Plasmonic Conglobation of Ultrathin Ag Nanofilms Far below Their Melting Points by Infrared Illumination
Abstract
:1. Introduction
2. Materials and Methods
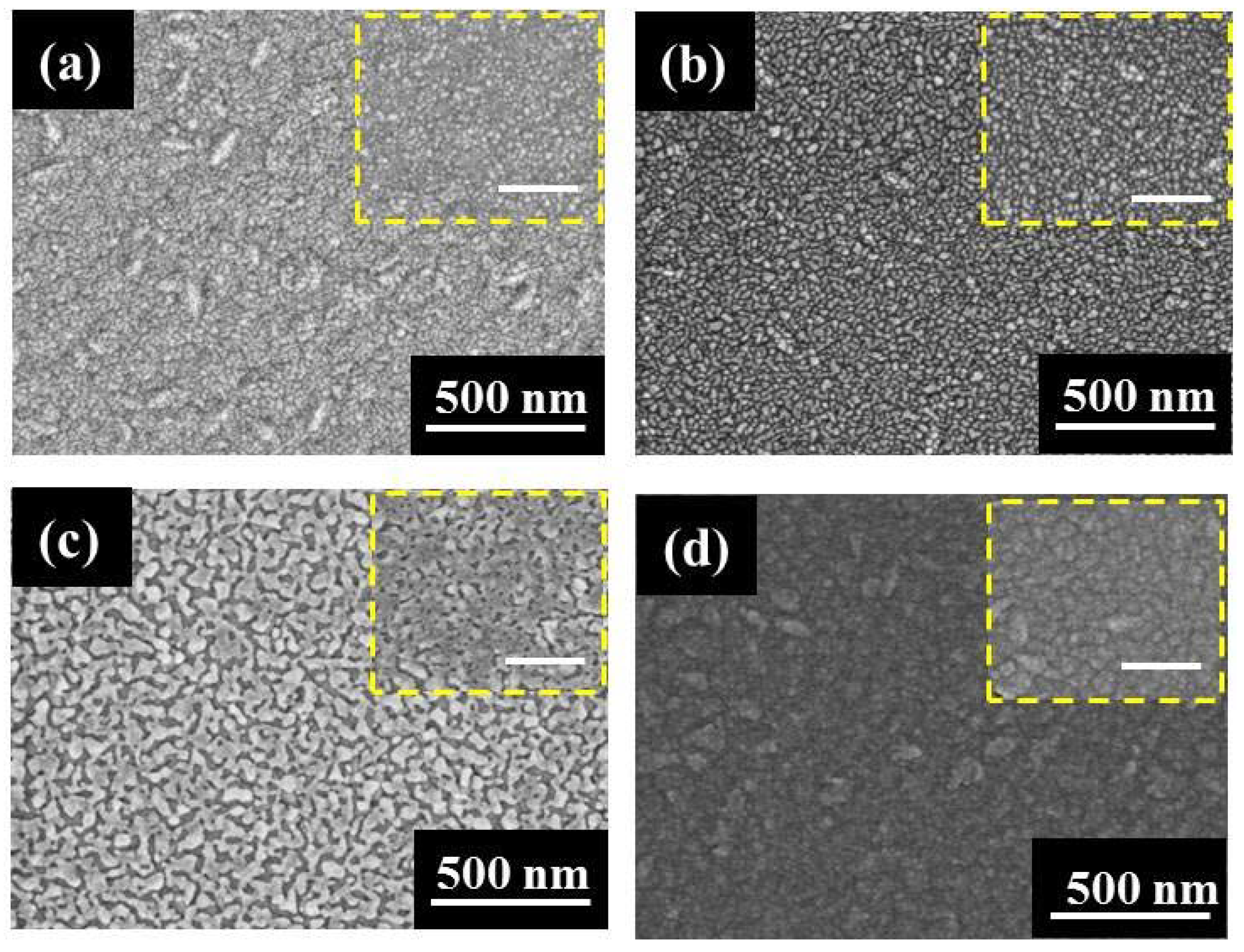
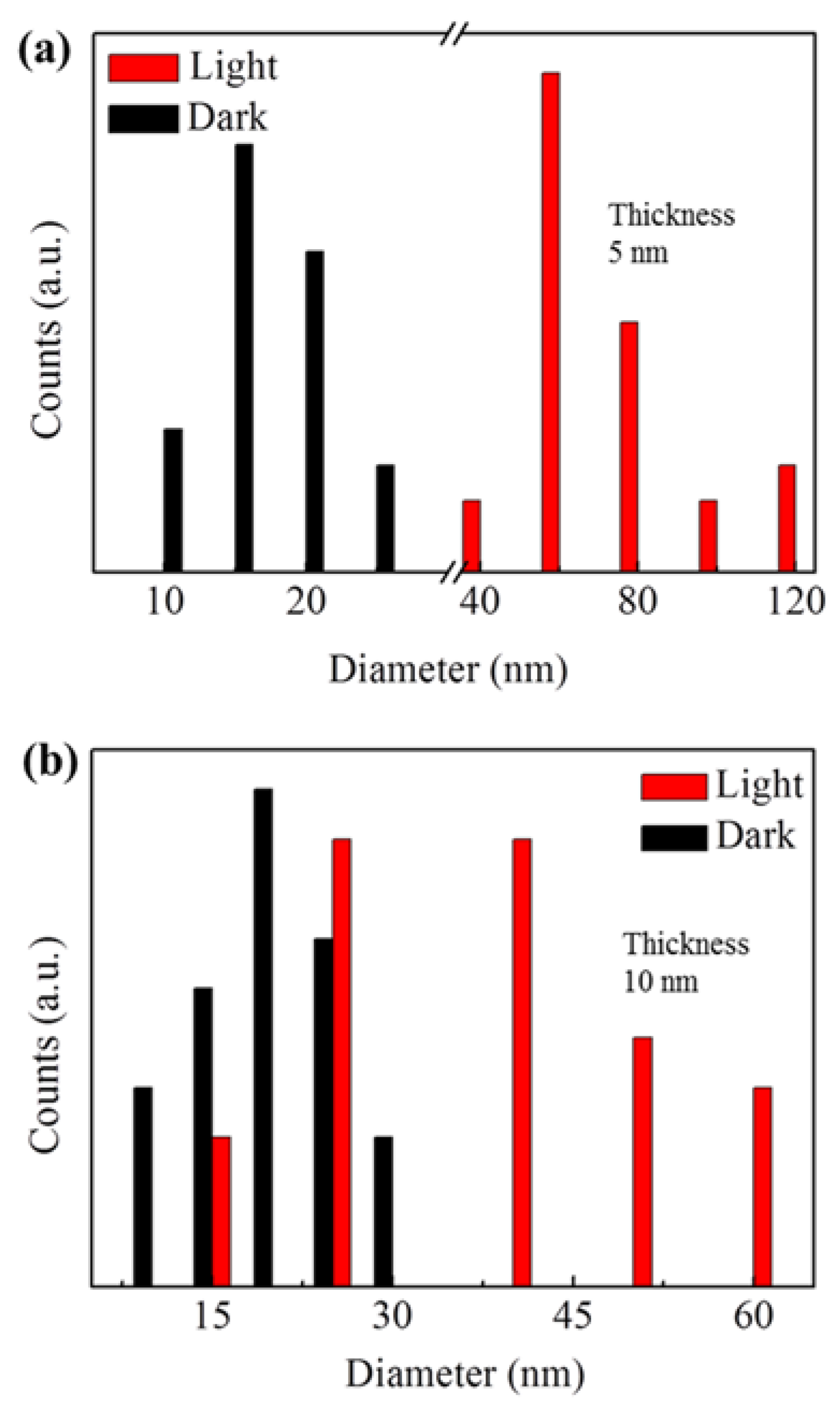
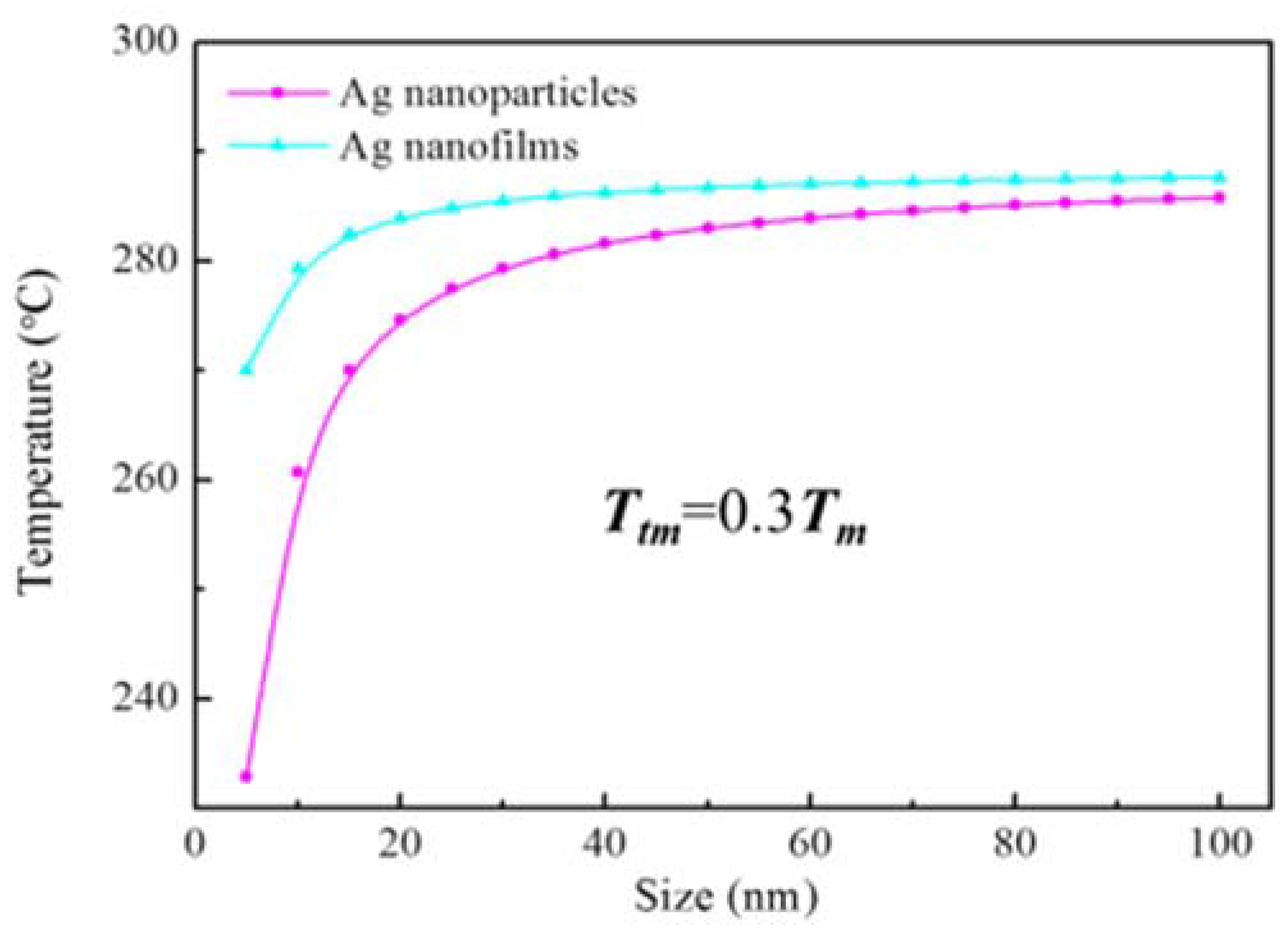
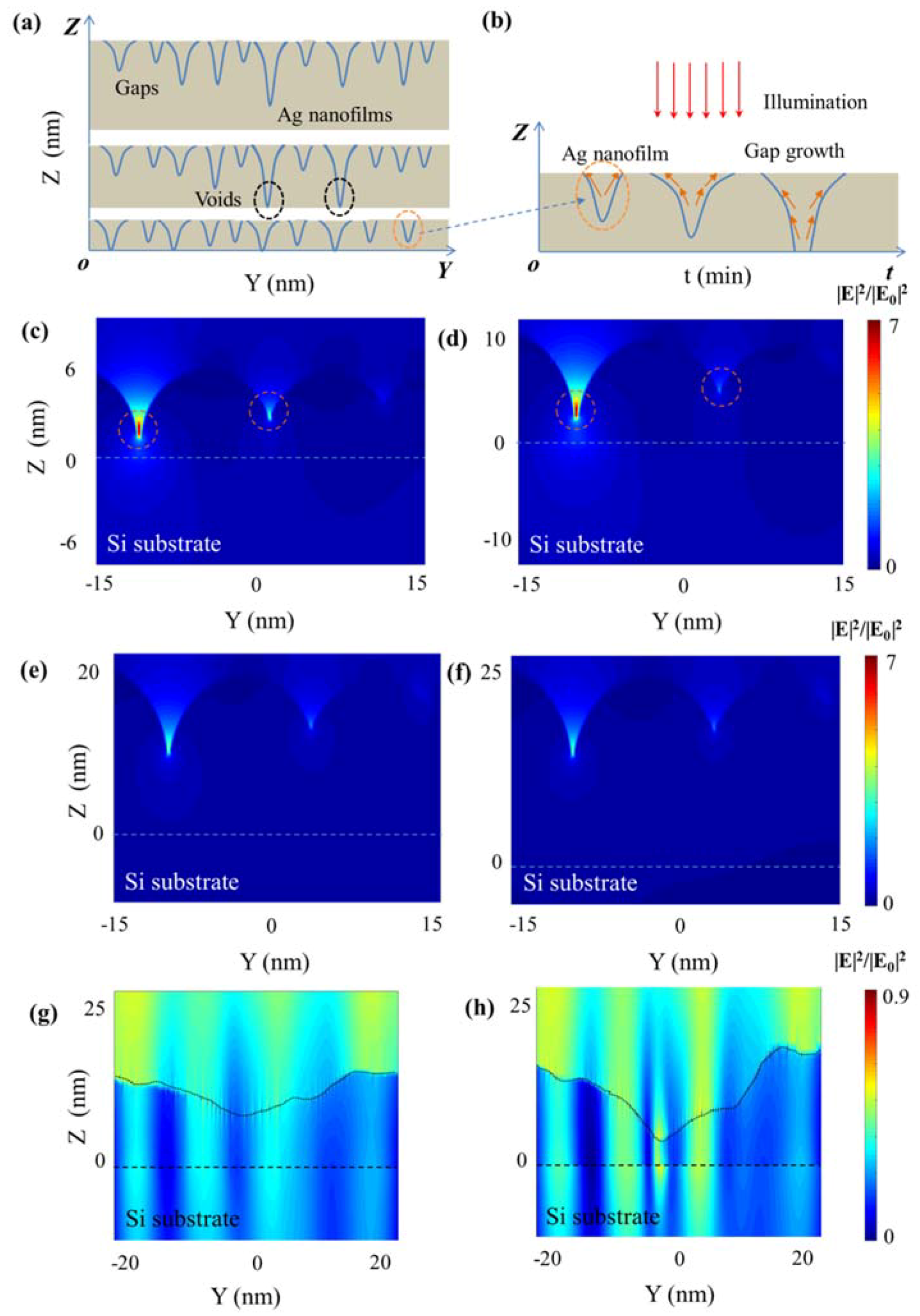
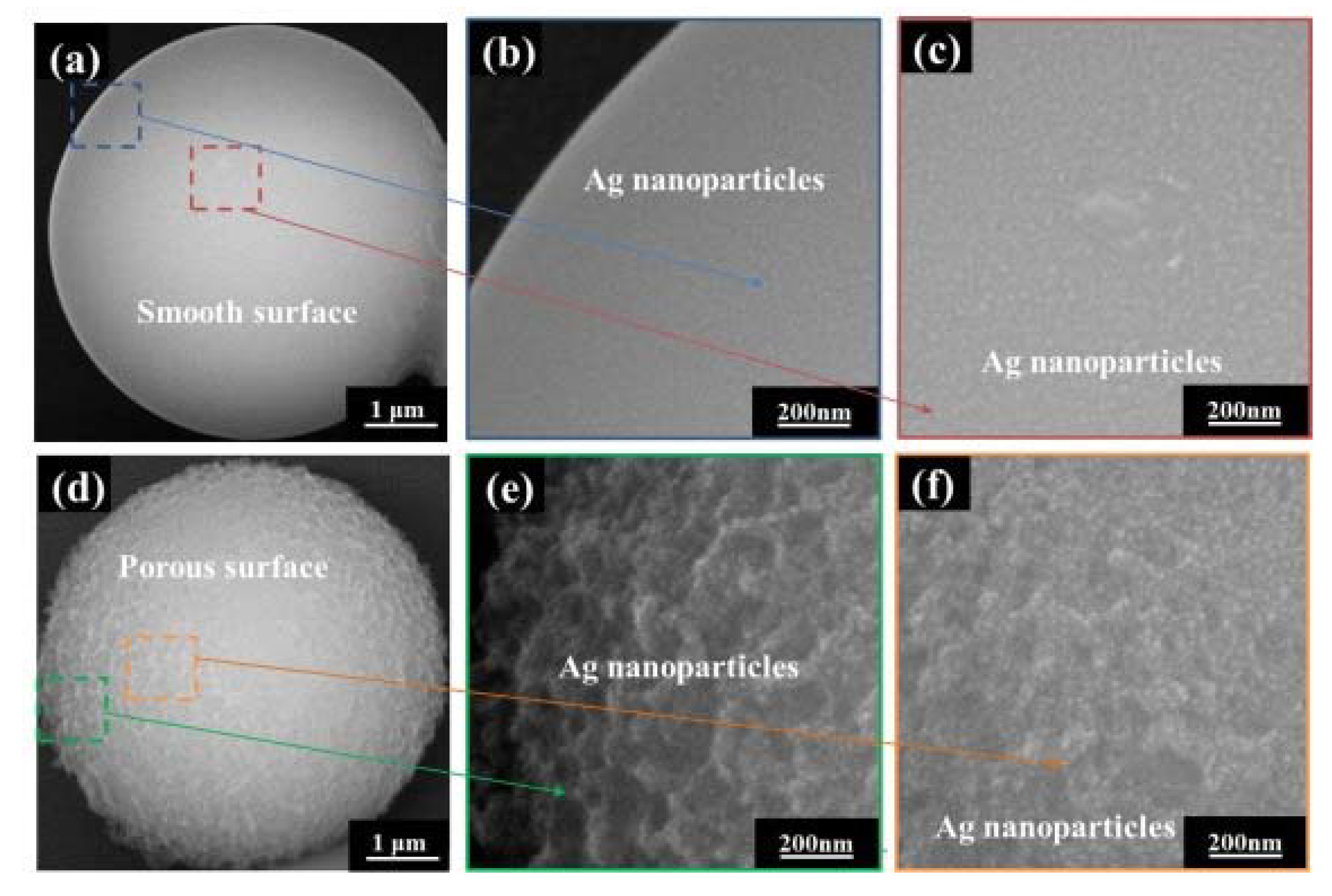
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franzen, S.; Folmer, J.C.W.; And, W.R.G.; O’Neal, R. Optical properties of dye molecules adsorbed on single gold and silver nanoparticles. J. Phys. Chem. A 2002, 106, 6533–6540. [Google Scholar] [CrossRef]
- Lu, L.; Luo, Z.; Xu, T.; Yu, L. Cooperative plasmonic effect of Ag and Au nanoparticles on enhancing performance of polymer solar cells. Nano Lett. 2013, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.P.; Kim, D.; Choe, M.; Lee, T.; Park, S.J.; Lee, D.S. A self-assembled Ag nanoparticle agglomeration process on graphene for enhanced light output in GaN-based LEDs. Nanotechnology 2012, 23, 255201. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ma, J.; Sun, X.; Chen, Z.; Jiang, X.; Guo, Z.; Huang, L.; Li, Y.; Wang, M.; Wang, C.; et al. Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res. 2009, 19, 1031–1304. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Choi, Y.J.; Park, H.H. Investigation of Ag-poly(3,4-ethylenedioxythiophene):polystyrene sulfonate nanocomposite films prepared by a one-step aqueous method. J. Appl. Phys. 2011, 109, 124902. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. Smart core−shell hybrid nanogels with ag nanoparticle core for cancer cell imaging and gel shell for PH-regulated drug delivery. Chem. Mater. 2010, 22, 1966–1976. [Google Scholar] [CrossRef]
- Norrman, K.; Ghanbari-Siahkali, A.; Larsen, N.B. Studies of spin-coated polymer films. Annu. Rep. Prog. Chem. Sect. C 2005, 101, 174–201. [Google Scholar] [CrossRef]
- Schilinsky, P.; Waldauf, C.; Brabec, C. Performance analysis of printed bulk heterojunction solar cells. Adv. Funct. Mater. 2010, 16, 1669–1672. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Garnett, E.C.; Cai, W.; Cha, J.J.; Mahmood, F.; Connor, S.T.; Greyson, C.M.; Yi, C.; McGehee, M.D.; Brongersma, M.L. Self-limited plasmonic welding of silver nanowire junctions. Nat. Mater. 2012, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.R.; Hüser, F.; Pauly, F.; Cuevas, J.C.; Natelson, D. Optical rectification and field enhancement in a plasmonic nanogap. Nat. Nanotechnol. 2010, 5, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hwang, G.T.; Kim, S.; Seo, J.; Park, H.J.; Yu, K.; Kim, T.S.; Lee, K.J. Flash-induced self-limited plasmonic welding of silver nanowire network for transparent flexible energy harvester. Adv. Mater. 2017, 29, 1603473. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Han, S.; Kim, D.; You, B.K.; Joe, D.J.; Hong, S.; Seo, J.; Kwon, J.; Jeong, C.K.; Park, H.J.; et al. Plasmonic-tuned flash Cu nanowelding with ultrafast photochemical-reducing and interlocking on flexible plastics. Adv. Funct. Mater. 2017, 27, 1701138. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Teo, E.J.; Toyoda, N.; Yang, C.; Wang, B.; Zhang, N.; Bettiol, A.A.; Teng, J.H. Sub-30 nm thick plasmonic films and structures with ultralow loss. Nanoscale 2014, 6, 3243–3249. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.B.; Siddiquee, A.M.; Chon, J.W. Below melting point photothermal reshaping of single gold nanorods driven by surface diffusion. ACS Nano 2014, 8, 12071–12079. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, T.; Saito, M.J. Effect of pretreatment on the 2-dimensional condensation of adsorbed krypton on alkali chlorides. Phys. Chem. 1967, 71, 453–454. [Google Scholar] [CrossRef]
- Nanda, K.K.; Sahu, S.N.; Behera, S.N. Liquid-drop model for the size-dependent melting of low-dimensional systems. Phys. Rev. A. 2002, 66, 013208. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.Y.; Wang, C.; Sun, S.; Lou, J. Cold welding of ultrathin gold nanowires. Nat. Nanotechnol. 2010, 5, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Ströbel, R.; Garche, J.; Moseley, P.T.; Jörissen, L.; Wolf, G. Hydrogen storage by carbon materials. J. Power Sources 2006, 159, 781–801. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhao, H.; Cai, W. Micro/nano-scaled carbon spheres based on hydrothermal carbonization of agarose. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 386–393. [Google Scholar] [CrossRef]
- Lu, A.H.; Zhang, X.Q.; Sun, Q.; Zhang, Y.; Song, Q.; Schüth, F.; Cheng, F. Precise synthesis of discrete and dispersible carbon-protected magnetic nanoparticles for efficient magnetic resonance imaging and photothermal therapy. Nano Res. 2016, 9, 1460–1469. [Google Scholar] [CrossRef]
- Li, D.; Han, D.; Qu, S.N.; Liu, L.; Jing, P.T.; Zhou, D.; Ji, W.Y.; Wang, X.Y.; Zhang, T.F.; Shen, D.Z. Supra-(carbon nanodots) with a strong visible to near-infrared absorption band and efficient photothermal conversion. Light Sci. Appl. 2016, 5, e16120. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Zhao, J.; Huang, T.; Yu, X.; Sun, J.; Fang, H.; Zhu, Z.; Zhang, M.; Yu, K. Plasmonic Conglobation of Ultrathin Ag Nanofilms Far below Their Melting Points by Infrared Illumination. Appl. Sci. 2018, 8, 897. https://doi.org/10.3390/app8060897
Dai H, Zhao J, Huang T, Yu X, Sun J, Fang H, Zhu Z, Zhang M, Yu K. Plasmonic Conglobation of Ultrathin Ag Nanofilms Far below Their Melting Points by Infrared Illumination. Applied Sciences. 2018; 8(6):897. https://doi.org/10.3390/app8060897
Chicago/Turabian StyleDai, Han, Junfeng Zhao, Tongjian Huang, Xinxiang Yu, Jie Sun, Hongjie Fang, Zhenfeng Zhu, Mingfu Zhang, and Kun Yu. 2018. "Plasmonic Conglobation of Ultrathin Ag Nanofilms Far below Their Melting Points by Infrared Illumination" Applied Sciences 8, no. 6: 897. https://doi.org/10.3390/app8060897



