both of these metals, quite low droplet cooling efficiencies were recorded at temperatures above 300 °C.
5.4. NORSOK Requirements versus Spray Efficiency
Applying a certain spray flux
(kg/s·m
2) with a cooling efficiency of
to a hot surface above the boiling point of the liquid, the cooling potential is given by:
Assuming that at e.g., 400 °C the cooling efficiency is about 0.10, a NORSOK [
19] spray application rate of 10 L/min·m
2, i.e., 0.167 kg/s·m
2, would be able to withdraw 43 kW/m
2 from the fire exposed surface. This is much lower than the heat flux from an industrial fire to an exposed object at 400 °C. Dependent on the fire scenario, this object may experience net heat flux levels between 200–350 kW/m
2.
The low cooling efficiency experienced above the Leidenfrost temperature explains why it is very important to activate deluge systems early in order to cool pressurized pipes and process equipment in order to keep the temperature development under control. If not, the water spray may have limited effects, and may not be able to prevent hot spot formation and a possible loss of containment, possibly resulting in a severely increased fire scenario.
5.5. Error Analysis
It was not possible to get exactly the same water application rate and exactly the same droplet diameters for each measurement series with the current setup. However, the standard deviation was within 2% for the water application rate and within ±1% for the droplet diameter. The 25.0-cm elevation for droplet acceleration was measured to be within 1 mm, i.e., representing less than 1% error in the droplet speed. Given the rather large standard deviations observed for the stainless steel discs, the droplet diameter, application rate, and droplet speed can be considered sufficiently constant for comparing the results from the different measurement series.
The discs were aligned horizontally to within 0.5° before each measurement series. However, there may be some slight misalignment during the heating and cooling cycles. This may to some extent influence the results. However, misalignment larger than 1° after the completion of each measurement series was not discovered. It is therefore reasonable to assume that the differences recorded in the water droplet cooling efficiencies were due to the different metals used, and for some temperature regions, the surface roughness.
The reason for the larger standard deviation in the recorded efficiency for stainless steel compared with aluminum may be due to the lower thermal conductivity/diffusivity of the stainless steel. When two consecutive droplets hit exactly the same spot, which ideally was the goal, the stainless steel surface did not recover thermally as much between each droplet impact as was the case for the aluminum discs. If a droplet then hit just off the impact point of the previous droplets, it would be partly exposed to a higher surface temperature. The one order of magnitude higher heat transport properties of aluminum better evened out the cooling impacts by the previous droplets. When the temperature gradients for aluminum were less steep, it did not make much difference whether the subsequent droplets hit the same location, or were slightly off from that location. Therefore, it was expected that the standard deviations experienced for the droplets impacting onto the aluminum discs would be lower than for the stainless steel discs.
6. Discussion
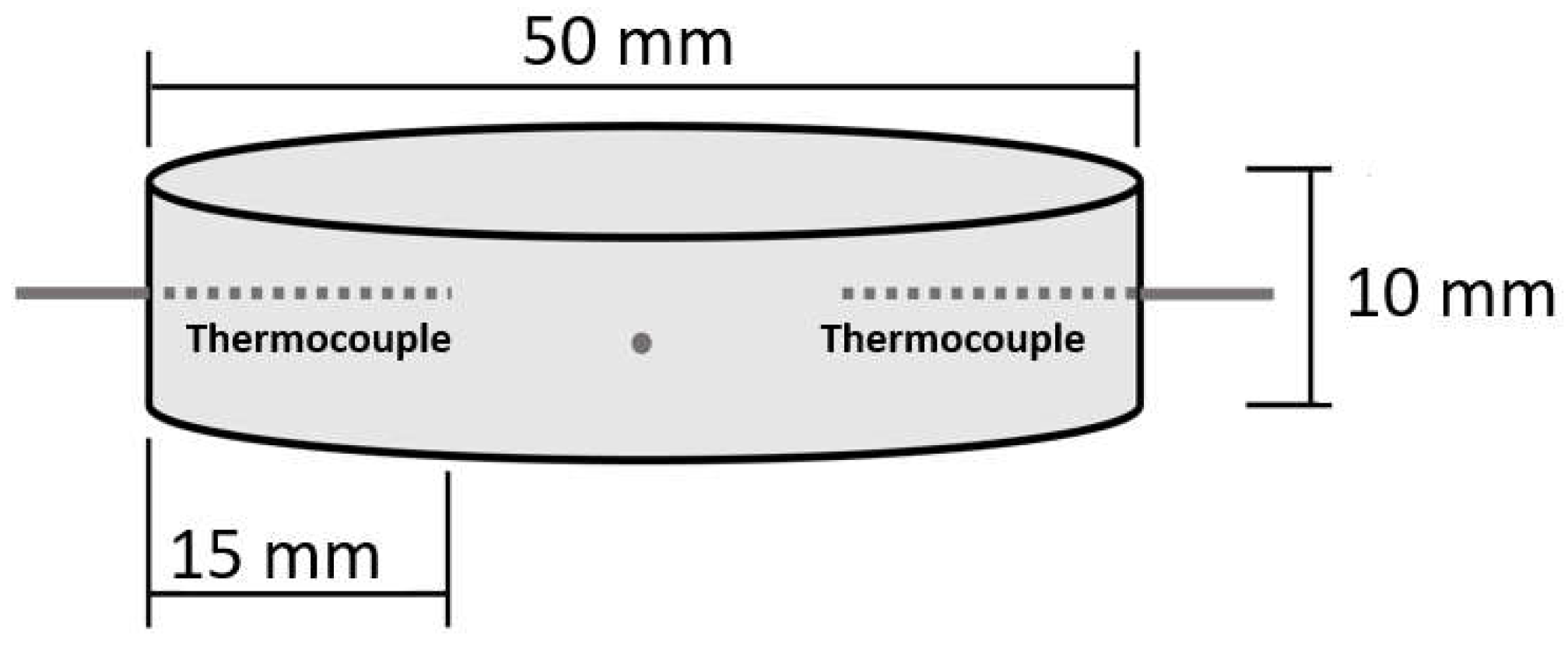
A method for measuring the cooling efficiency of water droplets impinging onto hot metal surfaces was developed. The test apparatus was described, measurements were performed on stainless steel and aluminum discs, and the recordings were processed to reveal the cooling efficiency in the temperature range between 85–400 °C. It was also investigated whether the method can reveal any differences in the cooling efficiency as a function of the surface roughness. Therefore, the 50-mm diameter and 10-mm thick metal discs tested were prepared with roughnesses of Ra 0.4 and Ra 3 for both materials. The test setup was validated by droplets (with a 2.4-mm diameter) generated by the acceleration of gravity from a hypodermic injection needle impacting onto the disc center at a speed of 2.2 m/s and a rate of 0.02 g/s, i.e., about three droplets per second.
The horizontally aligned disc was suspended by four thermocouples penetrating the disc 15-mm radially, along the horizontal symmetry plan. This solution minimized contact between the disc and other objects, and allowed for recording the disc temperature during the cooling phase with and without cooling the disc by water droplets. Several researchers placed a thermocouple junction either flush with or 0.5 mm below the droplets’ impact point [
11,
14]. This allowed them to record the surface temperature at the impact point. The thermocouple then induces a disturbance in the metal exactly at the point where the droplets hit the surface. The present method aimed at recording the temperature within the metal disc, with four symmetrically located thermocouples giving minimal disturbances to the droplet impact point. This gave a better representation of the water-cooling process of metallic objects, as such, during the cooling process. The tradeoff by losing information about the impact point surface temperature was thus outweighed by the possibility for better determining the water droplet cooling efficiency.
For the aluminum discs, the water droplet cooling efficiency associated with the critical heat flux, i.e., at about 125 °C, was in the range of 80% to 85%. The lowest water droplet cooling efficiency, i.e., in the range of 6% to 10%, was observed at a disc temperature of about 230 °C. Since this temperature was associated with a minimum in the cooling efficiency, it may be defined as the Leidenfrost temperature. The cooling efficiency then slightly increased with increasing temperature up to 400 °C due to the increasing temperature difference between the disc surface and the evaporating levitated droplet.
For stainless steel discs, at the recorded temperatures associated with the critical heat flux, i.e., about 150 °C to 180 °C, the droplet cooling efficiency was found to be in the range of 70% to 80%. The reason for this slightly lower maximum cooling efficiency compared to aluminum is likely due to the differences in thermal properties, i.e., a thermal conductivity of 150 W/mK for aluminum versus 15 W/mK for stainless steel.
For aluminum, the surface temperatures recovered more between each droplet impact compared to stainless steel, i.e., the impingement point was more thermally disturbed by the time the next droplet hit the stainless steel surface. Small variations in droplet impact point and droplet movements just after impact likely explained the higher standard deviations in the recorded efficiency for the stainless steel discs. Large standard deviations in measurements from the temperature regions near the temperature associated with the critical heat flux are also reported in other studies [
16], although those researchers did not focus on droplet cooling efficiency.
Clear differences in cooling efficiency were demonstrated between aluminum and stainless steel (same roughness for both metal disc surfaces). It was more challenging to reveal the differences in cooling efficiency within the same material, i.e., for the two different roughnesses studied (Ra 0.4 and Ra 3). The Student’s t-test (alpha < 0.05) was applied to indicate whether there were statistically significant differences between the water droplet cooling efficiency obtained for Ra 0.4 and Ra 3. The observed water droplet cooling efficiency differences for Ra 0.4 and Ra 3 were for the aluminum discs significant (alpha > 0.05) only for temperatures between 220–310 °C, where the higher surface roughness (Ra 3) gave a slightly higher cooling efficiency. This may be explained by the better possibilities for the water vapor generated to escape through the crevices below the droplet, i.e., resulting in less distance between the droplet and the aluminum surface peaks.
Due to the larger standard deviation in cooling efficiency observed for the stainless steel discs, any observed differences between the surface roughnesses of Ra 0.4 and Ra 3 were not statistically significant, i.e., Student’s
t-test alpha > 0.05. Indeed, for temperatures above 265 °C, the cooling efficiency was practically equal for stainless steel surface roughnesses of Ra 0.4 and Ra 3. It is more difficult to explain this observation. However, many parameters do control the heat and mass transfer in the film boiling regime [
7]. For stainless steel, the influence of crevices and peaks may be less prominent due to the heat transfer capacity being 10 times lower than that of similar peaks on the aluminum surface.
The results indicate that the application of fire water early in a fire scenario is very important, i.e., while the water cooling will likely be most efficient and thereby may prevent further temperature increase past the temperature associated with a boiling crisis. Upon reaching the film boiling regime, the cooling efficiency of the stainless steel discs was shown to become as low as about 12% to 14% due to the stable vapor film below the droplet. Even with application of the recommended 10 L/min·m
2, i.e., 0.167 kg/s·m
2 recommended by NORSOK [
19], the spray would only be able to withdraw 50–60 kW/m
2 from the fire exposed surface. This is significantly lower than the heat flux that objects may be exposed to in industrial fires, i.e., 250 kW/m
2 to 350 kW/m
2. This is a paradox for fire safety engineering.
The literature study did not reveal research papers investigating the absolute cooling efficiency of water droplets applied to hot metal surfaces of e.g., 10-mm wall thickness. Therefore, the present work may be the first to attempt quantifying this for objects of representative wall thicknesses. However, it should be noted that a variety of metal qualities are used in process plants, and that the present study is limited to horizontally aligned stainless steel discs of 10-mm thickness. In the process industry, equipment and pipe work may also be thermally insulated and shielded by stainless steel cladding. Such cladding, with thicknesses of 0.5 mm or 0.7 mm, is unfortunately too thin to be analyzed by the presented setup.
Omitting the fine mesh screen and moving the core setup to the classroom for demonstrating water droplet behavior and cooling efficiency worked well for demonstrating the mentioned physical phenomena to BSc and MSc students in fire safety engineering. The students expressed an increased understanding of water droplets’ cooling efficiency as a function of substrate temperature. The potential of very low water cooling efficiency in fire scenarios was noted. Therefore, it can be concluded that this simple setup serves both scientific and educational purposes.